Network analysis of sleep bruxism in the EPISONO adult general population
Summary
Sleep bruxism (SB) has been associated with biological and psychosocial factors. The assessment of SB includes self-report, clinical evaluation, and polysomnography. This study aimed to investigate the associations of self-reported SB with other sleep disorders and demographic, psychological, and lifestyle factors in the adult general population, and to investigate whether self-reported SB and polysomnographically (PSG) confirmed SB provide similar outcomes in terms of their associated factors. We recruited 915 adults from the general population in Sao Paulo, Brazil. All participants underwent a one-night PSG recording and answered questions about sex, age, BMI, insomnia, OSA risk, anxiety, depression, average caffeine consumption, smoking frequency, and alcohol consumption frequency. We investigated the link between SB and the other variables in univariate, multivariate, and network models, and we repeated each model once with self-reported SB and once with PSG-confirmed SB. Self-reported SB was only significantly associated with sex (p = 0.042), anxiety (p = 0.002), and depression (p = 0.03) in the univariate analysis, and was associated with insomnia in the univariate (p < 0.001) and multivariate (β = 1.054, 95%CI 1.018–1.092, p = 0.003) analyses. Network analysis showed that self-reported SB had a direct positive edge to insomnia, while PSG-confirmed SB was not significantly associated with any of the other variables. Thus, sleep bruxism was positively associated with insomnia only when self-reported, while PSG-confirmed SB was not associated with any of the included factors.
1 INTRODUCTION
Sleep bruxism (SB) is a repetitive jaw-muscle activity during sleep. Polysomnography (PSG), including electromyographic (EMG) recording of the jaw muscles, is the gold standard for SB assessment (Lobbezoo et al., 2018). Other means for SB assessment, albeit less accurate than PSG, are self-report and clinical assessments. On the basis of self-reports, the prevalence of sleep bruxism in the adult general population has been estimated at 8%–31.4% (Manfredini et al., 2013), while the only general population study so far using PSG has estimated the prevalence of sleep bruxism in adults at 7.4% (Maluly et al., 2013).
Sleep bruxism has been associated with several biological and psychosocial factors. Previous studies reported associations between sleep bruxism and psychological factors such as anxiety, depression (Goulart et al., 2021), and stress (van Selms et al., 2020), and between SB and lifestyle factors, such as smoking, alcohol consumption, and caffeine intake (Rintakoski & Kaprio, 2013). However, these studies were based on multivariate analyses with a single dependent variable. Network analysis, on the other hand, is a novel statistical method that assesses the associations between all variables that are potentially involved in a possible comorbidity network, without categorising them a priori as predictor or outcome (Epskamp et al., 2018). Using this technique in the Dutch National Sleep Registry (NSR) population, we found that self-reported SB was indirectly associated with insomnia, and that anxiety was a bridge factor between SB and insomnia (Chattrattrai et al., 2022). However, compared with an EMG device, the validity of self-reported SB was found to be lower (Stuginski-Barbosa et al., 2017). In addition, since the NSR population had a high prevalence of insomnia, it cannot be considered representative for the general population. Consequently, confirmation of our previous findings is needed in a general population sample that employs polysomnography to confirm sleep bruxism status.
Therefore, this study had two aims. The first aim was to investigate the associations of self-reported SB with other sleep disorders and demographic, psychological, and lifestyle factors in the adult general population. The hypothesis was that sleep bruxism in the adult general population is associated with insomnia via anxiety, as was observed previously in the NSR population. The second aim was to investigate whether self-reported SB and PSG-confirmed SB provide similar outcomes in terms of their associated factors. The hypothesis was that self-reported SB and PSG-confirmed SB yield the same associations with other variables.
2 METHOD
In this cross-sectional study, all participants, aged 20–79 years old, were recruited in 2007 for participation in the Sao Paulo Epidemiologic Sleep Study (EPISONO); a sample representative for the adult general population of Sao Paolo, Brazil (Maluly et al., 2013). Details of the recruitment procedure and methods have been described in detail previously (Santos-Silva et al., 2009). In total, 1042 participants underwent single-night PSG and 915 of them (88%) were finally included in the present study, because they completed all questionnaires included in the analysis (section 2.1.3). The study protocol was approved by the Ethics Committee of the Universidade Federal de Sao Paulo (CAAE: 01570712.4.0000.5505) and registered at ClinicalTrials.gov (NCT00596713).
2.1 Measures
Sleep bruxism (present or absent) was categorised in two ways, using self-report or polysomnography. This yielded two different groupings: self-reported (non-)SB groups and PSG-confirmed (non-)SB groups.
2.1.1 Self-reported (non-)SB groups
Self-reported SB was screened by a single question: “How often do you currently grind your teeth?” (Maluly et al., 2013). The answer options were: never, <1, 1, and 2–3 times/month, 1–2, 3–6 times/week, and daily. The self-reported non-SB group consisted of participants who reported having bruxed 2–3 times/month or less. The self-reported SB group consisted of participants who reported having bruxed 1–2 times/week or more.
2.1.2 PSG-confirmed (non-)SB groups
Participants who had <2 SB episodes/hour of sleep were included in the PSG-confirmed non-SB group; those with ≥2 SB episodes/hour of sleep, in the PSG-confirmed SB group (Rompre et al., 2007).
2.1.3 Associated factors
Sex (male, female), age, and body mass index (BMI) were derived from the demographic data. We used “female” as a reference in the multivariate and network analyses. Age was used as a continuous variable in all analyses. Body mass index was used as a continuous variable in all analyses.
Insomnia was assessed by the insomnia severity index (ISI) (Bastien et al., 2001), with total scores ranging from 0 to 28. The sum score of the ISI was used as a continuous variable in all analyses.
Obstructive sleep apnea (OSA) risk was obtained from the Berlin questionnaire (BQ) (Netzer et al., 1999), which consists of three categories related to snoring, daytime sleepiness, and high blood pressure. Participants with at least two symptom categories were categorised as having “high risk” of OSA; those with no or one symptom category, as having “low risk”. OSA risk was used as a categorical variable in all analyses.
Anxiety and depression were assessed by the Beck Anxiety Inventory (BAI) (Beck et al., 1988) and the Beck Depression Inventory (BDI) (Beck et al., 1961), respectively, both with total scores ranging from 0 to 63. The sum scores were used as continuous variables in all analyses.
Average caffeine consumption was derived from a question about average caffeine consumption per day (glass or cup/day). Caffeine-containing beverages included coffee, black tea, and cola soft drink.
Smoking frequency in the past 3 months was derived from a question with five answer options, namely: no, 1–2 times/3 months, monthly, weekly, and daily or almost daily. In addition, alcohol consumption frequency in the past 3 months was derived from a question with the same answer options. Because there were few participants who answered smoking 1–2 times/3 months, monthly, and weekly, we combined these as: no; sometimes (from smoking 1–2 times/3 months, monthly, and weekly); and daily or almost daily; both for smoking frequency and for alcohol consumption.
2.2 Associated factors of the self-reported and PSG-confirmed SB parts
Sex, age, BMI, insomnia, OSA risk, anxiety, depression, average caffeine consumption, smoking frequency, and alcohol consumption frequency were included in the analyses for both self-reported SB and PSG-confirmed SB.
2.3 Statistical analyses
Normality was assessed by the Kolmogorov–Smirnov test for all continuous variables, that is age, BMI, ISI score, anxiety and depression scores, and average caffeine consumption.
For both self-reported SB and PSG-confirmed SB, we estimated their association to the other variables in three steps: univariate, multivariate, and network analyses. We performed each analysis twice: once with self-reported SB and once with PSG-confirmed SB. First, we conducted univariate analyses, to investigate the pairwise associations. Second, to investigate the associations between all predictors and sleep bruxism simultaneously, we performed two multivariate analyses (one model for self-reported SB and another for PSG-confirmed SB) by which we predicted SB by all other variables. While these multivariate analyses shed light on which of the variables predict sleep bruxism while taking all other predictors into account, they do not inform us on how the different predictors relate among each other. Therefore, finally, we also estimated two network models by which we investigated the relations among all variables, allowing us to distinguish between variables that related not only directly but also indirectly to sleep bruxism. Details of the three steps are given below.
First, Mann–Whitney U tests were used to assess the associations between sleep bruxism and the continuous variables. Chi-square tests were used to investigate the association between SB and the categorical variables, that is sex, sleep bruxism, OSA risk, smoking frequency, and alcohol consumption frequency.
Second, multivariate logistic regression analyses were performed. The following predictors were entered into the self-reported SB and PSG-confirmed SB regression models in a single step: sex, age, BMI, insomnia, OSA risk, anxiety, depression, average caffeine consumption, smoking frequency, and alcohol consumption frequency.
Third, network analyses were conducted. There were 11 variables included in the analyses: age, BMI, ISI, anxiety, depression, and average caffeine consumption as continuous variables; and sex (female, male), SB (non-SB, SB), OSA risk (low risk, high risk), smoking frequency (no, sometimes, daily/almost daily), and alcohol consumption frequency (no, sometimes, daily/almost daily) as categorical variables. Since there were both categorical and continuous variables included in the model, we estimated a Mixed Graphical Model (MGM) (Haslbeck & Waldorp, 2018). To minimise false-positive edges, we used the regularisation technique “Least Absolute Shrinkage and Selection Operator” (LASSO). We used tuning parameter to adjust the level of regularization. However, this parameter cannot be set directly, it is determined by the Extended Bayesian Information Criterion (EBIC). The model selection using EBIC is good in terms of precision, that is the associations included in the network model are true (Isvoranu & Epskamp, 2021). The gamma (ɣ) hyperparameter in the EBIC model selection is generally set between 0 and 0.5 (Epskamp & Fried, 2018). Setting the ɣ hyperparameter to 0 yields denser networks with higher sensitivity, while setting ɣ to 0.5 yields sparser networks with higher specificity. In this study, we set the hyperparameter to 0.5 to minimise false-positive edges. Then, we visualized the network models in which we included all variables as “nodes” and the conditional dependence association between two connected nodes after controlling for all other variables are shown as “edges”, using the R-package qgraph (version 1.9) (Epskamp et al., 2012).
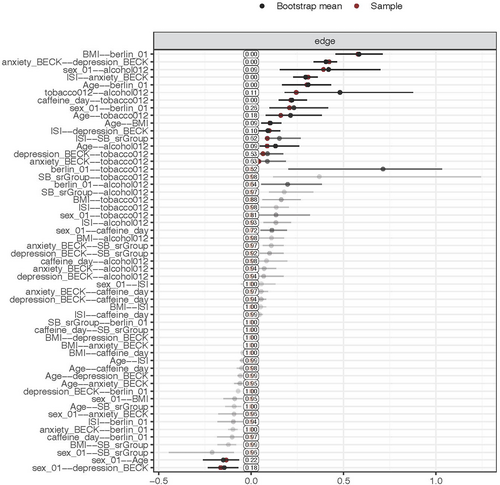
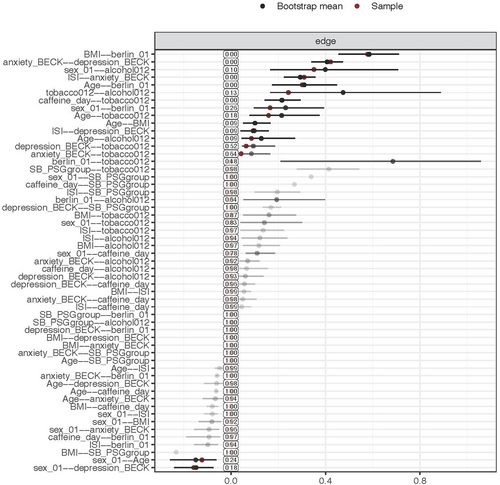
To assess the accuracy of network estimation, we used nonparametric bootstrap with 1000 bootstrap samples, using the R-package bootnet (version 1.5) (Epskamp et al., 2018). The bootstrapped confidence intervals (CI) of edge-weights accuracy for self-reported SB and PSG-confirmed SB are shown in the appendix (Figures A1 and A2). Last, we used the R-package NetworkComparisonTest (version 2.2.1) to investigate the differences between the self-reported and PSG-confirmed SB network models (van Borkulo, 2018). This method evaluates whether global strength, which is the overall connectivity of the networks or weighted absolute sum of all edges in the network, differs across the networks (van Borkulo et al., 2022). IBM SPSS Statistics (v.28; IBM Corp, Armonk, NY, USA) was used to perform univariate and multivariate analyses. R (v. 4.1.2; R Core Team 2021) was used to perform the network analysis.
3 RESULTS
In total, 915 participants were included in the analyses; 510 females and 405 males. There were 781 self-reported and PSG-confirmed non-bruxers, 16 participants who were self-reported non-bruxers but PSG-confirmed bruxers, 76 participants who were self-reported bruxers but PSG-confirmed non-bruxers, and 42 participants who were self-reported and PSG-confirmed bruxers.
3.1 Univariate analyses
For self-reported sleep bruxism, there were 797 non-bruxer (87.1%) and 118 self-reported sleep bruxers (12.9%). The mean ages of non-SB and SB groups did not differ significantly (non-SB 42.8 ± 14.4; self-reported SB 40.9 ± 14.5). Table 1 shows that, in the univariate analysis, self-reported SB was significantly associated with sex, insomnia, anxiety, and depression. There were only a few individuals with self-reported SB with smoking-sometimes and with drinking alcohol-daily/almost daily.
| Predictors | Non-bruxer (n = 797) | Sleep bruxer (n = 118) | Total (n = 915) | p |
|---|---|---|---|---|
| Sex | ||||
| Female | 434 (54.5%) | 76 (64.4%) | 510 (55.7%) | 0.042a,* |
| Male | 363 (45.5%) | 42 (35.6%) | 405 (35.6%) | |
| Age | ||||
| Median (IQR) | 42 (21) | 38.5 (22) | 0.164b | |
| BMI | ||||
| Median (IQR) | 26.48 (6.62) | 25.41 (5.73) | 0.079b | |
| ISI | ||||
| Median (IQR) | 6 (10) | 9.5 (11) | <0.001b,* | |
| OSA risk | ||||
| Low risk | 579 (72.6%) | 89 (75.4%) | 668 (73.0%) | 0.526a |
| High risk | 218 (27.4%) | 29 (24.6%) | 247 (27.0%) | |
| Anxiety | ||||
| Median (IQR) | 6 (10) | 9 (13) | 0.002b,* | |
| Depression | ||||
| Median (IQR) | 8 (9) | 8 (11) | 0.03b,* | |
| Average caffeine consumption | ||||
| Median (IQR) | 2 (4) | 2 (3) | 0.51b | |
| Smoking frequency | ||||
| No | 585 (73.4%) | 81 (68.6%) | 666 (72.8%) | 0.230a |
| Sometimes | 34 (4.3%) | 3 (2.5%) | 37 (4.0%) | |
| Daily/almost daily | 178 (22.3%) | 34 (28.8%) | 212 (23.2%) | |
| Alcohol consumption frequency | ||||
| No | 284 (35.6%) | 36 (30.5%) | 320 (35.0%) | 0.373a |
| Sometimes | 467 (58.6%) | 77 (65.3%) | 544 (59.5%) | |
| Daily/almost daily | 46 (5.8%) | 5 (4.2%) | 51 (5.6%) | |
- a Chi-square test.
- b Mann–Whitney U test.
- * Significant at 0.05.
For PSG-confirmed sleep bruxism, there were 857 non-bruxers (93.7%) and 58 PSG-confirmed sleep bruxers (6.3%). The mean ages of non-SB and SB groups were not significantly different (non-SB 42.7 ± 14.3; PSG-confirmed SB 41.2 ± 15.8). Table 2 shows that, in the univariate analysis, there was no significant association of PSG-confirmed SB with any of the other variables. Similar to self-reported SB, there were only a few individuals with PSG-confirmed SB with smoking-sometimes and with drinking alcohol-daily/almost daily.
| Predictors | Non-bruxer (n = 857) | Sleep bruxer (n = 58) | Total (n = 915) | p |
|---|---|---|---|---|
| Sex | ||||
| Female | 480 (56.0%) | 30 (51.7%) | 510 (55.7%) | 0.525a |
| Male | 377 (44.0%) | 28 (48.3%) | 405 (44.3%) | |
| Age | ||||
| Median (IQR) | 42 (21) | 36.5 (28) | 0.305b | |
| BMI | ||||
| Median (IQR) | 26.4 (6.49) | 25.77 (5.45) | 0.592b | |
| ISI | ||||
| Median (IQR) | 6 (9) | 7 (9) | 0.066b | |
| OSA risk | ||||
| Low risk | 624 (72.8%) | 44 (75.9%) | 668 (73.0%) | 0.613a |
| High risk | 233 (27.2%) | 14 (24.1%) | 247 (27.0%) | |
| Anxiety | ||||
| Median (IQR) | 6 (10) | 7.5 (10) | 0.508b | |
| Depression | ||||
| Median (IQR) | 8 (9) | 8 (11) | 0.646b | |
| Average caffeine consumption | ||||
| Median (IQR) | 2 (4) | 2 (4) | 0.998b | |
| Smoking frequency | ||||
| No | 629 (73.4%) | 37 (63.8%) | 666 (72.8%) | 0.230a |
| Sometimes | 33 (3.9%) | 4 (6.9%) | 37 (4.0%) | |
| Daily/almost daily | 195 (22.8%) | 17 (29.3%) | 212 (23.2%) | |
| Alcohol consumption frequency | ||||
| No | 301 (35.1%) | 19 (32.8%) | 320 (35.0%) | 0.675a |
| Sometimes | 507 (59.2%) | 37 (63.8%) | 544 (59.5%) | |
| Daily/almost daily | 49 (5.7%) | 2 (3.4%) | 51 (5.6%) | |
- a Chi-square test.
- b Mann–Whitney U test.
3.2 Multivariate analyses
In the multivariate analysis, only insomnia remained significantly associated to self-reported SB (Table 3). There was no significant association of PSG-confirmed sleep bruxism with any of the other variables in the multivariate analyses (Table 4).
| Predictors | Sleep bruxer (n = 118) versus non-bruxer (n = 797)a | |||
|---|---|---|---|---|
| B (SE) | OR | 95% CI | p | |
| Sex | ||||
| Female | Reference | - | - | - |
| Male | −0.389 (0.220) | 0.678 | 0.441–1.043 | 0.077 |
| Age | −0.006 (0.008) | 0.994 | 0.979–1.010 | 0.467 |
| BMI | −0.043 (0.022) | 0.958 | 0.917–1.001 | 0.055 |
| ISI | 0.053 (0.018) | 1.054 | 1.018–1.092 | 0.003* |
| OSA risk | ||||
| Low risk | Reference | - | - | - |
| High risk | 0.198 (0.267) | 1.220 | 0.723–2.058 | 0.457 |
| Anxiety | 0.011 (0.014) | 1.011 | 0.984–1.039 | 0.420 |
| Depression | 0.012 (0.014) | 1.012 | 0.984–1.041 | 0.398 |
| Average caffeine consumption | −0.022 (0.034) | 0.979 | 0.915–1.046 | 0.528 |
| Smoking frequency | ||||
| No | Reference | - | - | - |
| Sometimes | −0.751 (0.630) | 0.472 | 0.137–1.621 | 0.233 |
| Daily/almost daily | 0.145 (0.246) | 1.156 | 0.714–1.874 | 0.555 |
| Alcohol consumption frequency | ||||
| No | Reference | - | - | - |
| Sometimes | 0.383 (0.227) | 1.466 | 0.939–2.289 | 0.092 |
| Daily/almost daily | 0.174 (0.527) | 1.190 | 0.424–3.344 | 0.741 |
- Abbreviations: B, regression coefficient; CI, confidence interval; OR, odds ratio; SE, standard error.
- a Reference category.
- * Significant at 0.05.
| Predictors | Sleep bruxer (n = 58) versus non-bruxer (n = 857)a | |||
|---|---|---|---|---|
| B (SE) | OR | 95% CI | p | |
| Sex | ||||
| Female | Reference | - | - | - |
| Male | 0.227 (0.287) | 1.255 | 0.714–2.204 | 0.430 |
| Age | −0.001 (0.011) | 0.999 | 0.978–1.020 | 0.920 |
| BMI | −0.011 (0.030) | 0.989 | 0.934–1.049 | 0.721 |
| ISI | 0.030 (0.024) | 1.030 | 0.983–1.080 | 0.218 |
| OSA risk | ||||
| Low risk | Reference | - | - | - |
| High risk | −0.009 (0.366) | 0.991 | 0.484–2.029 | 0.980 |
| Anxiety | −0.007 (0.020) | 0.994 | 0.956–1.033 | 0.743 |
| Depression | 0.012 (0.020) | 1.012 | 0.973–1.052 | 0.555 |
| Average caffeine consumption | 0.008 (0.044) | 1.008 | 0.925–1.098 | 0.855 |
| Smoking frequency | ||||
| No | Reference | - | - | - |
| Sometimes | 0.625 (0.576) | 1.867 | 0.604–5.778 | 0.278 |
| Daily/almost daily | 0.324 (0.331) | 1.382 | 0.723–2.643 | 0.327 |
| Alcohol consumption frequency | ||||
| No | Reference | - | - | - |
| Sometimes | 0.077 (0.302) | 1.080 | 0.597–1.953 | 0.799 |
| Daily/almost daily | −0.552 (0.780) | 0.576 | 0.125–2.659 | 0.480 |
- Abbreviations: B, regression coefficient; SE, standard error; OR, odds ratio; CI, confidence interval.
- a Reference category.
3.3 Network analyses
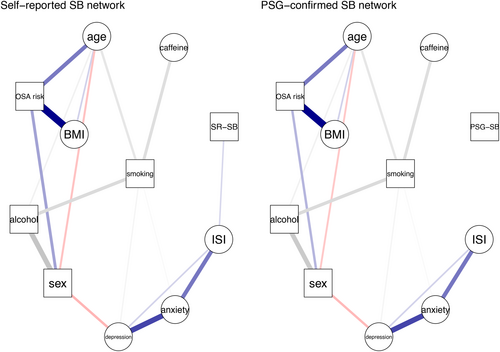
The network model for self-reported SB showed that sleep bruxism had a direct and positive link to insomnia (Figure 1), suggesting that self-reported SB has a positive association with insomnia when taking all the other variables into account. PSG-confirmed SB did not have any edge with any of the other variables. Interestingly, the network model showed that anxiety and depression were indirectly and positively associated with self-reported SB through insomnia. The network comparison test showed that there was no significant difference in global strength (0.12, p = 0.851) between both networks.
4 DISCUSSION
The current study aimed to investigate the associations of sleep bruxism with sleep disorders, demographic, psychological, and lifestyle factors in the adult general population. We investigated these associations via univariate, multivariate, and network analyses. We found that self-reported SB was significantly associated with insomnia. The association between self-reported sleep bruxism and insomnia is consistent with our NSR study (Chattrattrai et al., 2022). In addition, anxiety and depression were indirectly connected with self-reported SB via insomnia, which was not found in the multivariate analysis but only in the univariate analysis. These results show that sleep bruxism was associated with psychological factors such as anxiety and depression as found previously (Chattrattrai et al., 2022). Thus, the first hypothesis, that SB in the adult general population was associated with insomnia via anxiety, was partly accepted since there is a direct positive association between self-reported SB and insomnia in the network model, which was consistent with the multivariate analysis. On the other hand, PSG-confirmed SB did not have a significant association with any of the variables in any of the analyses. Thus, the second hypothesis, that self-reported SB and PSG-confirmed SB would yield the same associations with other variables, was rejected.
The network models show that sleep bruxism, insomnia, anxiety, and depression are grouped in the same cluster. This cluster appears to be the same phenotypic group in the EPISONO study (Castro et al., 2013) and in the NSR study (Chattrattrai et al., 2022). The association between insomnia, anxiety, and depression has been described previously. Individuals with anxiety or depression tend to have insomnia symptoms more often than subjects without such conditions (Oh et al., 2019). Another study supported that insomnia, anxiety, and depression may have some genetic overlapping (Gehrman et al., 2011). In addition, they share the same mechanism, viz., dysregulation of serotonin and dopamine genes (Blake et al., 2018) and of the hypothalamic–pituitary–adrenal axis (Vgontzas et al., 2001). At the same time, SB has been associated with arousal response (Huynh et al., 2006; Kato et al., 2003), central dopaminergic system, and serotoninergic pathway (de Baat et al., 2021). Severe bruxers showed lower blood serotonin levels compared with non-to-moderate sleep bruxers (Smardz et al., 2022). Therefore, this phenotypic group may confirm that SB has multiple aetiological factors, including psychosocial factors and sleep disorders. Consequently, the management of sleep bruxism requires a multidisciplinary approach, including exploring psychosocial factors and sleep disorders.
The present study showed different results between subjective (self-report) SB and objective (PSG-confirmed) sleep bruxism. At the individual level, it should be noted that some participants were classified as having sleep bruxism using self-report, but as non-SB using PSG, and vice versa. This may have affected the outcomes. However, when including only those participants that were classified as either SB or non-SB based on both self-report and PSG (n = 823; non-SB = 781, SB = 42), the results were similar to those of the PSG-confirmed sleep bruxism network model. The significant results in the self-report SB regression and network models may thus be related to the 60 participants (6.6%) who were excluded from the PSG-confirmed SB model. These self-reported sleep bruxers may have associations with insomnia and psychosocial factors, even though their SB activity was not confirmed with PSG. While self-report assesses the frequency of sleep bruxism over an indetermined (i.e., “currently”), most likely relatively long period, PSG-confirmed SB was based on a single night's result only. SB, however, is known to show a considerable night-to-night variability (Ohlmann et al., 2022; Van Der Zaag et al., 2008). Therefore, it would be premature to consider the 60 excluded participants as being actual non-bruxers, only because of them not having a positive PSG. More research is needed to further unravel the accuracy of the different assessment tools for sleep bruxism.
One interesting finding is that an association of insomnia was found with self-reported SB but not with PSG-confirmed SB. This leads to the question whether people with insomnia are sensitive to any symptom and interpret this as having sleep bruxism. It is noted that the diagnosis of insomnia needed to be evaluated based on clinical relevance and not to be diagnosed with PSG only (Schutte-Rodin et al., 2008). Sleep deprivation disturbs the pain inhibitory system and increases the pain sensitivity to cold and pressure (Staffe et al., 2019). In addition, insomnia may increase pain sensitivity (Haack et al., 2012). Thus, participants with insomnia may indeed be more sensitive to pain symptoms than those without insomnia. On the other hand, sleep disruption in insomnia patients could make them recognise actual SB events during their sleep, although those sleep bruxism activities are not always higher than the SB cut-off point. A related question is whether sleep bruxism is also involved with increasing pain sensitivity and discomfort. Non-painful muscle symptoms are part of the suggested subject-based assessment for SB, that are self-report and history report of bruxism status and patient's complaint related to bruxism (Manfredini et al., 2023). These symptoms decreased after reducing jaw-muscle activity with contingent electrical stimulation (Shimada et al., 2019). This may imply that there is indeed an increased sensitivity to non-pain symptoms in self-reported sleep bruxers. Similarly, experimental tooth grinding in healthy individuals could provoke jaw-muscle pain and fatigue after the test and 24 h after provocation (Koutris et al., 2018). This prolonged pain is associated with micro-trauma-related inflammatory processes and results in peripheral sensitisation (Koutris et al., 2018). While jaw-muscle symptoms were frequently reported in clinically confirmed SB, there was no association between such symptoms and muscle activity during sleep (Thymi et al., 2019). In short, participants with insomnia may be sensitive to any discomfort and pain, and interpret these symptoms as sleep bruxism. They could also notice actual sleep bruxism events during their sleep, while objectively those activities could not be always be detected or pass the cut-off point. For future studies, it is suggested to recognise the presence or absence of sleep bruxism on a continuum spectrum instead of using cut-off points (Manfredini et al., 2019).
In the present study, no associations were found between sleep bruxism and lifestyle factors such as average caffeine consumption, smoking, and alcohol consumption frequency. This is in contrast with previous studies in which these factors were positively associated with sleep bruxism (Rintakoski et al., 2010; Rintakoski & Kaprio, 2013). The different characteristics and lifestyles across population samples and the applied measurement tools may have affected the results. In addition, average caffeine consumption in this study included various caffeine-containing drinks such as coffee, tea, and cola, while previous studies included coffee only. So, the amount of caffeine consumption would be different between studies. Furthermore, this study assessed the frequency of smoking and alcohol consumption. To gain more insight into the association between sleep bruxism and lifestyle factors, it is suggested using the average amount of cigarette smoking, alcohol, and caffeine intake per day instead of the frequency.
This study has several strengths. First, it represents the adult general population. Second, we used polysomnography, which is still the current gold standard to assess sleep bruxism (Lobbezoo et al., 2018). Third, we used a novel statistical method in the dentistry research field, namely, network analysis. This method could reveal any possibly hidden associations among the variables included in the network model. Network analysis can show more information about how each variable connects to other variables, while multivariate regression analysis needs to define a dependent variable and can show only the associations of that dependent variable with other variables but not the associations among predictors themselves. For example, the phenotypic group that was found in the network analysis could not have been obtained from multivariate regression analysis.
This study has the following limitations. First, we used the Berlin questionnaire for the assessment of OSA risk in both self-reported and PSG-confirmed SB models to make these two models comparable. Using an instrumentally assessed variable, such as the apnea–hypopnea index, is recommended for investigating the associations between OSA and PSG-confirmed SB in further research. Second, due to differences in setting, parameters, methods, and population between the EPISONO and NSR populations, we could not compare the network models between the general population (EPISONO) and an insomnia-based population (NSR) directly, as we could do for the comparison between self-reported SB and PSG-assessed SB network models. To have standardised questionnaires and measurements is recommended for future research in order to compare the network models across the populations. Last, this is a cross-sectional study, so causal associations cannot be implied. Longitudinal data collection is needed to investigate the causal relationship between each of the factors.
5 CONCLUSION
Sleep bruxism was positively associated with insomnia only in individuals with self-reported SB, while PSG-confirmed SB was not associated with any of the included factors.
AUTHOR CONTRIBUTIONS
Thiprawee Chattrattrai: Conceptualization; formal analysis; investigation; methodology; project administration; software; validation; visualization; writing – original draft; writing – review and editing. Ghizlane Aarab: Conceptualization; methodology; writing – review and editing. Tessa F. Blanken: Conceptualization; formal analysis; methodology; software; validation; visualization; writing – review and editing. Gabriel Natan Pires: Conceptualization; data curation; formal analysis; investigation; methodology; resources; validation; visualization; writing – review and editing. Alberto Herrero Babiloni: Conceptualization; writing – review and editing. Cibele Dal Fabbro: Conceptualization; writing – review and editing. Eus J. W. Van Someren: Conceptualization; methodology; writing – review and editing. Gilles J. Lavigne: Conceptualization; writing – review and editing. Milton Maluly: Conceptualization; formal analysis; methodology; writing – review and editing. Monica L Andersen: Data curation; investigation; resources; writing – review and editing. Sergio Tufik: Data curation; investigation; resources; writing – review and editing. Frank Lobbezoo: Conceptualization; methodology; project administration; supervision; writing – original draft; writing – review and editing.
ACKNOWLEDGEMENTS
This study was supported by grants from the Associação Fundo de Incentivo à Pesquisa (AFIP). Monica L. Andersen and Sergio Tufik are recipients of CNPq Fellowships. Thiprawee Chattrattrai has been supported by a Mahidol University's Academic Development Scholarship.
CONFLICT OF INTEREST STATEMENT
GNP is a shareholder at SleepUp™, a Braziliand digital CBTi company, but attests that this position has no relationship with the aims, preparation, or execution of this study. The other authors declare that they have no competing interests to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES