Effects of (non)deceptive placebos on reported sleep quality and food cue reactivity
Summary
A lack of sleep can increase appetite, particularly for high-calorie food. The current study tested the effects of an open-label placebo for improving sleep quality and reducing food cue reactivity. In open-label placebo interventions, placebo recipients are informed that they are receiving a placebo without a pharmacologically active substance. Participants (n = 150) were randomly allocated to one of three groups that received either an open-label placebo to improve sleep quality, a deceptive placebo (“melatonin”), or no placebo. The placebo was administered daily before bedtime for 1 week. Sleep quality and reactivity to high-calorie food cues (appetite, visual attention to food images) were assessed. The deceptive placebo (but not the open-label placebo) reduced reported sleep-onset latency. The open-label placebo decreased perceived sleep efficiency. The placebo interventions did not change food cue reactivity. This study demonstrated that open-label placebos do not present an alternative to deceptive placebos for improving sleep quality. The undesirable open-label placebo effects found warrant further exploration.
1 INTRODUCTION
Poor sleep quality not only impacts how tired we are during the day but can also lead to adverse health outcomes, such as an increased appetite for high-calorie foods and subsequent weight gain (Brondel et al., 2010; Gangwisch et al., 2005). Several studies have documented that short sleep duration (< 6 hr per day) is associated with an increase in the incidence of obesity even after controlling for confounding variables like education or physical activity (Hasler et al., 2004; for a review and meta-analysis, see Itani et al., 2017). Furthermore, experimental studies suggest a causal effect of insufficient sleep on overeating (Broussard & Klein, 2022).
To avoid these negative health effects, scientific societies like the National Sleep Foundation recommend 7–9 hr of sleep per day for adults aged 18–64 years (Hirshkowitz et al., 2015). However, in a recent survey on sleep habits conducted in Austria, only about half of the participants reported that they got the recommended amount of sleep (Blume et al., 2020). Nonetheless, for some people even < 6 hr of sleep per night may be sufficient (Hor & Tafti, 2009). Therefore, when studying health outcomes, not only the total sleep duration but also sleep quality (e.g. problems falling asleep or subjectively perceived sleep quality) should be considered (Hinz et al., 2017; van de Langenberg et al., 2022). A meta-analysis has shown that the global subjective sleep quality (as measured by the Pittsburgh Sleep Quality Index [PSQI]; Buysse et al., 1989) has decreased during the COVID-19 pandemic (Scarpelli et al., 2022). This reveals a need for interventions to improve sleep quality that are easy to implement on a large scale.
There is increasing evidence gathered from placebo control groups of clinical trials on primary insomnia that even sham treatments can improve sleep quality (Jiang et al., 2019). Further, a meta-analysis found that deceptive placebo interventions (i.e. where participants were led to believe they had received a sleep-improving medication) could increase not only total sleep duration but also improve subjective sleep quality and decrease the perceived latency of sleep onset (Yeung et al., 2018). However, the use of deceptive placebo interventions presents an ethical dilemma (Annoni, 2018). In addition, deceiving patients may impair the trust relationship between patients and healthcare providers; without trust, a meaningful therapeutic relationship may become very difficult. Despite evidence that placebo effects are substantial and reliable in improving subjective sleep quality, if placebo recipients discover the deception, they may feel deceived or distrustful.
Over the past few years, open-label placebos (OLPs) have been investigated as a potential alternative for leveraging placebo effects without deceiving placebo recipients (von Wernsdorff et al., 2021). In OLP interventions, placebo recipients are fully aware and informed that they are receiving a placebo and not a medication with a pharmacologically active substance. It has been shown that such OLP interventions can, for example, decrease patients' pain or cancer-related fatigue (von Wernsdorff et al., 2021). In healthy participants, OLPs have reduced emotional distress (Guevarra et al., 2020; Schienle et al., 2022), and increased health-promoting behaviours (e.g. the practice of relaxation training; Schienle & Unger, 2021).
The development and evaluation of OLP interventions for improving sleep quality are still in their infancy. An initial study investigated the effects of OLPs at different dosages on various well-being indicators, including sleep quality (El Brihi et al., 2019). Participants in that study who received OLP treatment (taking for 5 days either one OLP pill in the low-dose group or four pills in the high-dose group) reported better sleep quality compared with a control group that received no treatment. In another study, two OLP interventions were compared that did not differ in the number of pills administered, but in the information given to the placebo recipients (Haas et al., 2022). One group of participants received the information that they would receive a placebo pill without an active ingredient, but then no further information on beneficial placebo effects. The second OLP group was (in addition to receiving a placebo pill) informed: (1) that placebos can have beneficial effects on different symptoms; (2) that the body can respond automatically to the pill even if the person taking the pill knows that it is a placebo; and (3) that a positive attitude towards the placebo is beneficial but not necessary to experience the desired effects. Neither of the two OLP groups showed an improved sleep quality compared with the control group that did not receive any treatment. The authors of that study argue that longer-term OLP interventions (instead of single applications) may be necessary to improve sleep quality.
As mentioned above, there is evidence that deceptive placebos can change the quality, duration and onset latency of sleep (Jiang et al., 2019; Yeung et al., 2018). However, there is mixed evidence for the efficacy of OLP approaches for improving sleep (El Brihi et al., 2019; Haas et al., 2022). The present study is the first to compare the effects of a 1-week deceptive placebo and OLP intervention on sleep quality. Further, based on the associations between sleep quality and eating behaviour (Brondel et al., 2010; Gangwisch et al., 2005), the present study also investigated the effects of these placebo interventions on appetite as well as attention to images of high-calorie food. So far, there has been only some evidence showing that deceptive placebos can decrease food cue reactivity (Hoffmann et al., 2018; Potthoff et al., 2019). However, to our knowledge, there has been no investigation carried out into whether an OLP can achieve the same effects.
It was hypothesized that compared with a control group, the placebos (deceptive, non-deceptive) would increase subjective sleep quality and estimated sleep duration, as well as reduce sleep-onset latency (assessed via the German PSQI; Hinz et al., 2017). Further, the placebos should decrease appetite for high-calorie food as well as visual attention to high-calorie food cues. The present study was preregistered in the Open Science Framework (OSF): https://osf.io/34kcx.
2 METHODS
2.1 Sample
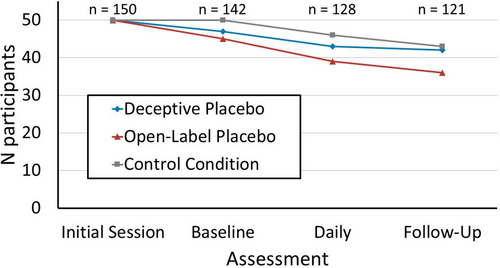
A total of 150 participants (age: M = 29.7 years, SD = 12.1 years; gender: 71% female, 29% male, < 1% other; PSQI sum score before intervention: M = 5.0, SD = 2.7; body mass index [BMI]: M = 22.7 kg/m2, SD = 3.7 kg/m2)1 were invited to participate in a study about sleep and eating behaviour. Exclusion criteria were reported somatic diseases, mental disorders, and medication affecting sleep or eating behaviour. Participants were randomly assigned to one of three groups (each n = 50): an OLP group; a deceptive placebo group; and a control group without placebo intervention. A total of 29 participants did not complete the study (Figure 1). Participants were recruited via posts on social media and at the university campus. The study was approved by the ethics committee of the university (ethics approval code: GZ. 39/73/63 ex 2021/22) and conducted following the Declaration of Helsinki.
2.2 Procedure
Participants were invited to a first session, where the general procedure was explained and written informed consent was obtained. Participants in the placebo groups received a bottle with 50 ml of a blue, mint-flavoured spray that was labelled as either a placebo (OLP group) or melatonin (deceptive placebo group). A spray for oral administration was used to mimic common over-the-counter melatonin supplements (Erland & Saxena, 2017). Participants of both placebo groups received a leaflet that explained that the spray (placebo or “melatonin”) would help them to fall asleep faster and to improve overall sleep quality. The deceptive placebo leaflet was based on an over-the-counter melatonin spray leaflet. The OLP group leaflet was based on established recommendations for OLP administration (Kaptchuk et al., 2010) informing participants: (1) that placebos can have beneficial effects on various symptoms; (2) that the body can respond automatically to the placebo; and (3) that an optimistic attitude towards the placebo is beneficial but not necessary (Haas et al., 2022). The control group was provided information about the general procedure of the study.
On the morning after the initial meeting before breakfast, participants completed an online survey including questions concerning demographic information, the PSQI (Hinz et al., 2017), and assessments of both appetite and food cue attention biases (Kakoschke et al., 2015). For the following seven evenings, participants in the placebo groups sprayed two puffs of the liquid on their tongues 30 min before bedtime. Each subsequent morning, all participants estimated the number of hours slept, rated their subjective sleep quality on a seven-point scale (“very good” to “very poor”) and appetite on a five-point scale (“How much would you like to eat something right now?”, from 0 “not at all” to 4 “very much”). After 1 week, the PSQI, appetite and food cue attention biases were reassessed in the morning before breakfast.
2.3 Materials
2.3.1 Pittsburgh Sleep Quality Index
The PSQI (Hinz et al., 2017) assesses seven components of sleep quality (subjective sleep quality; sleep latency; sleep duration; habitual sleep efficiency; sleep disturbances; use of sleep medication; and daytime dysfunction). The items of the original PSQI refer to the previous month (e.g. “During the past month, how would you rate your sleep quality overall?”). The German PSQI has been validated within a community sample of 9284 people (Hinz et al., 2017). A modified instruction for the PSQI was used that matched the duration of the intervention (i.e. 1 week; El Brihi et al., 2019).
The present study focused on PSQI components 2 (sleep-onset latency; based on the estimated minutes it took to fall asleep and frequency of the latency being above 30 min) and 1 (subjective sleep quality; from 0: “very good” to 3 “very bad”) based on the verbal placebo suggestions. Higher values (from 0 to 3) indicate poorer sleep quality and that it takes participants longer to fall asleep.
2.3.2 Appetite and food cue attention bias
Before and after the 1-week intervention, participants reported their general appetite (“How much would you like to eat something right now?”; 0: “not at all”; 4: “very much”) and specific appetite for high-calorie (e.g. pancakes) and low-calorie foods (e.g. fruits). Such single-item Likert-scale assessments of food liking or appetite are a well-established and time-efficient method for recording individual food preferences (Potthoff & Schienle, 2020; van Alebeek et al., 2023). A modified dot-probe task was then employed, using images from the food.pics database (Blechert et al., 2014; Blechert et al., 2019).2 This task presented a food- and non-food image pair for either 500 ms or 1500 ms. After each image pair, an arrow appeared in one of the locations (food or non-food). Participants responded to the arrow by pressing either “t” (upwards arrow) or “v” (downwards arrow). In total, 160 trials were conducted (80 high-calorie–non-food pairs and 80 low-calorie–non-food pairs; 80,500 ms trials and 1500 ms trials). Faster reaction times for trials with the arrow appearing in the food location indicate a food cue attention bias (Kakoschke et al., 2015). Trials with 500 ms image-pair duration assessed early, automatic attention biases; 1500 ms trials assessed later, more voluntarily controlled attention (Potthoff & Schienle, 2020).
2.3.3 Statistical analysis
Three by two analyses of variance (ANOVAs) were calculated to test the effects of Intervention (between-subjects: OLP, deceptive placebo, control group) and Time (within-subjects: before and after intervention) on PSQI scores, and food cue attention biases (dot-probe task). Daily sleep and appetite ratings during the intervention were averaged and were compared via ANOVAs with Intervention being the only between-subjects factor. Data from participants who conducted less than half of these daily assessments (n = 21) were excluded from the analysis (Figure 1).
3 RESULTS
3.1 Pittsburgh Sleep Quality Index
3.1.1 Sleep latency (PSQI component 2)
There was a significant medium-sized interaction between Time and Intervention (F(2,118) = 4.03, p = 0.020, ηpart2 = 0.064). The deceptive placebo group reported falling asleep faster after the intervention (t(41) = 3.84, pHolm < 0.001, d = 0.593). This effect was not present in the OLP group (t(35) = −0.312, pHolm = 0.757, d = −0.052) and control group (t(42) = 0.850, pHolm = 0.800, d = 0.130). There was a significant but small effect of Time (F(1,118) = 4.73, p = 0.032, ηpart2 = 0.039). Sleep-onset latency decreased over time (t(120) = 2.26, p =0 .026, d = 0.205). The main effect of Intervention was not significant (F(2,118) = 0.318, p = 0.728, ηpart2 = 0.005).
3.1.2 Sleep duration (PSQI component 3)
There was a significant medium-sized interaction Time × Intervention (F(2,118) = 4.03, p = 0.020, ηpart2 = 0.064). However, the within-group effects were not significant (placebo: t(41) = 1.95, puncorrected = 0.058, d = 0.300; OLP: t(35) = −1.75, puncorrected = 0.090, d = −0.291; control: t(42) = 0.703, puncorrected = 0.486, d = 0.107). Neither the main effect of Time (F(1,118) = 0.071, p = 0.790, ηpart2 = 0.001) nor Intervention (F(2,118) = 0.668, p = 0.515, ηpart2 = 0.011) were significant.
3.1.3 Habitual sleep efficiency (PSQI component 4)
The interaction effect (F(2,118) = 3.81, p = 0.025, ηpart2 = 0.061) was statistically significant. Habitual sleep efficiency decreased in the OLP group (t(35) = −2.05, puncorrected = 0.048, d = −0.342), but there was neither a significant effect in the deceptive placebo group (t(41) = 1.72, puncorrected = 0.094, d = 0.265) nor in the control group (t(42) = 0.443, puncorrected = 0.660, d = 0.068). Neither the main effect of Time (F(1,118) = 0.068, p = 0.794, ηpart2 = 0.001) nor Intervention (F(2,118) = 0.215, p = 0.807, ηpart2 = 0.004) were significant.
3.1.4 Other PSQI components
For the remaining PSQI components (subjective sleep quality, sleep disturbances, sleep medication, daytime disturbances), there were no significant main or interaction effects (all p > 0.05; Table 1).
| Component | Intervention | Pre mean (SD) | Post mean (SD) | ANOVA |
|---|---|---|---|---|
| 1. Subjective sleep quality | Deceptive: | 1.02 (0.71) | 0.83 (0.70) | Intervention: F(2,118) = 0.91, p = 0.405, ηpart2 = 0.015 |
| OLP | 1.10 (0.64) | 1.06 (0.63) | Time: F(1,118) = 3.90, p = 0.051, ηpart2 = 0.032 | |
| Control | 1.09 (0.51) | 1.00 (0.54) | Interaction: F(2,118) = 0.57, p = 0.567, ηpart2 = 0.010 | |
| 2. Sleep latency | Deceptive** | 1.26 (1.00) | 0.81 (0.89) | Intervention: F(2,118) = 0.32, p = 0.728, ηpart2 = 0.005 |
| OLP | 0.90 (1.00) | 1.00 (0.93) | Time:* F(1,118) = 4.73, p = 0.032, ηpart2 = 0.039 | |
| Control | 1.00 (0.94) | 0.86 (0.80) | Interaction:* F(2,118) = 4.03, p = 0.020, ηpart2 = 0.064 | |
| 3. Sleep duration | Deceptive | 0.30 (0.64) | 0.12 (0.33) | Intervention: F(2,118) = 0.67, p = 0.515, ηpart2 = 0.011 |
| OLP | 0.21 (0.52) | 0.39 (0.65) | Time: F(1,118) = 0.07, p = 0.790, ηpart2 = 0.001 | |
| Control | 0.22 (0.42) | 0.16 (0.43) | Interaction:* F(2,118) = 4.28, p = 0.016, ηpart2 = 0.068 | |
| 4. Habitual sleep efficiency | Deceptive | 0.65 (0.81) | 0.41 (0.73) | Intervention: F(2,118) = 0.22, p = 0.807, ηpart2 = 0.004 |
| OLP* | 0.36 (0.71) | 0.64 (0.93) | Time: F(1,118) = 0.07, p = 0.794, ηpart2 = 0.001 | |
| Control | 0.52 (0.84) | 0.42 (0.82) | Interaction:* F(2,118) = 3.81, p = 0.025, ηpart2 = 0.061 | |
| 5. Sleep disturbances | Deceptive | 0.95 (0.58) | 0.95 (0.49) | Intervention: F(2,118) = 0.41, p = 0.665, ηpart2 = 0.007 |
| OLP | 1.05 (0.51) | 0.89 (0.58) | Time: F(1,118) = 0.95, p = 0.331, ηpart2 = 0.008 | |
| Control | 1.04 (0.47) | 1.05 (0.49) | Interaction: F(2,118) = 1.42, p = 0.246, ηpart2 = 0.023 | |
| 6. Use of sleeping medication | Deceptive | 0.05 (0.31) | 0.14 (0.65) | Intervention: F(2,118) = 0.25, p = 0.780, ηpart2 = 0.004 |
| OLP | 0.05 (0.32) | 0.56 (0.33) | Time: F(1,118) = 0.15, p = 0.702, ηpart2 = 0.001 | |
| Control | 0.17 (0.57) | 0.09 (0.43) | Interaction: F(2,118) = 1.02, p = 0.364, ηpart2 = 0.017 | |
| 7. Daytime dysfunction | Deceptive | 0.95 (0.79) | 1.02 (0.84) | Intervention: F(2,118) = 0.09, p = 0.913, ηpart2 = 0.002 |
| OLP | 1.03 (0.71) | 0.94 (0.79) | Time: F(1,118) = 0.07, p = 0.796, ηpart2 = 0.001 | |
| Control | 0.96 (0.76) | 0.93 (0.74) | Interaction: F(2,118) = 0.24, p = 0.785, ηpart2 = 0.004 |
- Note: Intervention: Deceptive: deceptive placebo group; OLP: open-label placebo group; Control: control group; PSQI scores from 0 (highest sleep quality) to 3 (lowest sleep quality). *puncorrected < 0.05, **pHolm < 0.05 for pairwise comparison within group; *p < 0.05 for effect in ANOVA.
3.2 Daily ratings (sleep duration, subjective sleep quality and appetite)
For the averaged daily ratings, there was no effect of Intervention on the estimated sleep duration (F(2,125) = 0.12, p = 0.886), sleep quality (F(2,125) = 0.15, p = 0.862) and appetite (F(2,125) = 1.76, p = 0.176).
3.3 Food cue attention and specific appetite for high-calorie food
For none of the assessed high-calorie food assessments (food biases in dot-probe task and appetite), the interaction between Time and Intervention was significant (p > 0.638). All participants consistently showed an attention bias towards images of food that was not affected by the intervention, time or type of food depicted in the images (see Supplementary Table S1).
4 DISCUSSION
This study is the first to compare the efficacy of OLPs and deceptive placebos for improving sleep duration and quality. The participants were issued a take-home placebo spray that was administered daily before bedtime over 1 week. After 1 week, the deceptive placebo (“melatonin”) was found to reduce the time it took participants to fall asleep. This effect was not present in the OLP group (and in the control group). Thus, the placebo effect required deception.
It was only recently that research has begun to compare general acceptance and outcome expectations concerning deceptive and non-deceptive placebo treatment (Haas et al., 2021; von Wernsdorff et al., 2021). To produce positive health effects, deception may be necessary for some areas (e.g. depression; Schienle & Jurinec, 2022) but not for others (e.g. pain reduction; Disley et al., 2021). For the domain of sleep quality, future studies need to investigate if the rationale of OLP treatment is clear and plausible for the placebo recipients. In the present study, the deceptive placebo intervention was mimicking melatonin treatment. Melatonin is sold as an over-the-counter dietary supplement, and its use is rising among adults to improve sleep quality (Costello et al., 2014; Xie et al., 2017). Therefore, the deceptive suggestion used in the current experiment may have been very believable and thus effective even in the present sample whose sleep quality was already relatively high before the intervention (Hinz et al., 2017). In contrast, not all participants seemed to have been convinced by the OLP rationale. This can be inferred from the dropout rate immediately after the random group assignment, which was significantly higher in the OLP group (10%) compared with the control group (0%). Future research should investigate factors that potentially affect the attitude towards OLP interventions and motivation to take an OLP (Forkmann et al., 2023).
Habitual sleep efficiency, which is based on the ratio of time spent in bed and hours slept (Buysse et al., 1989), even decreased slightly in the OLP group (but not in the other groups). Furthermore, there was no significant improvement in sleep duration in any of the groups. These findings suggest that the OLP treatment did not have the intended effect of improving sleep duration and efficiency. The PSQI responses of participants even pointed in the opposite direction of the OLP suggestion, with a reduction in sleep efficiency. In OLP interventions, the expectancy or hope that a placebo treatment can improve symptoms must overcome the knowledge that there is no active ingredient in the treatment (Ballou et al., 2017; Kaptchuk, 2018). This discrepancy may cause cognitive dissonance, which may lead, at least in the domain of sleep quality, to unintended effects in some people.
No placebo effects were found for the other components of the PSQI, including subjective sleep quality. Although one other study observed similar effects as in the present investigation (Haas et al., 2022), other studies suggest that OLP interventions may be a promising tool to improve sleep quality (El Brihi et al., 2019; Rogev & Pillar, 2013). Thus, the evidence is mixed. As stated previously, little is known about why OLP interventions sometimes do not produce the desired effects. Variables such as the interaction between the OLP provider and recipient may influence the efficacy of an OLP (Blease et al., 2020). In the present study, the placebo provider and recipient interacted only in an initial session but not during the 1-week intervention. Similarly, Haas et al. (2022) limited the interaction between the placebo provider and recipient by using pre-recorded videos for instruction. In contrast, El Brihi et al. (2019) did have contact with participants during the treatment through reminder emails sent to the participants. Moreover, the authors of that study chose to create a brand name for the OLP (“Plaxibax”); previous research has indicated that branding can increase the effects of deceptive placebos (Faasse et al., 2016). Thus, future research could investigate further the conditions under which OLP effects are fostered.
Participants did not report decreased appetite for high-calorie food, nor did they display decreased attention to food cues as a result of this intervention. While prior research has suggested that lower sleep quality is associated with increased food cravings (Lv et al., 2018), the relatively high sleep quality of our participants (Hinz et al., 2017) might have prevented sleep-problem-associated cravings. It is, however, possible that food cravings might be able to be reduced in samples with severe insomnia or lower sleep quality, which subsequently should reduce appetite for high-calorie foods. Moreover, future studies should explore if certain components of sleep quality are more related to appetite and cravings than others.
The present study has several limitations. First, participants were drawn from a healthy convenience sample. Larger intervention effects may be observed in clinical samples with insomnia. Second, participants of the present study may have been sceptical whether an OLP could further improve their already high sleep quality. Third, subjective components of sleep quality were assessed via the PSQI; for the assessment of take-home placebos, future studies could use sleep trackers to measure physiological correlates of sleep quality. Fourth, additional subjective measures that are not covered by the PSQI like satisfaction with sleep quality (van de Langenberg et al., 2022) should be considered.
In conclusion, the deceptive placebo in the current study decreased sleep latency and thus helped fall asleep faster even in the current sample without severe sleep problems. The OLP was found to not have any desirable effects on sleep quality.
AUTHOR CONTRIBUTIONS
Jonas Potthoff: Conceptualization; data curation; formal analysis; methodology; project administration; validation; visualization; writing – original draft. Anne Schienle: Conceptualization; project administration; resources; supervision; validation; writing – original draft; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Endnotes
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the open science framework at https://osf.io/uvhac/.




