European guideline for the diagnosis and treatment of insomnia
Summary
This European guideline for the diagnosis and treatment of insomnia was developed by a task force of the European Sleep Research Society, with the aim of providing clinical recommendations for the management of adult patients with insomnia. The guideline is based on a systematic review of relevant meta-analyses published till June 2016. The target audience for this guideline includes all clinicians involved in the management of insomnia, and the target patient population includes adults with chronic insomnia disorder. The GRADE (Grading of Recommendations Assessment, Development and Evaluation) system was used to grade the evidence and guide recommendations. The diagnostic procedure for insomnia, and its co-morbidities, should include a clinical interview consisting of a sleep history (sleep habits, sleep environment, work schedules, circadian factors), the use of sleep questionnaires and sleep diaries, questions about somatic and mental health, a physical examination and additional measures if indicated (i.e. blood tests, electrocardiogram, electroencephalogram; strong recommendation, moderate- to high-quality evidence). Polysomnography can be used to evaluate other sleep disorders if suspected (i.e. periodic limb movement disorder, sleep-related breathing disorders), in treatment-resistant insomnia, for professional at-risk populations and when substantial sleep state misperception is suspected (strong recommendation, high-quality evidence). Cognitive behavioural therapy for insomnia is recommended as the first-line treatment for chronic insomnia in adults of any age (strong recommendation, high-quality evidence). A pharmacological intervention can be offered if cognitive behavioural therapy for insomnia is not sufficiently effective or not available. Benzodiazepines, benzodiazepine receptor agonists and some antidepressants are effective in the short-term treatment of insomnia (≤4 weeks; weak recommendation, moderate-quality evidence). Antihistamines, antipsychotics, melatonin and phytotherapeutics are not recommended for insomnia treatment (strong to weak recommendations, low- to very-low-quality evidence). Light therapy and exercise need to be further evaluated to judge their usefulness in the treatment of insomnia (weak recommendation, low-quality evidence). Complementary and alternative treatments (e.g. homeopathy, acupuncture) are not recommended for insomnia treatment (weak recommendation, very-low-quality evidence).
Guideline Report And Methods
This European guideline for the diagnosis and treatment of insomnia was developed on the basis of the guideline for insomnia by the German Sleep Society (Riemann et al., 2017), and has been modified and extended through the involvement of experts from various European countries and the European Insomnia Network under the umbrella of the European Sleep Research Society (ESRS). A more detailed version of this guideline's report can be found in the supplemental material.
The guideline focuses on insomnia, defined as difficulties initiating or maintaining sleep, or early morning awakening associated with impaired daytime functioning, for example, reduced cognitive performance, fatigue or mood disturbances. Thus, the target population of this guideline comprises patients suffering from insomnia as defined by ICD-10/ICSD-3. This includes all subtypes of insomnia, for example, non-organic insomnia and insomnia co-morbid with somatic or mental disorders. The guideline addresses adult patients (≥18 years). The literature on insomnia in children and adolescents was not reviewed. This guideline reviews the available literature with a special focus on the situation in Europe. The guideline is meant for physicians and clinical psychologists/psychotherapists who diagnose and treat patients with insomnia.
Computer-based searches using PubMed (www.ncbi.nlm.nih.gov/pubmed/) and the Cochrane Library (www.cochranelibrary.com) were conducted with the following keywords: (psychotherapy OR sleep hygiene OR relaxation OR mindfulness OR behaviour therapy OR cognitive therapy OR cognitive behavioural therapy OR stimulus control OR sleep restriction OR placebo OR benzodiazepine OR benzodiazepine receptor agonist OR sedating antidepressant OR antipsychotic OR neuroleptic OR antihistamine OR herbal therapy OR phytotherapy OR melatonin OR complementary alternative therapy OR homeopathy) AND insomnia (search filter set to meta-analysis). Furthermore, all issues of the journal ‘Sleep Medicine Reviews’ (until June 2016) were screened for additional relevant publications, and the search was expanded through identifying further publications from references in the screened full-texts. The search included studies conducted from January 1966 to June 2016. Studies were required to be written in English to be included. The first author conducted the literature search, screened titles and abstracts, and examined the full texts with the help of the third and last authors. Concerning the translation of effect sizes (Cohen's d) into text form, effect sizes <0.4 were defined as a small effect; effect sizes >0.4–0.8 as a good effect; effect sizes >0.8 as a very good effect.
The GRADE (Grading of Recommendations Assessment, Development and Evaluation; Atkins et al., 2004; Guyatt et al., 2008) system was used to grade the evidence and inform recommendations. The published evidence was rated as high quality if the examined meta-analyses suggested it to be very unlikely that further research would change our confidence in the estimate of an observed effect. In contrast, the verdict low quality was given when the examined meta-analyses suggested that any estimate of effect is uncertain. Table S1 shows the classification system for the quality of evidence according to Guyatt et al. (2008) in detail. Two grades of recommendations were used: ‘strong’ and ‘weak’. The transformation of levels of evidence into grades of recommendation was based on a consensus being reached between the authors.
Insomnia
Aetiology and pathophysiology
This guideline primarily targets insomnia as an independent disorder, and not as an isolated symptom or a syndrome closely related to, or even directly caused by, other somatic or mental disorders. The type of insomnia addressed here closely resembles the concept of ‘psychophysiological’ insomnia as conceptualized decades ago (Hauri and Fisher, 1986). Given recent developments in the DSM-5 (2013) and ICSD-3 (2014), we will use the terms insomnia and insomnia disorder interchangeably throughout this guideline. Instead of the previously used dichotomy primary versus secondary insomnia, we will follow the concept of co-morbidity.
Several research groups have suggested aetiological and pathophysiological models of insomnia (Espie, 2002; Espie et al., 2006; Harvey, 2002; Levenson et al., 2015; Morin, 1993; Riemann et al., 2012, 2015). Most of these are explicitly or implicitly based on the so-called ‘3P’ model of insomnia by Spielman et al. (1987), which postulates that predisposing, precipitating and perpetuating factors are involved in the aetiology of insomnia. For example, genetic influences (Palagini et al., 2014) or personality characteristics like neuroticism or maladaptive perfectionism are seen as predisposing factors.
Acute stressors, for example, stress at work or interpersonal conflicts, usually trigger acute insomnia. Acute insomnia is very common and often a transient phenomenon, which is relieved after cessation of the stressor (Ellis et al., 2012a; Espie, 2002). Chronic stress exposure may also be seen as an underlying cause for chronic insomnia. In many cases, perpetuating factors have to come into play during the transition from acute to chronic insomnia. Spielman et al. (1987) suggested that maladaptive coping strategies are perpetuating factors, for example, prolonged time in bed or napping in order to catch up on lost sleep. While these behaviours may appear reasonable, they can reduce sleep pressure and may lead to chronic insomnia in the long run. Additionally, Espie et al. (2006) have emphasized the development of a maladaptive focus upon sleep in patients with insomnia, whereby sleep-related attentional biases and direct attempts to control sleep disturb the two-process bioregulation of sleep (Borbély, 1982; Borbély and Achermann, 1999), interfering with the expected default recovery to normal sleep, following episodic stress.
The hyperarousal model of insomnia postulates that increased arousal levels in the cognitive, emotional and physiological domains represent both predisposing ‘and’ perpetuating factors (Perlis et al., 1997; Riemann et al., 2010, 2015). Central to this model are results showing that patients with insomnia have increased power in fast electroencephalographic (EEG) frequencies during non-rapid eye movement sleep. This might also be reflected by an increased cyclic alternating pattern rate (Chouvarda et al., 2012). An increased frequency of microarousals during rapid eye movement (REM) sleep, which contributes to the perception of parts of REM sleep as wakefulness, has also been observed in patients with insomnia, relative to normal sleepers (Feige et al., 2013; Riemann et al., 2012). Neurobiologically, hyperarousal may be driven by a dominance of arousal-generating brain areas relative to sleep-inducing brain areas (Saper et al., 2005).
Cognitive models of insomnia stress the relevance of worry and rumination in the development and maintenance of insomnia (Harvey, 2002). Moreover, Baglioni et al. (2010) have emphasized that patients with insomnia have an increased emotional reactivity, which may also be of aetiological relevance.
Circadian factors are important in a subgroup of individuals, for example in those who undertake shift work or in blind patients, where desynchronization of the sleep–wake pattern and the circadian phase contributes to sleep initiation and sleep maintenance difficulties. This also applies to some cases of sleep-onset insomnia in adolescents/young adults, whereby a circadian phase delay may be the underlying factor, and to elderly patients with early awakening, whereby a phase advance may play a role (Abbott et al., 2016).
Definition of insomnia – diagnostic classification systems
In most European countries, use of the International Classification of Diseases (ICD-10, 1992) is mandatory for physicians and clinical psychologists/psychotherapists in order to get reimbursed through health insurance. For the diagnosis of insomnia, the diagnostic categories ‘Non-organic insomnia’ (F51.0) and ‘Disorders of initiating and maintaining sleep (insomnias)’ (G47.0) are relevant. The definition for non-organic insomnia is presented in Table 1.
|
The diagnosis of ‘non-organic insomnia’, according to ICD-10, is based solely on the subjective experience of afflicted individuals. No quantitative criteria for sleep-onset latency, sleep duration, or the frequency, or duration, of nocturnal awakenings is required. The term ‘non-organic insomnia’ refers to the fact that this sleep disorder does not have a specific recognizable somatic disorder at its core. However, the use of this term has been discussed critically over the last few years in light of documented neurobiological alterations in patients with insomnia.
DSM-5 (2013) has removed the distinction between primary and secondary insomnia. This distinction was aimed at differentiating ‘pure’ independent insomnia from ‘secondary’ insomnia, i.e. insomnia being related to or even hypothetically being caused by another somatic/mental disorder. Instead, the new umbrella category ‘insomnia disorder’ was introduced, which is also used in the third version of the International Classification of Sleep Disorders (ICSD-3; AASM, 2014). The decision to remove the distinction between primary and secondary insomnia was based on an NIH conference on insomnia in 2005 (National Institutes of Health, 2005), with the lack of evidence that treating the primary disorder would relieve insomnia accordingly, for example in cases of insomnia associated with depression, being the main reason for this change.
The definition of insomnia within the ICSD-3 largely follows that of the DSM-5. Table 2 shows the diagnostic criteria for insomnia according to the ICSD-3. In order to receive the diagnosis, there must be a disturbance of nocturnal sleep (criterion A) and related daytime impairment (criterion B). Furthermore, the sleep disorder has to occur at least 3 nights a week for a period of 3 months to be diagnosed as a clinically relevant disorder. If diagnostic criteria are fulfilled co-morbid with a mental or somatic disorder, both disorders are diagnosed.
|
As already mentioned, acute insomnia is very common and does not need a specific treatment in all cases (Ellis et al., 2012b). Chronic insomnia, instead, needs to be treated. The definitions for chronicity are, however, varying. ICD-10 requires a minimum duration of 1 month, whereas the ICSD-3 specifies 3 months. The authors of this guideline endorse the use of ICSD-3 for diagnostic purposes, and expect the development of ICD-11 will most likely follow the conceptual innovations of ICSD-3.
Diagnostic procedure
The recommended procedure for the diagnostic management of insomnia disorder, and its co-morbidities, is shown in Table 3.
|
|
- CRP, C-reactive protein; CT, Computed Tomography; ECG, electrocardiogram; EEG, electroencephalogram; MRT, Magnetic Resonance Tomography.
A medical and psychiatric/psychological anamnesis is mandatory, and has to be tailored to the clinical picture of the patient and his/her symptomatology. With respect to the assessment of medical disorders, it needs to be borne in mind that some somatic causes of insomnia can be specifically treated, for example hyperthyroidism. However, even in the case of a clear somatic cause, many patients with insomnia develop a psychophysiological vicious cycle of insomnia, which includes rumination, worry about the consequences of poor sleep and increased physiological tension. These processes can be successfully treated in these co-morbid cases of insomnia.
Similar considerations should be made for substance use (e.g. alcohol/caffeine), which is important to evaluate in patients with insomnia. In particular, alcohol consumption is a common maladaptive self-treatment strategy in patients with insomnia, and can contribute to sleep-maintenance difficulties. Thus, alcohol consumption should be actively evaluated and considered during treatment planning. Furthermore, many medications can interfere with sleep. Therefore, the use, dosage and timing of medication should also be evaluated.
Mental disorders, especially depression, bipolar disorder or psychosis are also frequently accompanied by sleep-onset or sleep-maintenance difficulties or early morning awakening. A recent meta-analysis (Baglioni et al., 2016) showed that disturbances of sleep continuity (prolonged sleep latency, increased frequency of nocturnal awakenings, prolonged periods of wakefulness after sleep onset) occur transdiagnostically in almost all mental disorders. Patients with chronic insomnia often suffer from a co-morbid mental disorder, which they do not spontaneously report. This may be due to the fact that it is easier for some patients to talk about sleep than to talk about emotional distress. Thus, the presence of mental disorders should also be actively examined. Tiredness/fatigue also occurs in many mental or neurodegenerative disorders. Sleepiness (presumably experienced as a consequence of sleep loss) is usually not a symptom of insomnia per se, but may be due to an accumulation of sleep loss in these patients. As such, tiredness/fatigue and sleepiness should also be assessed.
Table 4 summarizes the major somatic and mental co-morbidities of insomnia.
| Psychiatric | Medical | Neurological | Substance use/dependence |
|---|---|---|---|
| Depressive disorders | Chronic obstructive pulmonary diseases | Neurodegenerative diseases | Alcohol |
| Bipolar disorders | Diabetes mellitus | Fatal familial insomnia | Nicotine |
| Generalized anxiety disorder | Chronic kidney diseases | Cerebrovascular diseases | Caffeine |
| Panic disorder | Human immunodeficiency virus infection | Multiple sclerosis | Marijuana |
| Posttraumatic stress disorder |
Malignancy Rheumatic disorders |
Traumatic brain injury | Opioids |
| Schizophrenia | Chronic pain | RLS | Designer drugs |
| Sleep apnea | Cocaine | ||
| Amphetamine |
- RLS, Restless Legs Syndrome.
The diagnostic procedure should also include a clinical interview consisting of a thorough sleep history (to assess sleep hygiene behaviour, sleep habits, sleep environment – including co-sleeping arrangements, work schedules, circadian factors and indications of other sleep disorders, e.g. restless legs syndrome, sleep apnea, circadian sleep–wake disorder, etc.). The consensus sleep diary (Carney et al., 2012), for 7–14 days, is also strongly recommended. Moreover, the Pittsburgh Sleep Quality Index (PSQI) can be used to assess subjective sleep during the previous month (Buysse et al., 1989). The PSQI, however, is not a specific instrument for diagnosing insomnia and should not be used for that purpose. The Insomnia Severity Index (ISI) has been developed to assess the severity of the disorder, and has also been shown to be a reliable and valid instrument to detect patients with insomnia (Bastien et al., 2001). In addition, the Bergen Insomnia Scale (Pallesen et al., 2008) and the Sleep Condition Indicator (Espie et al., 2012) have promising psychometric properties. An overview of the available scales for assessing sleep, and sleep disorders, is provided by Shahid et al. (2012). Furthermore, if indicated, actigraphy or polysomnography should be considered.
A meta-analysis of polysomnographic studies showed that patients with insomnia have a significantly reduced total sleep time, significantly prolonged sleep-onset latencies, and an increased number of nocturnal awakenings and amount of time awake during the night (Baglioni et al., 2014). Furthermore, slow-wave sleep and REM sleep percentages are reduced compared with good sleepers. However, the differences were not very pronounced, for example, total sleep time was reduced by about 25 min. In contrast, subjective total sleep time is reduced by about 2 h in patients with insomnia compared with good sleepers (Feige et al., 2008). This has led to the use of the terms ‘pseudoinsomnia’, ‘sleep state misperception’ or ‘paradoxical insomnia’. Many experts argue that polysomnography is not helpful in the assessment of insomnia because it does not correlate with the subjective perceptions of patients. However, we suggest polysomnography may have an additional diagnostic value ‘because’ it does not correlate with subjective measures and thus may deliver information not inherent in the subjective patient report. In addition, objective measures are mandatory to diagnose potential co-morbid disorders (e.g. PLMD = Periodic Leg Movement Disorder, sleep apnea), which are common. Sleep apnea may have a complex relationship with insomnia, thus being more than a mere co-morbidity (Sweetman et al., 2017). Several studies suggest that the polysomnographically determined microstructure of sleep is altered in insomnia, with increases in fast frequency power and in the number of microarousals. These phenomena are partly independent of the subjective experience of sleep (Riemann et al., 2015), and may become relevant for treatment decisions in the future (see ‘Outlook for the future’). Another recent discovery concerns differences between insomnia with, and without, an objective short sleep duration (Fernandez-Mendoza, 2017; Vgontzas et al., 2013). It is hypothesized that insomnia with polysomnographically documented short sleep duration has primarily biological roots and would thus respond better to biological treatments. If this hypothesis turns out to be true, polysomnography may become even more important in the diagnostic procedure for insomnia.
Epidemiology
Approximately 6% of the adults in industrialized countries suffer from chronic insomnia as a disorder (for overview, see Ohayon, 2002), with a clear-cut preponderance of females compared with males (Zhang and Wing, 2006) and an age-related increase in prevalence rates. More recent data (e.g. from Norway, the UK and Germany) indicate an increase in the prevalence of insomnia, to about 10% of the population, in recent years (Calem et al., 2012; Marschall et al., 2017; Pallesen et al., 2014). Moreover, it appears that the use of hypnotic agents has also increased significantly over a 10-year period (e.g. from 7% to 11% in Norway; Pallesen et al., 2001, 2014). Table 5 shows epidemiological data on the prevalence of insomnia, as a disorder, in 10 European countries (no such data are available for insomnia, on the disorder level, for the other European countries).
| Country | Author (year) | Sample size | % Insomnia diagnosis |
|---|---|---|---|
| England | Calem et al. (2012) | 20 503 | 5.8% |
| Finland | Ohayon and Partinen (2002) | 982 | 11.7% |
| France | Léger et al. (2000) | 12 778 | 19% |
| Germany | Schlack et al. (2013) | 7988 | 5.7% |
| Hungary | Novak et al. (2004) | 12 643 | 9% |
| Italy | Ohayon and Smirne (2002) | 3970 | 7% |
| Norway | Pallesen et al. (2001, 2014) | 2000 | 15.5% |
| Romania | Voinescu and Szentágotai (2013) | 588 | 15.8% |
| Spain | Ohayon and Sagales (2010) | 4065 | 6.4% |
| Sweden | Mallon et al. (2014) | 1550 | 10.5% |
Table 5 demonstrates that the prevalence of insomnia varies largely from one European country to the other. This may be, in part, due to differences in methodological quality between studies. At present the prevalence of insomnia, as a disorder, in Europe, seems to vary from a minimum of 5.7% in Germany to a maximum of 19% in France. There is only one comprehensive epidemiological study (Van de Straat and Bracke, 2015) that employed a cross-national approach and studied sleep problems across 16 European countries, but only in older adults. This study did not specifically include questions to derive insomnia diagnoses, just a single item measure of sleep problems. This study showed that the prevalence rate for this type of sleep problem varies from a minimum of 16.6% in Denmark to a maximum of 31.2% in Poland. Our literature search and the study by van de Straat and Bracke suggest an urgent need for Pan-European cross-sectional studies to better understand the size of the problem in Europe, also with respect to co-morbidities.
Studies in general practice or medical specialty settings deliver substantially higher prevalence rates: data from general practice in Germany (Wittchen et al., 2001) indicate that one-fifth of the patients consulting a GP suffer from insomnia; whereas in Norway more than 50% of GP patients have insomnia (Bjorvatn et al., 2017).
In terms of the persistence of insomnia, there is very little information from Europe. However, Morin et al. (2009a) provided data on the natural course of insomnia in Canada, and showed that approximately 70% of the patients show persistent symptoms over the course of 1 year. In this study, 46% of those suffering from insomnia showed persistent symptoms over the course of 3 years.
The prevalence of hypnotic usage, i.e. usage of benzodiazepines (BZ) and benzodiazepine receptor agonists (BZRAs), varies largely from one European country to the other. A UK study reported an increase in hypnotic usage from 0.4% to 0.8% in the general population from 1993 to 2000 – the data remained stable from 2000 to 2007 (Calem et al., 2012). A German study described the prevalence of having taken a hypnotic, at least once, increased from 4.7% to 9.2% from 2009 to 2016 (Marschall et al., 2017). In general, it is not clear how many patients with insomnia in Europe regularly take hypnotics – further research is necessary to determine the exact scale of this issue.
Health risks
Several meta-analyses show that insomnia is a significant risk factor for cardiovascular diseases (Li et al., 2014; Meng et al., 2013; Sofi et al., 2014). Specifically, insomnia is a risk factor for arterial hypertension, myocardial infarction and chronic heart failure (Laugsand et al., 2011, 2014a; Palagini et al., 2013). In addition, Anothaisintawee et al. (2015) showed that insomnia is a risk factor for type 2 diabetes.
Besides insomnia itself, there is evidence suggesting that short sleep duration (sleeping less than 6 h on average) is a risk factor for obesity, type 2 diabetes, hypertension and cardiovascular diseases (Bayon et al., 2014; Buxton and Marcelli, 2010; Cappuccio et al., 2010; Faraut et al., 2012; Patel and Hu, 2008). Consequently, short sleep duration also increases mortality (Liu et al., 2017). However, the association between a short sleep duration and insomnia is not yet fully understood.
Neurological disorders are frequently co-morbid with insomnia (Mayer et al., 2011), and insomnia may play a role in the development of cognitive impairment (Yaffe et al., 2014). In addition, one cross-sectional study suggests a relationship between impaired sleep quality and cortical atrophy in older adults (Sexton et al., 2014). More recent work points to a general involvement of insomnia in the development of neurodegenerative disease, especially dementia (Osorio et al., 2011). Bassetti et al. (2015) stress the bi-directional nature of the relationship between insomnia and brain disorders.
Significant evidence has been gathered with respect to the relationship between insomnia and mental disorders (Riemann and Voderholzer, 2003). In a meta-analysis, Baglioni et al. (2011) showed that people with insomnia have an increased risk for the development of major depressive disorder (odds ratio 2.1), which may also lead to early retirement (Paunio et al., 2015). Similar relationships have been documented for insomnia complaints and suicide ideation, suicide attempts and completed suicides (Malik et al., 2014; Pigeon et al., 2012).
Large epidemiological studies have also demonstrated that insomnia is a risk factor for sick leave, an increased number of accidents in the work place (Laugsand et al., 2014b; Sivertsen et al., 2009a,b) and motor-vehicle accidents (Léger et al., 2014).
Costs of insomnia
The question of the direct and indirect costs of insomnia has been dealt with in several large, well-designed, studies (Daley et al., 2009; Léger and Bayon, 2010; Ozminkowski et al., 2007). Of particular relevance to Europe, the costs of several brain disorders in Europe were compared in 2010 (Gustavsson et al., 2011). This study ranked sleep disorders ninth among all neuropsychiatric disorders with respect to direct and indirect costs. An average total sum (costs) of €790 per year, per patient, was calculated. These overall costs were based on individual costs calculated against the estimated prevalence of insomnia, ranging from 6% to 12%, in the European population (Wittchen et al., 2011). Concerning so-called DALYs (disability-adjusted life-years), a figure of 10.3/10 000 individuals was given for females, and 8.4/10 000 individuals for males – ranking ninth among all neuropsychiatric disorders studied. According to WHO data, insomnia ranked 11th in the list of most important brain disorders with respect to global burden (Collins et al., 2011). Thus, it can be concluded that insomnia represents a high financial burden to European healthcare systems, either through direct costs, i.e. costs for medication or psychotherapeutic treatment, or indirect costs, for example, due to sick leave or early retirement.
Treatment of insomnia
In the presence of co-morbidities, clinical judgement should decide whether the insomnia or the co-morbid condition is treated first, or whether both are treated at the same time. Of note, the grading and recommendations of all the treatment options outlined in this section are collectively summarized in Table 15.
Cognitive behavioural therapy for insomnia (CBT-I) and other psychotherapeutic approaches
Cognitive behavioural therapy for insomnia usually consists of psychoeducation/sleep hygiene, relaxation training, stimulus control therapy, sleep restriction therapy and cognitive therapy (Riemann and Perlis, 2009). Usually, CBT-I is applied face to face (either on an individual basis or in a group format) by a trained clinician in four-eight sessions. A number of manuals have been published in different languages (Dutch: Verbeek and van de Laar, 2014; English: Morin and Espie, 2004; Perlis et al., 2005; French: Goulet et al., 2013; German: Hertenstein et al., 2015; Spiegelhalder et al., 2011; Italian: Devoto and Violani, 2009; Norwegian: Bjorvatn, 2013; Portuguese: Paiva, 2008; and Slovakian: Backhaus and Riemann, 2003).
Psychoeducation/sleep hygiene. In the context of CBT-I, psychoeducation typically includes the so-called ‘sleep hygiene rules’ about health practices (e.g. clockwatching, physical exercise, substance use) and environmental factors (e.g. light, noise, temperature) that may promote or disrupt sleep (Hauri, 1991). Furthermore, psychoeducation includes basic information about normal sleep and age-related changes in sleep patterns.
Relaxation therapy. Relaxation therapy includes clinical procedures aimed at reducing somatic tension (e.g. progressive muscle relaxation, autogenic training) or intrusive thoughts at bedtime (e.g. imagery training, meditation).
Behavioural strategies (sleep restriction, stimulus control). Sleep restriction therapy is a method designed to curtail the time in bed to the actual amount of sleep being achieved (Spielman et al., 1987). For example, if a patient with insomnia reports sleeping 6.5 h per night on average, the initial recommended sleep window (the time from lights out to final arising time) would be restricted to 6.5 h (with a minimum sleep window of 4–6 h being advised, even when the average sleep time is lower; Kyle et al., 2015). On a weekly basis, adjustments to this sleep window are made. Time in bed is either increased by 15–30 min (when sleep efficiency is >85–90%), kept stable or decreased by 15–30 min (when sleep efficiency is <80%), until an optimal sleep duration is reached. It is strongly recommended that sleep diaries be used to estimate sleep time, both before starting sleep restriction therapy and also during follow-ups. Stimulus control therapy is a set of behavioural instructions designed to re-associate the bed/bedroom with sleep and to re-establish a consistent sleep–wake schedule (Bootzin, 1972): (1) go to bed only when sleepy; (2) get out of bed when unable to sleep; (3) use the bed/bedroom only for sleep and sex (e.g. no reading, no watching TV); (4) arise at the same time every morning; (5) do not nap during the day.
Cognitive therapy. Cognitive strategies are psychological methods designed to identify, challenge and change misconceptions about sleep and faulty beliefs about insomnia and its perceived daytime consequences (Morin and Espie, 2004). These strategies include methods aimed at reducing or preventing excessive monitoring of, and worrying about, insomnia and its correlates or consequences.
Other psychotherapeutic approaches. Other psychotherapeutic approaches that have been empirically investigated include mindfulness-based treatments and hypnotherapy. Mindfulness-based treatments are rooted in Buddhist philosophy, and include stress reduction techniques and cognitive elements (Crane et al., 2017). Hypnotherapy is also conceived as a mind–body intervention bearing similarities to meditation techniques. Hypnotherapy consists of verbal suggestions by the therapist, which are supposed to elicit subconscious changes (Facco, 2017; Terhune et al., ).
Grading of the evidence
There are 15 published meta-analyses on the efficacy of CBT-I (Table 6). These comprise meta-analyses of CBT-I for ‘primary’ insomnia as well as meta-analyses of CBT-I for co-morbid insomnia. In the latter, it was shown that CBT-I has a positive impact on both insomnia complaints and co-morbid symptoms.
| Author (year) | Population | Number of studies/number of patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Morin et al. (1994) | Insomnia | 59/2102 | CBT-I and single components | SOL, WASO, NOA, TST |
|
| Murtagh and Greenwood (1995) | Insomnia | 66/2007 | CBT-I and single components | SOL, NOA, TST, SQ |
|
| Pallesen et al. (1998) | Insomnia, age >50 years | 13/388 | CBT-I and single components | SOL, NOA, WASO, TST |
|
| Montgomery and Dennis (2004) | Primary insomnia, age >60 years | 7/322 | CBT-I, bright light and physical exercise | SOL, TST, SE, WASO |
|
| Irwin et al. (2006) | Insomnia, age >55 years versus younger patients | 23/NA | CBT-I and single components | SQ, SOL, TST, SE, WASO | Medium to strong effects in older patients |
| Belleville et al. (2011) | Insomnia with/without co-morbid anxiety | 50/2690 | CBT-I | Anxiety scales | Moderate effects on anxiety |
| Okajima et al. (2011) | Primary insomnia | 14/927 | CBT-I | SOL, WASO, EMA, SE, PSG, ACT |
|
| Miller et al. (2014) | Primary insomnia | 4/192 | Sleep restriction therapy | SOL, WASO, TST, NOA, SE, SQ | Sleep restriction alone is effective |
| Koffel et al. (2015) | Insomnia | 8/659 | Group CBT-I | SOL, WASO, SE, SQ, TST, pain, depression | Group CBT-I is effective |
| Trauer et al. (2015) | Chronic insomnia | 20/1162 | CBT-I | SOL, WASO, TST, SE | Clinically relevant efficacy without undesired side-effects |
| Geiger-Brown et al. (2015) | Co-morbid insomnia (somatic/mental) | 23/1379 | CBT-I | SOL, WASO, TST, SE, ISI, PSQI | Good efficacy; long-term effects at 18 months |
| Wu et al. (2015a) | Co-morbid insomnia (somatic/mental) | 37/2189 | CBT-I | SOL, WASO, SQ, TST, remission, co-morbid symptoms | Good efficacy; smaller effects on co-morbid symptoms; better effects for mental outcomes |
| Ho et al. (2016) | Insomnia + PTSD | 11/593 | CBT-I | SOL, WASO, SE, TST, PTSD symptoms | Good sleep effects, good effects on PTSD symptoms |
| Johnson et al. (2016) | Insomnia + cancer | 8/752 | CBT-I | SE, WASO, ISI, cancer symptoms | Good sleep effects, good effects on cancer symptoms |
| Tang et al. (2015) | Insomnia + pain | 11/1066 | CBT-I | SQ, fatigue, pain | Good sleep effects, good effects on co-morbid symptoms |
- ACT, actigraphy; CBT-I, cognitive behavioural therapy for insomnia; EMA, early morning awakening; ISI, insomnia severity index; NOA, number of awakenings; PSG, polysomnography; PSQI, Pittsburgh Sleep Quality Index; PTSD, posttraumatic stress disorder; SE, sleep efficiency; SOL, sleep-onset latency; SQ, sleep quality; TST, total sleep time; WASO, wake time after sleep onset.
The first five meta-analyses (Irwin et al., 2006; Montgomery and Dennis, 2004; Morin et al., 1994; Murtagh and Greenwood, 1995; Pallesen et al., 1998) and the meta-analysis provided by Trauer et al. (2015) dealt with the efficacy of CBT-I, or its components, in patients with primary insomnia. All these meta-analyses demonstrated good efficacy for CBT-I (according to our translated definition of effect sizes) on sleep-related outcome parameters, and a good stability of the results at follow-up assessments.
Belleville et al. (2011) showed that CBT-I has a small to moderate effect on anxiety levels in patients with or without clinically relevant co-morbid anxiety. Miller et al. (2014) investigated one component of CBT-I, i.e. sleep restriction therapy. This meta-analysis was based on only four studies, but showed good efficacy for sleep restriction therapy. Group CBT-I was investigated by Koffel et al. (2015). This meta-analysis demonstrated a good efficacy for group format; however, only eight original studies could be included. The most recent meta-analyses addressed CBT-I for co-morbid insomnia, i.e. insomnia in the context of mental or somatic disorders. Geiger-Brown et al. (2015) and Wu et al. (2015a,b) dealt with a variety of co-morbid conditions, whereas Ho et al. (2016), Johnson et al. (2016) and Tang et al. (2015) specifically investigated insomnia in the context of posttraumatic stress disorder, cancer and chronic pain. These meta-analyses showed that co-morbid insomnia also responds well to CBT-I. Of particular importance, CBT-I, though focusing exclusively on sleep, also had good effects on the co-morbid conditions.
There is also evidence supporting the efficacy of brief versions of CBT-I, for example, using two face-to-face sessions and two telephone calls (Buysse et al., 2011) or just one session for acute insomnia (Ellis et al., 2015). There are also other forms of application, for example, group CBT-I courses delivered by nurses (Espie et al., 2007).
Table 7 shows meta-analyses on the efficacy of self-help and internet-based CBT-I. These six meta-analyses focus on self-help CBT-I approaches (Ho et al., 2015; Van Straten and Cuijpers, 2009), or internet-based CBT-I, for example, the programmes ‘sleep healthy using the internet’ (SHUTi; Ritterband et al., 2009) or SLEEPIO (Espie et al., 2012). The four meta-analyses on internet-based CBT-I showed good treatment efficacy; however, the efficacy was lower than for face-to-face CBT-I. One of these meta-analyses investigated the effects of internet-based CBT-I on anxiety and depression levels, and showed small to moderate effects (Ye et al., 2015). A recent large randomized controlled trial also suggested that internet-based CBT-I reduced subclinical depression levels and may thus be used for the prevention of depression (Christensen et al., 2016). Moreover, Thiart et al. (2016) investigated the health economic effects of computerized CBT-I (cCBT-I), concluding that it was associated with an 87% probability of being more effective than treatment as usual.
| Author (year) | Population | Number of studies/number of patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Van Straten and Cuijpers (2009) | Insomnia | 10/1000 | Self-help CBT-I | SOL, WASO, SE, SQ, TST | Small to moderate effects |
| Cheng and Dizon (2012) | Insomnia | 6/433 | cCBT-I | SOL, WASO, SE, SQ, TST | Small to moderate effects |
| Ho et al. (2015) | Insomnia | 20/2411 | Self-help + cCBT-I | SOL, WASO, SE, SQ, TST | Self-help CBT-I is effective and acceptable as a starter for treatment |
| Ye et al. (2015) | Insomnia with co-morbid conditions | 9/776 | cCBT-I | Anxiety, depression | Moderate effect sizes for co-morbid symptoms |
| Zachariae et al. (2017) | Insomnia | 11/1460 | cCBT-I | ISI, SOL, WASO, NOA, TST, SQ | Comparable to face-to-face CBT-I |
| Seyffert et al. (2016) | Insomnia | 15/2392 | cCBT-I | ISI, SOL, TST, WASO, NOA, SQ, PSQI | Good efficacy for sleep parameters, good follow-up results |
- CBT-I, cognitive behavioural therapy for insomnia; cCBT-I, computerized cognitive behavioural therapy for insomnia; ISI, insomnia severity index; NOA, number of awakenings; PSQI, Pittsburgh Sleep Quality Index; SE, sleep efficiency; SOL, sleep-onset latency; SQ, sleep quality; TST, total sleep time; WASO, wake time after sleep onset.
Two meta-analyses have been published comparing CBT-I with pharmacotherapy. Smith et al. (2002) compared pharmacological studies using BZ or BZRAs with psychotherapeutic studies, and concluded that both options are comparably effective in the short term. Mitchell et al. (2012) analysed studies that directly compared CBT-I with pharmacotherapy; only five studies fulfilled the inclusion criteria. Based on this evidence, the authors concluded that CBT-I and hypnotics have comparable efficacy in the short term, and that CBT-I is superior in the long term.
An interesting question is whether a combination of CBT-I with medication has synergistic effects. Two randomized controlled trials addressed this issue using CBT-I with temazepam or zolpidem (Morin et al., 1999, 2009b). In the acute treatment phase, the combination of CBT-I and pharmacotherapy appears to be slightly superior compared with either treatment alone. However, during maintenance treatment, discontinuation of pharmacotherapy appears to be more favourable (Morin et al., 2009b). The authors also present their data in terms of response/remission criteria. According to this data evaluation, CBT-I alone led to a positive treatment response in 60% and remission in 40% of cases. These outcomes were stable at follow-ups or even improved (remission at 6 months follow-up: 67.8%).
With respect to mindfulness-based treatments and hypnotherapy, three meta-analyses have been published (Gong et al., 2016; Kanen et al., 2015; Lam et al., 2015). The meta-analyses on mindfulness-based treatments noted moderate to good effects (Gong et al., 2016; Kanen et al., 2015) on sleep parameters. Hypnotherapy had a positive impact on sleep-onset latency; however, the overall quality of the studies included was poor. Thus, these treatments may be promising but the evidence is less convincing than it is for CBT-I.
As will be discussed in more detail in the section on hypnotics, the placebo effect needs to be noted in the context of the efficacy of psychotherapy. In comparison with pharmacological research, placebo-controlled studies are more difficult to conduct in psychotherapy research, as therapists can usually not be blinded towards ‘sham’ therapies. Thus, due to this methodological difficulty, psychotherapy studies may overestimate treatment efficacy.
The aforementioned evidence suggests that CBT-I is recommended as first-line treatment for chronic insomnia in adults of any age (strong recommendation, high-quality evidence; see Tables 6, 7 and 15).
Pharmacotherapy
Several overviews of hypnotics for insomnia have been published (Riemann and Nissen, 2012). Available substances include BZ and BZRAs, antidepressants, antipsychotics, antihistamines, phytotherapeutic substances and melatonin (Table 8).
| BZ | Diazepam, flunitrazepam, flurazepam, lormetazepam, nitrazepam, oxazepam, temazepam, triazolam |
| BZRA | Zaleplone, zolpidem, zopiclone |
| Antidepressants | Agomelatine, amitriptyline, doxepin, mianserin, mirtazapine, trazodone, trimipramine |
| Antipsychotics | Chlorprothixene, levomepromazine, melperone, olanzapine, pipamperone, prothipendyl, quetiapine |
| Antihistamines | Diphenhydramine, doxylamine, hydroxyzine, promethazine |
| Phytotherapeutics | Hops, melissa, passiflora, valerian |
| Melatonin receptor agonists | Melatonin, ramelteon, slow-release melatonin |
- BZ, benzodiazepines; BZRA, benzodiazepine receptor agonists.
Before summarizing the efficacy of these different pharmacological substances, we present four meta-analyses on the placebo effects in this condition (Table 9). The three newest of these meta-analyses concluded that there are significant placebo effects in clinical trials of pharmacological treatments for insomnia. Most notably, Winkler and Rief (2015) analysed 32 studies with 3969 participants, and found that more than 60% of the response to medication (in most studies BZ and BZRAs) was also observed with placebo. This finding held true for both subjectively and polysomnographically measured sleep parameters.
| Author (year) | Population | Number of studies/number of patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Hróbjartsson and Gøtzsche (2001) | 40 clinical conditions including insomnia | 5/100 | Placebo versus active drug | Sleep parameters | Almost no evidence that placebo has strong effects |
| McCall et al. (2003) | Insomnia | 5/213 | Placebo versus active drug | SOL, TST | Significant placebo effects for SOL + TST (subjective) |
| Bélanger et al. (2007) | Primary insomnia | 34/1392 | Placebo/wait list versus active drug | SOL, TST, WASO, NOA, SE/subjective and objective | Significant placebo effects in pharmacological studies |
| Winkler and Rief (2015) | Insomnia | 32/3969 | Placebo versus active drug | Sleep parameters/objective and subjective | 63.5% of drug response was obtained with placebo |
- NOA, number of awakenings; SE, sleep efficiency; SOL, sleep-onset latency; TST, total sleep time; WASO, wake time after sleep onset.
Grading of the evidence
Table 10 summarizes the meta-analyses on the efficacy of BZ and BZRAs in the treatment of insomnia. These meta-analyses clearly show that BZ and BZRAs are effective in the short-term treatment (≤4 weeks) of insomnia. Pillai et al. (2017) analysed data from one randomized controlled trial with BZRAs according to definitions of treatment response/remission, and observed positive treatment responses in 76.7% of cases and remissions in 47.7% of participants.
| Author (year) | Population | Number of studies/number of patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Nowell et al. (1997) | Primary insomnia | 22/1894 | BZ + zolpidem versus placebo, short-term treatment | SOL, NOA, TST, SQ | Significant improvement of sleep |
| Holbrook et al. (2000) | Primary insomnia | 45/2672 | BZ + zopiclone versus placebo, short-term treatment | SOL, TST, USE |
|
| Dündar et al. (2004) | Insomnia | 24/3909 | BZ versus BZRA, short-term treatment | SOL, TST, NOA, WASO, SQ, USE |
|
| Glass et al. (2005) | Insomnia, age >60 years | 24/2417 | BZ + BZRA versus placebo, short-term treatment | SQ, SOL, TST, NOA, USE |
|
| Buscemi et al. (2007) | Chronic insomnia | 105/5582 | BZ + BZRA + sedating antidepressants | SOL + secondary outcomes, USE | BZ and BZRA are effective; more USE with active drugs versus placebo |
| Huedo-Medina et al. (2012) | Insomnia | 13/4378 | BZRA (zolpidem, zaleplone, eszopiclone) | SOL + secondary outcomes | Small but significant effects on subjective and objective SOL |
| Winkler et al. (2014) | Insomnia | 31/3820 | BZ, BZRA, sedating antidepressants, melatonin | Polysomnographic and subjective sleep parameters | BZ and BZRA have significant effects on subjective and objective outcomes; smaller effects for antidepressants |
- BZ, benzodiazepines; BZRA, benzodiazepine receptor agonists; NOA, number of awakenings; SOL, sleep-onset latency; SQ, sleep quality; TST, total sleep time; USE, undesired side-effects; WASO, wake time after sleep onset.
Table 11 (upper panel) summarizes the meta-analyses on the efficacy of antidepressants in the treatment of insomnia. It should be noted that dosages for antidepressants to treat insomnia are usually much lower than the recommended doses for depression. Only a few randomized controlled trials have evaluated the efficacy of these mostly sedating antidepressants. The authors of the first two meta-analyses concluded that the efficacy of sedating antidepressants is weaker than that for BZ/BZRAs. However, McCleery et al. (2014) described positive effects of trazodone for sleep disorders co-morbid with Alzheimer's disease. The meta-analysis by Yeung et al. (2015) dealt exclusively with low-dose doxepin, and showed that there are significant effects on subjective and polysomnographic parameters in the short term.
| Author (year) | Population | Number of studies/number of patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Sedating antidepressants | |||||
| Buscemi et al. (2007) | Chronic insomnia | 105/873 | BZ + BZRA + sedating antidepressants | SOL | Sedating antidepressants are less effective than BZ/BZRA |
| Winkler et al. (2014) | Insomnia | 31/3820 | BZ + BZRA + sedating antidepressants + melatonin | Subjective and objective sleep parameters | Sedating antidepressants are less effective than BZ/BZRA |
| McCleery et al. (2014) | Insomnia co-morbid with M. Alzheimer | 5/313 | Trazodone + melatonin + ramelteon | SOL, TST, WASO, SE | Trazodone improves TST and SE |
| Yeung et al. (2015) | Insomnia | 9/1983 | Low-dose doxepin | Subjective and objective sleep parameters | Small to moderate effects for sleep maintenance and TST, but no effects for SOL |
| Phytotherapeutic interventions | |||||
| Bent et al. (2006) | Insomnia | 16/1093 | Valerian versus placebo, short-term treatment | SQ, SOL |
|
| Fernández-San-Martín et al. (2010) | Insomnia | 18/1317 | Valerian versus placebo | SQ | No effects on quantitative parameters, slight effects for SQ |
| Leach and Page (2015) | Insomnia | 14/1602 | Valerian, chamomile, kava, wuling | SOL, SE, TST, SQ | No significant effects |
| Ni et al. (2015) | Insomnia | 76/7240 | CHM versus placebo versus BZ | PSQI, CGI | CHM better than placebo, but poor quality of studies |
- BZ, benzodiazepines; BZRA, benzodiazepine receptor agonists; CGI, clinical global impression; CHM, Chinese herbal medicine; PSQI, Pittsburgh Sleep Quality Index; SE, sleep efficiency; SOL, sleep-onset latency; SQ, sleep quality; TST, total sleep time; WASO, wake time after sleep onset.
There are no meta-analyses on the efficacy of antihistamines in insomnia, but one systematic review concluded that antihistamines have only a small to moderate efficacy in the treatment of insomnia and that tolerance to these substances develops quickly (Vande Griend and Anderson, 2012). Of note, many sedating antidepressants (Table 11, upper panel) probably exert their hypnotic effect through the histaminergic system.
There are no meta-analyses on the efficacy of antipsychotics in insomnia, but four related systematic reviews exist. Monti and Monti (2004; Monti et al., 2017) and Cohrs (2008) concluded that sedating antipsychotics increase total sleep time and the amount of slow-wave sleep in patients with schizophrenia. However, Anderson and Vande Griend (2014) and Thompson et al. (2016) conclude that the evidence on quetiapine is insufficient to recommend its use in the treatment of insomnia, in the absence of psychiatric disorders, particularly in light of its potential side-effects.
Table 11 (lower panel) summarizes the meta-analyses on the efficacy of phytotherapeutics in the treatment of insomnia. The authors of these publications came unanimously to the conclusion that the methodological quality of the studies included was poor and further studies are warranted. The meta-analyses did not show a clinically relevant efficacy of the investigated substances. A meta-analysis of studies investigating Chinese herbal medicine (CHM) concluded that CHM is superior to placebo with respect to its effect on subjective sleep parameters and equally effective as BZ. However, the authors of the meta-analysis emphasize the poor quality of the original studies, which cannot be independently assessed by the authors of this guideline because all the original manuscripts were published in Chinese.
Table 12 summarizes meta-analyses on the efficacy of melatonin (including mainly fast-release preparations, but also ramelteon and prolonged-release formulations) in the treatment of insomnia. These meta-analyses do not provide a uniform picture concerning the efficacy of melatonin and the melatonin receptor agonist ramelteon. Buscemi et al. (2005) and Ferracioli-Oda et al. (2013) reported that melatonin reduces sleep-onset latency, which was also demonstrated for ramelteon (Liu and Wang, 2012). Kuriyama et al. (2014) also found significant positive effects of melatonin on sleep-onset latency and sleep quality. However, the effects were small from a clinical point of view. Some of the original studies also investigated undesired side-effects and concluded that melatonin is a safe drug.
| Author (year) | Population | Number of studies/number of patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Brzezinski et al. (2005) | Different populations including insomnia | 17/284 | Melatonin 0.3–40 mg versus placebo | SOL, TST, SE | SOL ↓; TST ↑; SE ↑ |
| Buscemi et al. (2005) | Primary sleep disorders | 14/425 | Melatonin 1–5 mg versus placebo | SOL, WASO, TST, SE, SQ, USE | SOL ↓; best effect in sleep phase delay |
| Buscemi et al. (2006) | Secondary sleep disorders | 15/524 | Melatonin 1–10 mg versus placebo | SOL, USE |
No effect on SOL No USE |
| Braam et al. (2009) | Sleep problems with intellectual dysfunction | 9/183 | Melatonin 0.5–9 mg versus placebo | SOL, TST, NOA | SOL ↓; TST ↑; NOA ↑ |
| Geijlswijk et al. (2010) | Delayed sleep phase syndrome | 9/317 | Melatonin 0.3–5 mg versus placebo | DLMO, SOL, TST | Phase advance DLMO, improved sleep |
| Ferracioli-Oda et al. (2013) | Primary sleep disorders | 19/1683 | Melatonin 1– 10 mg versus placebo | SOL, TST, SQ | Moderate effects on sleep continuity |
| Liu and Wang (2012) | Chronic insomnia | 8/4055 | Ramelteon 4–32 mg versus placebo | SOL, USE | Positive effects on subjective/objective SOL/no USE |
| McCleery et al. (2014) | Insomnia with M. Alzheimer | 5/313 | Trazodon, melatonin, ramelteon | SOL, TST, WASO, SE | No evidence supporting melatonin/ramelteon |
| Kuriyama et al. (2014) | Insomnia | 13/5812 | Ramelteon | SOL, TST, SQ | SOL ↓; SQ ↑; clinically small effects |
| Zhang et al. (2016) | Sleep disorders with neurodegenerative disorders | 9/370 | Melatonin | PSQI | Positive effects on PSQI and RBD |
- DLMO, dim light melatonin onset; NOA, number of awakenings; PSQI, Pittsburgh Sleep Quality Index; RBD, rapid eye movement sleep behaviour disorder; SE, sleep efficiency; SOL, sleep-onset latency; SQ, sleep quality; TST, total sleep time; USE, undesired side-effects; WASO, wake time after sleep onset.
The aforementioned evidence suggests that BZ and BZRAs may be used in the short term if the first-line treatment (CBT-I) is ineffective or unavailable (high-quality evidence). Some sedating antidepressants too may be used for short-term treatment (moderate-quality evidence). Further, antihistamines and antipsychotics are not recommended for the treatment of insomnia (strong recommendation – low- to very-low-quality evidence), and melatonin and phytotherapy are not recommended for insomnia (weak recommendation – low-quality evidence; Tables 8–12 and 15).
Light therapy and exercise
Light exposure has been used as a powerful experimental tool in animal research on sleep–wake and circadian rhythms, with clear-cut effects being observed on a variety of biological outcome variables. In humans, light therapy has been used as a treatment for seasonal affective disorders and circadian rhythm disorders with supposedly good clinical efficacy (Huck et al., 2014). Exercise doubtlessly has positive effects on psychological and physical health, and many studies show that regular exercise reduces mortality (Hupin et al., 2015). Of particular importance for the current guideline, both light therapy and exercise have also been suggested to be efficacious in patients with insomnia.
Grading of the evidence
Van Maanen et al. (2016) investigated the impact of light therapy on insomnia, and found small to moderate effects of this treatment on sleep parameters. Kredlow et al. (2015) investigated the effects of different exercise regimes on sleep in good and poor sleepers. While moderately positive effects were shown on several sleep parameters, it has to be stressed that most original studies did not focus on clinically relevant insomnia. Given the fact that both light therapy and exercise are supported by extensive basic and public health research, further studies should be devoted to delineate their effects in patients with insomnia.
The aforementioned evidence suggests that light therapy and/or exercise may be useful adjuvant therapies for insomnia (weak recommendation – low-quality evidence; Table 15).
Complementary and alternative medicine
In the area of complementary and alternative medicine, several treatments for insomnia have been suggested, including acupuncture, acupressure, aromatherapy, foot reflexology, homeopathy, meditative movement therapies, moxibustion, music therapy and yoga.
Grading of the evidence
Table 13 summarizes meta-analyses and systematic reviews on the efficacy of complementary and alternative treatments for insomnia. Overall, the studies underlying this evidence are methodologically poor and thus difficult to evaluate. There is some evidence suggesting that acupuncture is effective (Cao et al., 2009; Cheuk et al., 2012; Lan et al., 2015; Sarris and Byrne, 2011). However, evaluation of the studies on this topic is difficult for the authors of this guideline because most of the original articles are published in Chinese. The authors of all of the above-mentioned meta-analyses have stressed caution due to the quality of the original studies. There is no evidence supporting the efficacy of aromatherapy or homeopathy. Three meta-analyses on music therapy (Jespersen et al., 2015; de Niet et al., 2009; Wang et al., 2016) exist and suggest a potential positive effect of this treatment. However, the methodological quality of these studies is questionable. A similar picture arises for foot reflexology, moxibustion and meditative movement therapies, including yoga. These treatments may have potential; however, the poor quality of many of the original studies (as noted by the authors of the meta-analyses) makes it difficult to reach clear conclusions.
| Author (year) | Population | Number of studies/number of patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Acupuncture | |||||
| Chen et al. (2007) | Insomnia (primary and secondary) | 6/673 | Auricular acupuncture | TST, reduction of insomnia | Positive effects for acupuncture, but poor quality of studies |
| Cheuk et al. (2012) | Insomnia | 33/2293 | Acupuncture versus no treatment versus pseudo-acupuncture | PSQI | Not interpretable because of poor quality of studies |
| Yeung et al. (2015) | Insomnia (primary and secondary) | 40/4115 | Acupuncture, reflexology, ear acupuncture versus school medicine/sham/sleep hygiene/music therapy/routine treatment | PSQI, SRSS, effect rate, GHQ-28, STAI, AIS, BDI, PFS, sleep diary | Acupuncture marginally better than sham treatment; ear acupuncture versus sham questionable; each intervention better than routine treatment |
| Lan et al. (2015) | Poor sleepers | 15/1429 | Auricular acupuncture versus sham acupuncture versus placebo | Response rate, PSG, sleep diaries | ‘positive’ effects of acupuncture, poor quality of studies |
| Lee and Lim (2016) | Insomnia post-stroke | 13/1051 | Acupuncture (TCM) versus sham acupuncture versus drugs | PSQI, ISI, AIS, TCM standards | Acupuncture better than drugs, poor quality of studies |
| Aromatherapy | |||||
| Hwang and Shin (2015) | Different groups | 12/704 | Aromatherapy versus control | Sleep disorder | Highly significant improvement of sleep (poor quality of studies) |
| Homeopathy | |||||
| Cooper and Relton (2010) | Insomnia | 4/199 | Individualized homeopathy versus placebo | SOL, TST, SQ, etc. | ‘Trends’ for homeopathic medicine, no significant changes of sleep, poor quality of studies |
| Ernst et al. (2011) | Insomnia | 6/263 | Individualized homeopathy versus placebo | TST, SQ, etc. | No effects, poor quality of studies |
| Moxibustion | |||||
| Sun et al. (2016ab) | Primary insomnia | 22/1971 | Moxibustion versus ‘Western medications’, TCM | ‘Clinical effective rate’ | Moderate effects, poor quality of studies |
| Music therapy | |||||
| Wang et al. (2016) | Heterogenous samples with acute or chronic sleep problems | 10/557 | Passive music consumption | RCSQ, PSG, VAS, VSH, PSQI | Positive effects on sleep quality |
| Jespersen et al. (2015) | Insomnia | 6/340 | Music therapy versus no treatment versus TAU | PSQI | Increase of sleep quality, reduction of PSQI scores |
| Oil | |||||
| Lillehei and Halcon (2014) | ‘Sleep disturbances’ | 15/? | Essential oil | Different outcomes | Essential oils could be helpful with minor sleep problems |
| Reflex zone massage | |||||
| Lee et al. (2011) | Different target groups | 44/1860 | Reflex zone massage versus control | Fatigue, pain, sleep | Good effect strengths for sleep |
| Yoga/Tai Chi/Chi Gong | |||||
| Wang et al. (2016) | Insomnia | 17/1880 | MM versus wait list | PSQI, SQ | Increase of sleep quality, poor quality of studies |
| Wu et al. (2015ab) | Insomnia (>60 years) | 14/1225 | MM versus control group | PSQI | Improved sleep quality, heterogeneous quality of studies |
- AIS, Athens Insomnia Scale; BDI, Beck Depression Inventory; GHQ-28, General Health Questionnaire; ISI, Insomnia Severity Index; MM, meditative ‘movement’ = yoga, Tai Chi, Chi Gong; PFS, Piper Fatigue Scale; PSG, polysomnography; PSQI, Pittsburgh Sleep Quality Index; RCSQ, Richards–Campbell Sleep Questionnaire; SOL, sleep-onset latency; SQ, sleep quality; SRSS, Self-Rating Scale on Sleep; STAI, State Trait Anxiety Inventory; TAU, Treatment As Usual; TCM, traditional Chinese medicine; TST, total sleep time; VAS, visual analogue scale; VSH, Verran Snyder–Halpern Sleep Scale.
The aforementioned evidence suggests that complementary and alternative treatments for insomnia are not recommended (weak recommendation – very-low-quality evidence; Tables 13 and 15).
Long-term treatment of insomnia with hypnotics
The pharmacological literature summarized above dealt with the short-term treatment of insomnia (≤4 weeks). The rationale for this is that the hypnotics available are exclusively indicated, and approved, only for short-term treatment in most European countries. Arguably, however, the long-term treatment of insomnia using hypnotics is clinically relevant because insomnia typically returns following withdrawal. Table 14 summarizes the results of studies that investigated the long-term use of hypnotics (for at least 12 weeks) for insomnia.
| Author (year) | Sample | Substance | Duration of treatment | Tolerance | Abuse dependency | Rebound | Other undesired side-effects |
|---|---|---|---|---|---|---|---|
| Krystal et al. (2003) |
N = 593 (ESZ) N = 195 (PLA) |
3 mg eszopiclone (39.5% dropouts) Placebo (43.3% dropouts) |
6 months | – | – | no (no detailed analysis) | moderate |
| Perlis et al. (2004) |
N = 98 (ZOLP) N = 101 (PLA) |
10 mg zolpidem (18.4% dropouts) Placebo (20.7% dropouts) |
12 weeks | – | – | no | moderate |
| Roth et al. (2005) | N = 471 (ESZ) |
Open label ext. ESZ: 17.8% dropouts PLA: 22.5% dropouts |
6 + 6 months | – | – | not indicated | moderate |
| Walsh et al. (2007) |
N = 548 (ESZ) N = 280 (PLA) |
3 mg eszopiclone (37% dropouts) Placebo (52% dropouts) |
6 months | – | – | no – questionable | moderate |
| Krystal et al. (2008) |
N = 669 (ZOLP) N = 349 (PLA) |
12.5 mg zolpidem SR (35.3% dropouts) Placebo (47.6% dropouts) |
24 weeks | – | – | no – questionable | moderate |
| Mayer et al. (2009) |
N = 227 (RAM) N = 224 (PLA) |
8 mg ramelteon (30% dropouts) Placebo (21.4% dropouts) |
6 months | – | – | no – questionable | moderate |
| Ancoli-Israel et al. (2010) |
N = 194 (ESZ) N = 194 (PLA) |
2 mg eszopiclone (24.2% dropouts) Placebo (elderly) (23.7% dropouts) |
12 weeks | – | – | no – questionable | moderate |
| Krystal et al. (2010) |
N = 159 (DOX) N = 81 (PLA) |
1/3 mg doxepin (10% dropouts) Placebo (14% dropouts) |
12 weeks | – | – | no | moderate |
| Roehrs et al. (2011) |
N = 17 (ZOLP) N = 16 (PLA) |
5/10 mg zolpidem (17.6% dropouts) Placebo (12.5% dropouts) |
12 months | – | no dose escalation | no indication | no indication |
| Randall et al. (2012) |
N = 60 (ZOLP) N = 65 (PLA) |
10 mg zolpidem (26.7% dropouts) Placebo (27.6% dropouts) |
8 months | – | – | no indication | no indication |
| Uchimura et al. (2012) |
N = 164 (ESZ) N = 161 (ESZ) |
1/2/3 mg eszopiclone (about 15% dropouts) | 24 weeks | – | – | no – questionable | moderate |
| Michelson et al. (2014) |
N = 522 (SUV) N = 259 (PLA) |
30/40 mg suvorexant (38% dropouts) Placebo (37% dropouts) |
12 months | – | – | no – but stronger under suvorexant | moderate – cave: hypersomnia |
These long-term studies show that the efficacy of hypnotics may remain stable over longer periods of administration. However, in some studies the effects decreased over time. Moreover, it has to be noted that some of the investigated substances, i.e. eszopiclone, zolpidem SR, ramelteon and suvorexant, are not available in Europe. To circumvent the possible risks of chronic hypnotic usage, such as dependence and rebound insomnia, some authors have suggested intermittent use especially for BZ and BZRAs (Parrino et al., 2008). However, there are no meta-analyses examining the effects of intermittent use of hypnotics on insomnia. An alternative solution, suggested by Voshaar et al. (2006), is to employ counselling interventions including, where necessary, CBT-I during discontinuation. In general, hypnotic discontinuation should be based on slowly tapering off medication, supporting patients during this sometimes difficult period with counselling, CBT-I or, if necessary, alternative medications (e.g. sedating antidepressants).
Based upon the evidence, BZ and BZRAs are not recommended in the longer-term treatment of insomnia (strong recommendation – low-quality evidence; Tables 14 and 15).
Diagnostic management of insomnia and its co-morbidities
|
|
Treatment In the presence of co-morbidities, clinical judgement should decide whether insomnia or the co-morbid condition is treated first, or whether both are treated at the same time.CBT-I CBT-I is recommended as first-line treatment for chronic insomnia in adults of any age (strong recommendation, high-quality evidence). Pharmacological interventions A pharmacological intervention can be offered if CBT-I is not effective or not available. BZ and BZRA
|
- BZ, benzodiazepine; BZRA, benzodiazepine receptor agonist; CBT-I, cognitive behavioural therapy for insomnia; CT, Computed Tomography; ECG, electrocardiogram; EEG, electroencephalogram; MRT, Magnetic Resonance Tomography.
Risks and side-effects of insomnia treatment
The side-effects of CBT-I have not been thoroughly investigated yet. However, Kyle et al. (2011, 2014) stress that sleep restriction, as one component of CBT-I, leads to transient increases in somnolence and fatigue and objectively impaired vigilance. As such, sleep restriction therapy can only be recommended without restrictions when there are no safety concerns, for example, sleep restriction may be contraindicated in professional drivers. Similar side-effects can also be expected with stimulus control therapy. A more detailed and critical evaluation of the undesired effects of CBT-I is suggested.
With respect to hypnotics, a variety of side-effects have been reported, including hangover, nocturnal confusion, falls, rebound insomnia, tolerance and dependency (Hoffmann, 2013; Kapil et al., 2014; Uhlenhuth et al., 1999). These side-effects are often aggravated by multi-pharmacy, especially in older adults. It is undisputed that BZ and BZRA have the potential for tolerance and dependency. However, there are little data available on the number of patients who will become dependent when taking BZ or BZRA for a certain period of time. Hallfors and Saxe (1993) showed in one meta-analysis that substances with short half-lives induce dependency more quickly. Moreover, the acute cognitive effects of zopiclone, zolpidem, zaleplone and eszopiclone were examined in one meta-analysis by Stranks and Crowe (2014). On the basis of their findings, they suggest zolpidem and zopiclone have significant negative effects on next-day cognitive performance. Other notable results with respect to the negative impact of BZRAs include: Tom et al. (2016), who reported that use of zolpidem was associated with greater risk of hip fracture and traumatic brain injury than eszopiclone; Sun et al. (2016a) who demonstrated a significant relationship between zolpidem use and suicide attempts, as well as completed suicides; and Joya et al. (2009) who showed an increased risk for minor infections with the use of eszopiclone and zolpidem, compared with placebo. In terms of cognitive effects after withdrawal from long-term BZ use, one meta-analysis showed that negative effects might last up to 6 months (Barker et al., 2004). In light of the evidence, Glass et al. (2005) conclude that the undesired side-effects outweighed the benefits of BZ/BZRA use in the elderly >60 years.
Three meta-analyses have been published on the effects of BZ and BZRAs on driving abilities. Verster et al. (2006) showed that BZ and zopiclone lead to impaired driving abilities. Further, Rapoport et al. (2009) and Dassanayake et al. (2011) showed a significant correlation between BZ use and accidents. A combination of alcohol use and BZ intake further increases the risk for accidents. Of note, sedating antidepressants also increase the risk of accidents.
It has been discussed, albeit controversially, whether BZ and BZRA increase the risk for mortality. In terms of the existing evidence, Palmaro et al. (2015) conducted an analysis of two large cohort studies from France (n = 60 000 patients) and UK (n = 90 000 patients). These authors showed that the occasional intake of BZ was associated with an increase in mortality. Moreover, data from the American Cancer Society suggest that the combination of insomnia with the intake of hypnotics may be associated with an increased mortality (Kripke, 2009, 2011, 2013; Kripke et al., 1979, 2002). Further research (Frandsen et al., 2014; Jennum et al., 2015, 2016) investigated mortality associated with the use of BZ, antidepressants and antipsychotics in patients with Parkinson's disease, dementia and stroke. These studies also showed an increased mortality in those using psychotropic agents.
Recommendations
Our overall recommendations for the diagnosis and therapy of insomnia are presented in Table 15. Additionally, a clinical algorithm for the diagnostic and therapeutic process is summarized in Fig. 1.
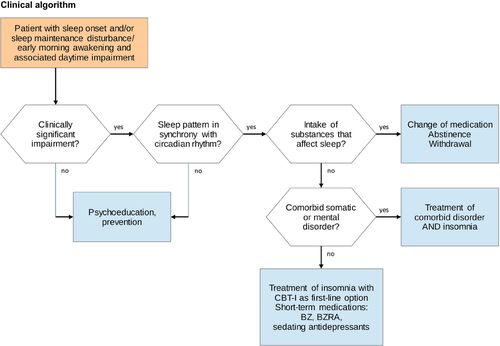
Please note that these recommendations largely correspond to the guidelines for insomnia treatment of the American College of Physicians (ACP, 2016). Both guidelines recommend CBT-I as first-line treatment for insomnia. Concerning the pharmacological treatment of insomnia, an American Academy of Sleep Medicine guideline gave a ‘weak’ recommendation for orexin receptor antagonists, BZ, BZRAs, doxepine and ramelteon to treat insomnia (Sateia et al., 2017). Substances like trazodone, tiagabine, diphenhydramine, melatonin, tryptophan and valerian were explicitly not recommended in this guideline.
Outlook For The Future
Cognitive behavioural therapy for insomnia, though being the first-line treatment for insomnia, is not easily available. It is assumed that only a minority of patients with chronic insomnia will receive this treatment in Europe. Thus, the widespread implementation of CBT-I will be a major challenge for the future. Apart from physicians and clinical psychologists/psychotherapists, other health professionals (e.g. nurses) should be trained in CBT-I. Furthermore, web-based delivery of CBT-I may offer a chance to improve the healthcare situation for patients with insomnia in Europe.
The efficacy of the different components of CBT-I as standalone interventions has been rarely investigated or compared. Thus, more work is necessary to dismantle the effects of these components in randomized controlled studies. In addition, the impact of CBT-I on daytime function in those with insomnia has been scarcely investigated.
With respect to new psychotherapeutic approaches, further research is needed to evaluate mindfulness-based treatments and hypnotherapy. Furthermore, these approaches, in addition to other techniques, should be explored, especially in those who do not respond to traditional CBT-I. For example, one pilot study indicated that Acceptance and Commitment Therapy (ACT; Hertenstein et al., 2015) might be a useful alternative for non-responders. Another innovative approach consists of intensive sleep retraining (Harris et al., 2012). This very brief therapeutic approach is realized in the sleep laboratory, and can be utilized over a period of 25 h and is thought to be based on a reconditioning of sleep. The positive effects of a first randomized controlled trial (Harris et al., 2012) also raise questions about the potential of sleep deprivation in the context of insomnia treatment.
With respect to the most frequently used drugs for insomnia, BZ and BZRAs, the question of efficacy and side-effects of long-term treatment should be addressed in naturalistic studies. It would be especially helpful to know before the first prescription, which patient will abuse these substances or become dependent on them.
Newer hypnotic drugs like ramelteon or suvorexant have been introduced into the healthcare system of the USA, but not in Europe. In particular, it remains an open question whether the orexin receptor antagonists will be available on the European market in the near future. Other drugs that are sometimes used for the treatment of insomnia, like tiagabine and pregabalin, have not been subjected to thorough testing concerning their efficacy and side-effects – further research is needed here.
Light therapy and exercise may be useful treatment approaches for insomnia, and it is unlikely that these treatments produce severe side-effects. Light therapy has clear effects on several biological parameters. In this context it is also suggested that further research into circadian underpinnings of insomnia might be helpful to gain new insights into its pathophysiology. However, the efficacy for those with insomnia remains to be seen. Similarly, exercise is a well-established strategy for improving general health. However, whether it has specific effects on insomnia remains unclear.
Very new treatments include brain cooling and electrostimulation. A brain-cooling device has been introduced on to the market in the USA recently (Nofzinger and Buysse, 2011). Electrostimulation has been shown to induce slow-wave sleep in experimental studies, and it has been tested in good sleepers and poor sleepers with mixed effects (Frase et al., 2016, 2017). Further research needs to be conducted and published on the efficacy of these treatments.
Acknowledgements
The authors would like to express their gratitude to the European Sleep Research Society and its current board members (Walter McNicholas, Tiina Paunio, Tom de Boer, Lino Nobili, Philippe Peigneux, Hans-Peter Landolt, Pierre-Hervé Luppi) for their confidence and the financial support provided (travel costs for the Frankfurt consensus meeting, 31 March, 2017), and critical feedback during the process of developing the guideline.
Conflict of Interests: European Insomnia Guideline
| Authors | Payments for speaking engagements (SE), advisory boards and consulting (ABC), royalties (R), etc. | Financial activities outside the topic | Patents/copyrights | Other unrelated payments |
|---|---|---|---|---|
| Arnardottir |
SE: Weinmann ABC: Nox Medical |
No | No | No |
| Baglioni | No | No | No | No |
| Bassetti | SE + ABC: Jazz, Servier, UCB, Zambon | No | No | Research support: ResMed, Respironics, Vifor Pharma, UCB Pharma |
| Bjorvatn | R: text books | No | No | No |
| Groselj | No | No | No | No |
| Ellis |
R: text books ABC: UCB pharma |
No | No | No |
| Espie |
SE: Big Health Ltd, Warnford Wellness R: text books |
No | Shareholder and co-founder Big Health Ltd | No |
| Garcia-Borreguero | No | No | No | No |
| Gjerstad | No | No | No | No |
| Gonçalves | No | No | No | No |
| Hertenstein | R: textbooks | No | No | No |
| Jansson-Fröjmark | No | No | No | No |
| Jennum | No | No | No | No |
| Leger | ABC: Biocodex, Philips, Vanda, Actelion, Jazz | No | No | No |
| Nissen | SE: Vanda Pharmaceuticals | No | No | No |
| Parrino | No | No | No | No |
| Paunio | No | No | No | No |
| Pevernagie | No | No | No | No |
| Riemann |
ABC: Institutes for Behavior Therapy R: text books |
No | No | No |
| Spiegelhalder |
R: text books SE: Institutes for Behavior Therapy |
No | No | No |
| Strazisar | No | No | No | No |
| Verbraecken | No | No | No | No |
| Weeß | No | No | No | No |
| Wichniak | SE: Angelini, Servier, Lundbeck | No | No | No |
| Zavalko | No | No | No | No |
| Zoetmulder | No | No | No | No |




