Cyclic alternating pattern in obstructive sleep apnea: A preliminary study
Valentina Gnoni and Panagis Drakatos have contributed equally to the preparation of the manuscript
Summary
Obstructive sleep apnea is linked to cardiovascular disease, metabolic disorders and dementia. The precise nature of the association between respiratory events in obstructive sleep apnea, cortical or subcortical arousals, and cognitive, autonomic and oxidative stress consequences remains incompletely elucidated. Previous studies have aimed to understand the relationship between obstructive sleep apnea and arousal patterns, as defined by the cyclic alternating pattern, but results have been inconsistent, in part likely due to the presence of associated comorbidities. To better define this relationship, we analysed cyclic alternating patterns in patients with obstructive sleep apnea without any additional comorbidities. We identified 18 adult male, non-obese subjects with obstructive sleep apnea and no other comorbidities or medication history, who underwent whole-night electroencephalography and polysomnography. Cyclic alternating pattern analysis was performed and verified by certified somnologists. Pairwise linear regression analysis demonstrated an inverse relationship between obstructive sleep apnea severity and cyclic alternating pattern subtype A1, and a direct correlation with cyclic alternating pattern subtype A3. Cyclic alternating pattern subtypes A1 prevail in milder obstructive sleep apnea phenotype, whilst cyclic alternating pattern subtypes A2 and A3 overcome among moderate-to-severe obstructive sleep apnea patients. The milder obstructive sleep apnea group also presented higher sleep efficiency, and increased percentages of non-rapid eye movement stage 3 and rapid eye movement sleep, as well as longer cyclic alternating pattern sequences in N3, while severe obstructive sleep apnea patients spent more time in lighter sleep stages. These results imply/suggest a balance between cyclic alternating pattern’s adaptive and maladaptive arousal processes in obstructive sleep apnea of differing severities. In milder obstructive sleep apnea (apnea–hypopnea index < 20), sleep continuity may be reinforced by cyclic alternating pattern subtype A1, whereas in more severe obstructive sleep apnea, decompensation of these sleep-stabilizing mechanisms may occur and more intrusive cyclic alternating pattern fluctuations disrupt sleep circuitry.
1 INTRODUCTION
Obstructive sleep apnea (OSA) is one of the most prevalent sleep and breathing disorders (Malhotra et al., 2020). Due to its links with major metabolic and cardiovascular comorbidities and dementia (Emamian et al., 2016; Jackson et al., 2020), it is also increasingly recognized as a major, if still under-diagnosed, public health concern (Polsek et al., 2020). In OSA, a sleep state-dependent reduction in the pharyngeal dilator muscle activity leads to a complete or partial closure of the upper airway in susceptible individuals (Malhotra et al., 2020). Ensuing intermittent hypoxia and hypercapnia trigger brief cortical arousals that restore airway patency (Kaur & Saper, 2019). However, the transient cortical arousals and sleep fragmentation are associated with cognitive impairment (Bucks et al., 2017), autonomic dysregulation, increased oxidative stress and haemodynamic changes during sleep (Malhotra et al., 2020). Although positive airway pressure (PAP) is considered to be an effective treatment for OSA, many patients do not tolerate it, and compliance is frequently poor (Rosenzweig et al., 2015). An alternative therapeutic approach may involve selective targeting and reduction of cortical arousals while maintaining or augmenting the respiratory drive during these respiratory events (Kaur & Saper, 2019).
However, arousal is a heterogeneous concept, and the exact cortical/subcortical origin of its sub-circuitries in humans is far from clear (Grady et al., 2020; Satpute et al., 2019). In non-rapid eye movement (NREM) sleep, arousals appear to be arranged in sequences known as the cyclic alternating pattern (CAP; Parrino et al., 2012). CAP represents an adaptive condition of sustained arousal instability oscillating between a greater arousal level and activation (phase A), and a lesser arousal level and deactivation (phase B; Parrino et al., 2012). There is growing evidence that CAP and arousals underwrite the basic mechanisms of sleep regulation, with subtype A1 contributing to the build-up and consolidation of deep slow-wave sleep (SWS), whilst subtypes A2 and A3 lightening sleep and contributing to the onset of rapid eye movement (REM) sleep or wakefulness (Terzano et al., 2005).
The severity of OSA has been, in past studies, variably linked to enhanced amounts of CAP, with the airway restoration linked with A phases, especially subtypes A2 and A3 (Milioli et al., 2015). Increased percentages of subtypes A3 and reduced percentages of subtypes A1 have been reported in patients with severe OSA, with PAP therapy shown to robustly curtail subtype A3, and to partly recover subtype A1 (Parrino et al., 2012). Similarly, daytime somnolence in OSA has been inconsistently linked to the duration and rate of subtype A2, and to reduction in phase B (Korkmaz et al., 2018).
Differences in results across past studies reflect methodological issues (e.g. diverse definition of apneic events; Bosi et al., 2018), as well as inherent differences in OSA phenotypes with associated comorbidities (Malhotra et al., 2020), all of which likely contribute to differential structural or functional adaptations of arousal circuitries (Rosenzweig et al., 2015). These may affect our reading of the interplay of intermittent hypoxia/hypercarbia and sleep fragmentation in OSA, which is fundamental to our understanding of its core neurobiology (Rosenzweig et al., 2014, 2015). To this end, we undertook to define heuristic associations between cortical arousal (sleep instability) parameters, and polysomnographic and respiratory measures in a (rare) group of patients with untreated OSA without comorbidities.
2 METHODS
Preliminary analysis of CAP parameters in patients with different OSA severities was undertaken; the patients’ clinical, demographic and video-polysomnography (vPSG) features were collected from the database of the multimodal clinical study InCOSA (Clinical.Trials.Gov, 2020), approved by the Research Ethics Committee (IRAS-Project-ID-170912;REC-REF16/L0/0893). Eighteen adult (≥ 35 ≤ 70 years old), non-obese (body mass index [BMI] < 30 kg m−2) male patients with a de novo diagnosis of OSA according to the ICSD criteria (International Classification of Sleep Disorders, III Edition; American Academy of Sleep Medicine, 2014) were included (American Academy of Sleep Medicine, 2014). The recommended hypopnea criteria, defined as a ≥ 30% decrease/drop in airflow with at least 3% SpO2 desaturation and/or associated electroencephalogram (EEG) arousal was used (Berry et al., 2017). All patients had a Respiratory Disturbance Index (RDI) of above 5 events per hour. Strict exclusion criteria (Clinical.Trials.Gov, 2020) stipulated no concomitant sleep disorders, no psychiatric, neurological or medical history or current illness, no history of alcohol or (recreational) drug abuse, and no concurrent use of psychotropic drugs. Similarly, patients with predominant or exclusive REM-related OSA were excluded. Smokers, professional drivers and shift workers were similarly excluded.
2.1 PSG evaluation
Full-night vPSG recordings were obtained following 1 night of habituation at the sleep laboratory. vPSG included 256-channel high-density (hd)EEG (Electrical Geodesics) with electrooculography, submental-electromyography, respiratory inductance plethysmography, nasal pressure sensor, pulse-oximeter, two-lead electrocardiogram, body position detector and synchronized audio-visual recording, as previously described (Rosenzweig et al., 2016). Of note is that the nasal pressure sensor was used in this study as per hospital clinical governance. Whilst this is permissible (Berry et al., 2017), the limitations include an overestimation of apnea–hypopnea index (AHI) in patients with predominant oral respiration. We also calculated the respiratory effort-related arousal (RERA) events. However, it should be noted that despite our best clinical practice efforts, some other types of EEG activation that can also be associated with an obstructive upper airway event, and which do not fulfill strict AASM-defined arousals, might have been missed (for further discussion, see Bosi et al., 2018; Thomas, 2003). For all participants, the bedtime was set at 22:30 hours.
For purposes of the PSG scoring, six hdEEG channels (i.e. F3,F4,C3,C4,O1,O2) were referenced to the mastoid, and the scoring was carried out according to AASM rules (Berry et al., 2017). Sleep and respiratory parameters were measured according to standard criteria (Berry et al., 2017).
2.1.1 Sleep microstructure
Cyclic alternating pattern was performed following standardized guidelines using Embla REM-logic software (Terzano et al., 2002). Two certified somnologists manually scored and verified all standard CAP parameters, as previously described (Terzano et al., 2002).
2.2 Statistics
Statistical analyses were performed using the IBM SPSS Statistics V26.0 (SPSS). All quantitative data were expressed as mean and standard deviation (SD). Due to non-normality of the data, non-parametric tests were used. Clinical and vPSG data in the two groups were compared by means of the Mann–Whitney U-test. Linear regression was used (e.g. a best-fit approach; Parrino et al., 2012) to measure the relationship between AHI and CAP rate in NREM sleep. Statistical significance was set at p < .05.
3 RESULTS
Eighteen OSA patients (mean AHI 26.9 ± 23.07 events per hr) with a mean age of 43.8 ± 10.3 years, mean BMI of 26.3 ± 4.1 kg m−2) and mean sleepiness (Epworth Sleepiness Scale [ESS]) score of 8.6 ± 4.9 were identified for the purposes of this preliminary analysis. Of those, eight patients were diagnosed with mild OSA (5 ≥ AHI ≤ 15 events per hr), five with moderate (15 ≥ AHI ≤ 30 events per hr), and five patients were diagnosed with severe sleep-disordered breathing (AHI ≥ 30 events per hr), according to the ICSD criteria (American Academy of Sleep Medicine, 2014; Figure 1a,b).
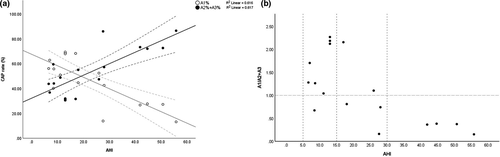
Pairwise linear regression analysis was subsequently undertaken to investigate the interaction between OSA severity and CAP (Figure 1a). AHI-related distribution of phase A subtypes in NREM is shown in Figure 1; notably, the percentages of subtype A1 are inversely correlated with OSA severity, mirrored by the reciprocal increase of subtypes A2 and (predominantly) A3 (Table 1). The opposite trends converged approximately at the mean AHI of 20 events per hr (Figure 1a), suggestive of two broad OSA severity-related CAP phenotypes.
| AHI < 20 events per hr (n = 10) | AHI ≥ 20 events per hr (n = 8) | Mann–Whitney | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | U-test | p | |
| Age (years) | 40.7 | 7.5 | 47.7 | 13.2 | 54.50 | .197 |
| BMI (kg m−2) | 24.6 | 4.7 | 28 | 4.2 | 44.00 | .180 |
| Education (years) | 18.9 | 2.5 | 18.7 | 2.6 | 36.50 | .748 |
| ESS | 6.9 | 4.9 | 10.8 | 4.5 | 55.50 | .166 |
| Sleep macrostructure | ||||||
| TST (min) | 402.45 | 48.44 | 352.56 | 79.7 | 27.00 | .250 |
| WASO (min) | 69.14 | 56.75 | 128.71 | 48.1 | 14.00 | .020* |
| SE (%) | 84.21 | 10.42 | 70.96 | 11.48 | 14.00 | .020* |
| N1% | 9.38 | 4.19 | 17.35 | 10.67 | 21.00 | .090 |
| N2% | 42.53 | 6.19 | 54.26 | 13.29 | 17.00 | .040* |
| N3% | 24.42 | 5.13 | 13.69 | 5.71 | 5.00 | .001* |
| REM% | 23.66 | 5.4 | 14.69 | 5.89 | 10.00 | .010* |
| ODI (events per hr) | 10.29 | 4.31 | 41.28 | 22.41 | 1.00 | .001* |
| Mean SpO2% | 95.31 | 0.88 | 94.09 | 0.97 | 11.50 | .010* |
| Lowest SpO2% | 85.98 | 4.31 | 81.13 | 7.57 | 22.50 | .120 |
| Sleep time with SpO2 below 90% (min) | 2.6 | 3.42 | 18.73 | 21.13 | 15.00 | .030* |
| AHI (events per hr) | 12.08 | 3.85 | 46.19 | 22.62 | 0.00 | .001* |
| Apnea index (events per hr) | 3.37 | 3.08 | 25.06 | 28.04 | 10.00 | .021 |
| Hypopnea index (events per hr) | 8.58 | 4.54 | 21.12 | 10.31 | 9.00 | .015 |
| AHI in NREM (events per hr) | 9.88 | 6.48 | 52.05 | 22.10 | 0.00 | .001 |
| RERA (events per hr) | 1.50 | 0.91 | 1.19 | 1.11 | 43.00 | .54 |
| RERA in NREM (events per hr) | 1.50 | 0.88 | 1.36 | 1.19 | 39.50 | .74 |
| RDI (events per hr) | 13.71 | 4.95 | 47.64 | 22.63 | 0.00 | .001* |
| Arousal index (events per hr) | 16.72 | 6.32 | 34.09 | 13.07 | 8.00 | .004* |
| Sleep microstructure | ||||||
| CAP time (min) | 175.20 | 49.22 | 190.50 | 67.57 | 34.00 | .594 |
| CAP rate (%) | 56.39 | 11.44 | 64.21 | 17.49 | 32.00 | .477 |
| CAP rate N1 (%) | 43.84 | 13.38 | 54.34 | 13.90 | 21.00 | .091 |
| CAP time N1 | 16.53 | 10.59 | 33.91 | 29.40 | 20.00 | .076 |
| CAP rate N2 (%) | 57.51 | 8.98 | 70.85 | 17.74 | 23.00 | .131 |
| CAP time N2 | 99.54 | 27.29 | 137.36 | 69.05 | 24.00 | .155 |
| CAP rate N3 (%) | 57.36 | 22.15 | 40.00 | 31.69 | 23.50 | .142 |
| CAP time N3 (min) | 56.80 | 24.02 | 15.76 | 10.27 | 5.00 | .002* |
| CAP cycles (n) | 373.60 | 99.12 | 351.00 | 112.54 | 34.00 | .594 |
| CAP cycles (s) | 29.28 | 1.95 | 34.11 | 3.56 | 10.00 | .008* |
| CAP sequences (n) | 39.60 | 9.01 | 41.63 | 11.98 | 39.50 | .965 |
| CAP sequences duration (min) | 4.42 | 1.02 | 5.23 | 2.65 | 40.00 | 1.000 |
| A1% (%) | 58.53 | 10.66 | 29.90 | 13.40 | 76.00 | .001* |
| A1 (n) | 238.40 | 64.51 | 125.38 | 56.27 | 7.00 | .003* |
| A1 mean duration (s) | 7.63 | 0.89 | 7.05 | 0.89 | 26.00 | .212 |
| A2% (%) | 14.11 | 4.63 | 16.10 | 8.89 | 34.00 | .633 |
| A2 (n) | 51.80 | 26.08 | 52.88 | 23.28 | 30.50 | .398 |
| A2 mean duration (s) | 9.64 | 1.29 | 10.04 | 1.87 | 35.00 | .656 |
| A3% (%) | 27.28 | 9.81 | 53.96 | 19.59 | 7.00 | .002* |
| A3 (n) | 83.40 | 42.92 | 172.75 | 101.10 | 16.00 | .033* |
| A3 mean duration (s) | 13.49 | 1.88 | 14.10 | 3.84 | 36.00 | .722 |
| B mean duration (s) | 19.91 | 2.23 | 23.23 | 3.17 | 12.00 | .025* |
| A1 index (n) | 46.13 | 10.03 | 25.47 | 10.65 | 75.00 | .001* |
| A2 + A3 index (n) | 26.16 | 10.25 | 45.45 | 15.00 | 11.00 | .009* |
| A1 + A2 + A3 index (n) | 72.29 | 15.36 | 70.93 | 16.45 | 39.00 | .965 |
- Abbreviations: A1 index, the number of subtype A1/NREM hours; A1 + A2 + A3 index, the number of subtype A1 + A2 + A3/NREM hours; A2 + A3 index, the number of subtype A2 + A3/NREM hours; AHI, apnea–hypopnea index; BMI, body mass index kg m−2; CAP, cyclic alternating pattern; CAP rate and CAP time in N1, N2 and N3; CAP rate, total CAP time/total NREM*100; CAP time, temporal sum of all CAP sequences in NREM sleep; ESS, Epworth Sleepiness Scale; n, number; NREM, non-rapid eye movement sleep; N1(X), non-rapid eye movement sleep stage 1(X); ODI, oxygen desaturation index; RDI, respiratory disturbance index; REM, rapid eye movement; RERA, respiratory effort-related arousal; SE, sleep efficiency; SpO2, oxygen saturation; TST, total sleep time; WASO, wake after sleep onset.
- Comparison is made between the two groups: AHI < 20 events per hr and AHI ≥ 20 events per hr using the Mann–Whitney test. Bold is used to denote those parameters that reached statistical significance.
Further comparisons were undertaken for purposes of better definition of the two phenotypes, and OSA patients were divided into a group with AHI < 20 events per hr (n = 10; milder OSA), and into a group with moderate to severe OSA and AHI ≥ 20 events per hr (n = 8). No significant differences were found between the two groups in age, weight (BMI), education, ESS and total sleep time (TST; Table 1). Moreover, overall CAP rate and time did not differ significantly between the two groups (Table 1).
However, several differences were recorded in patients’ sleep macro- and microarchitecture. For example, the group of patients with an AHI < 20 events per hr had better overall sleep efficiency. Their sleep was less fragmented, with an overall higher portion of sleep recorded in NREM3 and REM sleep (Table 1).
Notably, the NREM3 sleep stage of patients with milder OSA supported longer CAP sequences, with an overall higher rate of subtype A1 (A1%: 58.53 ± 10.66 versus 29.90 ± 13.40; p = .001) and lower rate of A3 subtype (A3%: 27.28 ± 9.81 versus 53.96 ± 19.59; p = .002). In keeping, RDI (RDI: 13.71 ± 4.95 versus 47.64 ± 22.63 events per hr; p = .001) and AI (AI:16.72 ± 6.32 versus 34.09 ± 13.07 events per hr; p = .004) were reported significantly lower in patients with milder OSA. RERA events in the NREM3 sleep were similarly experienced in both groups of patients (RERA:1.5 ± 0.88 versus 1.36 ± 1.19 events per hr; p = .74). Conversely, the group with AHI ≥ 20 events per hr had CAP cycles of longer duration, and they spent more time in NREM2.
4 DISCUSSION
Our results, to the best of our knowledge, for the first time showcase an unadulterated interplay between CAP’s adaptive and maladaptive arousal processes in OSA, of differing severities. We show that during mild arousing (hypercapnic) stimuli (e.g. AHI < 20 events per hr), sleep continuity is initially reinforced by the occurrence of K-complexes and delta bursts in the sleep EEG, as characterized by the CAP subtype A1 (Figure 1; Terzano et al., 1996). However, when the physiological stimuli become too intense (e.g. AHI ≥ 20 events per hr), this may overwhelm the brain's sleep gating mechanisms, and a cortical change is then increasingly translated by an alpha mixed or an alpha/beta frequency burst, as seen by an increase in aggregated ratio of subtypes A2 and A3 in patients with more severe OSA (Figure 1).
It is important to note that, whilst subtype A1 has been linked to a deeper and more efficient SWS that facilitates phase-amplitude coupling and processes of synaptic plasticity, and A3 subtype predominantly with cortical arousals and sleep fragmentation, all phase A subtypes were shown to be capable of reinstatement of breathing (Parrino et al., 2012), with the strongest effect nonetheless noted during A3 (Milioli et al., 2015; Parrino et al., 2012).
A deconstruction of arousal circuitries in the human brain is in its infancy, with its cortical and subcortical sources remaining elusive (Satpute et al., 2019). Conversely, in a series of seminal studies, Saper and colleagues have recently successfully argued the existence of several subcortical arousal circuitries in rodents (Anaclet et al., 2014; Kaur et al., 2013; Kaur & Saper, 2019). For example, they demonstrated ascending and descending projections from the subregions of pontine parabrachial nucleus (PBN), the hub with seemingly a major central role in inducing cortical arousals and respiratory regulation (Anaclet et al., 2014; Kaur & Saper, 2019). The emerging picture is that of two potentially functionally distinct arousal states, which may arise from PBN’s ascending projections to the basal forebrain, and other hypothalamic, insular and thalamo-cortical targets. In this model, the baseline arousal state of wakefulness is supported, at least in part, by the excitatory inputs from the medial PBN (Kaur & Saper, 2019). Its inhibition by sleep-active GABAergic neurons in the medullary parafacial zone leads to a powerful increase in synchronizing SWS (Anaclet et al., 2014) and the electrocortical signature of K-complexes and delta bursts, highly reminiscent of human CAP subtype A1 (Parrino et al., 2012). In contrast, a stimulation of the more lateral subregions of PBN under conditions of visceral sensory distress, such as pain or respiratory insufficiency, evokes a much more potent basal forebrain arousal (Kaur & Saper, 2019). Its electrocortical signature is that of higher (alpha/beta) frequency bursts, very much in keeping to human CAP A2/A3 response. Of note is that the (hypercapnic) stimulation of the lateral PBN in rodents can also lead to a powerful concomitant re-instatement of breathing via PBN’s descending medullary projections (Kaur & Saper, 2019; Yokota et al., 2015).
In human studies, and in patients with OSA, arousal has in the past been invariably shown to be correlated with increased respiratory effort (as sensed by vagal mechanoreceptors), to a lesser degree with the level of hypercapnia, and, debatably, least of all with the level of hypoxia (Gleeson et al., 1990; Kaur et al., 2013; Kaur & Saper, 2019; White, 2017). Building on this, we argue that CAP A2/A3 indices in our study likely reflect the severity of all obstructive events and RERAs, as recorded by patients’ RDI (Table 1). Thus, we similarly propose that comparatively lower CAP A2/A3 indices in our patients with milder OSA (Table 1) may reflect an insufficient power of milder (hypercapnic) stimuli in this group to consistently provoke a powerful EEG arousal via ascending branches of the lateral PBN region (or rather its human functional anatomical correlate/s). Similarly, its partial effects via descending branches may lead to an increased nocturnal respiratory effort (Kaur & Saper, 2019; Yokota et al., 2015). This may, in turn, stimulate a conditional expiratory oscillator in the medullary parafacial zone (Boutin et al., 2017), and further trigger the ascending rhythmic inhibition of the PBN-cortical circuitry (Anaclet et al., 2014; Boutin et al., 2017). We further propose that this activity, in parallel to its effects in rodents, may result in the series of EEG delta bursts. Moreover, we argue that this adaptive activity likely accounts for a significantly higher CAP subtype A1 index in our patients with milder OSA (Anaclet et al., 2014).
Whilst these hypothetical notions of competing arousal circuitries remain speculative, they are in rudimentary agreement with the core findings of rodent studies to date, as well as with some early experimental human data (Anaclet et al., 2014; Boutin et al., 2017; Kaur et al., 2013; Kaur & Saper, 2019). It is hoped that our findings, together with their theoretical construct, offer a judicious basis on which future in-depth multimodal imaging studies of OSA can be built.
In summary, despite its limitations, we believe that our small preliminary study significantly contributes to the understanding of the complex interplay between OSA severity and sleep instability dynamics. Moreover, our findings suggest that CAP parameters might serve as an important EEG biomarker of arousal-related phenomena that may in future enable characterizations of their appropriate cortical and subcortical sources, and hence as such enable a more personalized and targeted therapeutic approach in disorders such as OSA.
ACKNOWLEDGEMENTS
This work was supported by the Wellcome Trust [103952/Z/14/Z].
CONFLICT OF INTEREST
All authors contributed to the design of the study protocol. VG, SH, PD, IR collected data. All authors were involved in reviewing and drafting of the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the authors upon reasonable request and with permission of the Research Ethics Committee.




