Refractory neutrophil activation in type 2 diabetics with chronic periodontitis
Abstract
Background
Inflammation increases diabetes mellitus type 2 (T2DM) progression and severity. T2DM patients are at high risk of the rapid development of chronic periodontitis (CP). Topical presence, high numbers, and bactericidal effects of immune cells are challenged by augmented antigen-induced inflammation, which promotes both diseases.
Objectives
To investigate gingival cellular inflammatory responses in individuals with previously undiagnosed T2DM with CP or CP alone and in systemically and periodontally healthy controls (H) in vivo and to establish an ex vivo technique permitting quantitative and qualitative assessments of gingival crevicular immune cells.
Materials and Methods
T2DM + CP, CP, and H individuals (n = 10, each) received a 2-week oral hygiene regimen (OHR). Afterwards, a noninvasive sampling technique was performed to evaluate gingival inflammation induced under standardized conditions in vivo, that is, in the absence of severe periodontal destruction and inflammation at clinically healthy sites. Stimuli (casein/test or phosphate-buffered saline w/o. Ca2+ or Mg2+, PBS(−/−)/control) were randomly applied contralaterally in the gingival sulci of participants’ upper dentes canini. One day after completion of the OHR, gingival crevicular fluid (GCF) was kinetically assayed between the time of the baseline (BL) measurement and 55 minutes. Polymorphonuclear leukocyte (PMN) content (PMNGCF) was quantitated at an optimum time of 35 minutes. PMNGCF counts reflect local inflammation. Ex vivo samples were fluorimetrically labeled, gated according to the donor's peripheral blood polymorphonuclear neutrophils (PMNPB), and then counted, employing flow cytometry.
Results
PMNGCF counts in unstimulated gingival crevices (at BL) in the T2DM + CP group were higher than those in the CP and H groups. PMNGCF counts were elevated in casein vs PBS(−/−)-stimulated gingival crevices in all groups. Patients with T2DM + CP showed increased PMNGCF counts compared to those with CP (P = .035) according to scatter plots. CD45+ counts in the stimulated sites in T2DM + CP patients were higher than those in CP and H patients (P = .041). Under stimulation conditions, the CD45+ counts differed from those under placebo conditions (P = .019), indicating augmented, inducible inflammatory leukocyte infiltrate in T2DM + CP patients.
Conclusions
This noninvasive technique permits quantitative assessment of (experimental) gingival inflammation in vivo, revealing an influence of T2DM + CP on the number of primary immune cells in the gingival crevice. Patients who are challenged with (local) leukocytosis are likely at risk of collateral damage to the gingival crevice neighboring tissues, favoring the severity and progression of CP and consequently T2DM (www.clinicaltrials.gov NCT01848379).
1 INTRODUCTION
The innate and adaptive arms of the immune system become activated in patients with metabolic disorders, particularly type 2 diabetes mellitus (T2DM) patients.1 Substantial numbers of primary immune cells are recruited to the tissues of individuals affected by these diseases, even in the absence of foreign antigens, for example, bacteria, fungi, or particles.2
In T2DM, underlying pathophysiological mechanisms impair insulin production by pancreatic β-cells with or without insulin resistance in peripheral cells; the latter directly impacts excessive peripheral blood glucose or free fatty acids. During this metabolic shift, patients may suffer from spells of adephagia, further promoting obesity and adiposity.3 Interestingly, direct consequences of the responses and modulation of phagocyte populations strongly depend on the metabolic system.4
In this context, the roles of myeloid cells and primarily macrophages (MΨ) are well established.5 In addition to MΨ, other immune cells with altered functionality become modulated in early metabolic syndrome, a precursor of T2DM. Furthermore, zones of inflammation augment a variety of effects, such as the secretion of cyto/chemokines or the recruitment and activation of leukocyte populations.6
Evidence from murine models indicates that like MΨ, neutrophilic polymorphonuclear leukocytes (PMN) double their numbers within tissues when animals are fed a high-carbohydrate diet.7
Increased numbers of adhesion antigens on PMN membranes and enhanced leukocyte-endothelial interactions have been reported in T2DM patients8 and murine models.9, 10 These molecular changes most likely alter the magnitudes of PMN functions, such as adhesion, chemokinesis, chemotaxis, phagocytosis, and antigen degradation.11
The shared pathophysiologies of T2DM and chronic periodontitis (CP) have been reviewed elsewhere.12, 13 Hyperinflammation, particularly hyperreactive PMN, has a large impact on the underlying mechanisms of host tissue destruction in the pathogenesis of CP.14 Furthermore, such PMN phenotypes, among others, may influence metabolic (eg, T2DM) and central or peripheral artery obstructive diseases. PMN thus may act as putative pathogenic link, rendering CP capable of influencing comorbidities.
Harvested by venipuncture from vessels of the upper limbs, these venous neutrophils (PMNPB) should be naïve, not primed, and if at all, remotely challenged or activated. Most likely, they have not undergone stages of activation and recruitment, such as marginalization, extravasation, chemotaxis, or tissue migration. In contrast, morphological and (patho-) physiological differences due to topical gingival or periodontal neutrophils (PMNGCF) may be expected.
The objective of the assay was to measure stimulus-dependent inflammatory leukocyte responses in the gingival crevice. Differences in this experimentally induced inflammatory reaction between patients with newly diagnosed T2DM and periodontitis (T2DM + CP), patients with CP alone, and systemically and periodontally healthy individuals (H) were evaluated by quantifying the casein-stimulated influx of PMN in healthy gingival crevice collateral sites that were placebo controlled (PBS(−/−)).
The null hypothesis was as follows: T2DM + CP patients topically exhibit exhausted host responses upon inflammatory stimulation because PMN fail the experimental inflammatory challenge in T2DM + CP and CP individuals but not in healthy individuals.
2 MATERIAL AND METHODS
The presented data are part of a prospective, single-blinded, clinically controlled explorative study approved by the Institutional Review Board for human studies at the Medical Faculty at Justus Liebig University in Giessen (Germany) and registered with ClinicalTrails.gov (NCT01848379).
2.1 Study size and participants
All the participants were fully informed and gave their written consent. Patients without any diagnostic or therapeutic history of the disease were initially diagnosed with T2DM by an endocrinologist considering the fasting plasma glucose (FPG ≥ 160 mg/dL) level and glycosylated hemoglobin (HbA1c ≥ 7.5%) value, receiving the ICD10 code of E11.9(-1): diabetes mellitus type II without complications, uncontrolled to date and severe. These patients were hospitalized due to their acute metabolic disorder. All T2DM patients who were diagnosed according to ICD10 E11.9(-1) exhibited CP, supporting the findings of Lalla et al. 15 A matched T2DM group without CP could not be established.
After careful statistical evaluation of the parameter distributions and variabilities derived from pretest data, case numbers estimates were determined. In total, 10 patients with T2DM + CP, based on the criteria for test teeth (see below), respective numbers of systemically healthy individuals with CP and controls (H) were recruited.
The inclusion criteria were age between 18 and 70 years. To avoid the effects of long-term disease persistence and/or therapeutic intervention, a new diagnosis of T2DM was mandatory. The exclusion criteria were as follows: pregnancy, smoking habits, hypersensitivity to milk products, severe general medical conditions (except T2DM) leading to changes in the oral cavity, flu-like infections, and any use of antibiotics, antihistamines, or immunosuppressants in the past 6 months.
2.2 Periodontal examinations and oral hygiene phase
Periodontal examinations were performed in all participants; the examinations included periodontal probing depth (PPD), clinical attachment level (CAL), and bleeding on probing (BOP) at six sites/tooth using Florida Probe® (Florida Probe Corporation, Gainesville, FL). Severe CP was diagnosed according to Flemmig.16 Periodontal health was defined as the absence of BOP, PPD ≤ 3 mm, and CAL < 3.5 mm. The criteria for the T2DM + CP and CP groups were the presence of ≥ 12 teeth and untreated severe CP (at least 6 teeth with a PPDBL ≥ 5.5 mm and CALBL ≥ 6 mm). Further periodontal examination was performed using the periodontal screening record (PSR = 4). Individuals qualifying for group H exhibited neither systemic nor oral diseases/syndromes and a PSR ≤ 2.
To establish a standardized baseline (BL), any initial gingival inflammation was minimized with a 2-week oral hygiene regimen. All individuals received professional tooth cleaning and instructions once a week. Oral hygiene indices including the papillary bleeding index (PBI)17 and modified plaque index (mPLI)18 were recorded at all time points; obtaining these indices did not interfere with the assay procedure. Eventually, the evaluation of indices was performed after the assay.
Test and control teeth (dentes canini of the upper jaw) exhibited neither signs nor topical (risk) factors for gingival/periodontal inflammation (eg, PPD ≥ 3 mm, subgingival or marginal restorations, caries, clinically detectable calculus, or biofilm).
2.3 Metabolic and systemic parameters
Venous blood was taken from all participants at day baseline (BL) to assess FPG level, HbA1c value, erythrocyte sedimentation rate, and the number of peripheral neutrophil leukocytes. Purified peripheral blood PMN (PMNPB)19, 20 in specified gates were used as a reference for flow cytometric analysis of the PMNGCF populations. Weight and height were measured for all participants to calculate body mass index (BMI) at the beginning of the oral hygiene phase.
2.4 Local inflammation assay
Following a split-mouth design, one dens caninus of the upper jaw was randomly selected by lot for the application of an inflammatory stimulus (stimulated site), and the contralateral caninus served as a placebo (phosphate-buffered saline without divalent cations, PBS−/−) at the control site. Both dentes canini were isolated from saliva with cotton rolls and gently air-dried. The mesial tooth surfaces were marked, and to generate equal conditions before testing, both sulci were rinsed for 15 seconds with 2 mL of PBS−/− employing a single-use syringe with a blunt cannula inserted 1 mm into each sulcus at the marked position.21 Samples from the initial rinsing were discarded, and the gingival crevices were dried. Either 2 µL of the standardized inflammatory stimulus or the placebo was pipetted into the corresponding sulcus using a 1 to 10 µL reference pipette (Eppendorf-Vertrieb). In a pretest, BL sampling was followed by stimulus/placebo application. For kinetic assessments of neutrophil numbers, samplings were repeated from BL up to 55 minutes. An analysis revealed that 35 minutes was a feasible period for adequate PMN harvest. GCF was collected from both sites, stimulated and unstimulated, separately. Sulcular fluid containing PMN was aspirated into a falcon tube by a plastic cannula. The sterile filtrated milk protein casein, previously reported to generate chemotactic PMN responses in vitro and in vivo,22-24 was used as the inflammatory stimulus at 2 mg/mL in PBS−/−.
To compare the periodontal inflammatory burden of the three groups, analyses of PMNGCF were performed within 1 hour after the clinical procedures. GCF samples and purified peripheral blood PMN (PMNPB) were pelleted for 10 minutes at 200 × g at 4°C and carefully resuspended in 500 µL PBS−/−. Aliquots of 100 µL GCF samples were stained with 5 µL each of isotype control-FITC (tiso), (eBioscience), antibodies against panleukocyte marker (tpan) anti-human-CD45-FITC (Bio Legend), and leukocyte integrin anti-Human-CD11b-APC (eBioscience). Before running the GCF samples, 100 µL of diluted (1:10) counting beads (CytoCountTM, DakoCytomation) were added to each sample as a defined reference for quantification.25
For accuracy, in addition to each GCF sample, 100 µL of PMNPB from the corresponding patient were diluted in PBS (1:100-1:10 000, according to the cell concentration) and used to calibrate the flow cytometer. Individual gates around the PMNPB served as references for the PMNGCF population during analysis (Figure 2). Events were determined to be positive for a PMNGCF count if CD45+ markers were found within the gate. For the analysis of tCD11b+ fluorescence, an intensity proportional to the expressed cell surface antigen within the reference gate was used. Samples were analyzed using a CyAnTM ADP flow cytometer (Beckmann-Coulter, Krefeld, Germany) with 5000 events. The threshold was set to ≥ 0.5 µm forward scatter. Samples assayed were depicted as a dot-plot diagram with the populations of interest gated (eg, PMNGCF, PMNPB, counting beads).
2.5 Statistical analysis
The statistical analyses were performed by the Institute of Medical Statistics with SAS statistical software (version 9.3). Each patient was set as a statistical unit, and the significance threshold was stated as 0.05. The primary outcome variable of the study was the number of PMN in the GCF samples (PMNGCF). The secondary outcome variables were PPD, CAL, oral hygiene parameters, and metabolic and systemic parameters, which were assumed to be nonnormally distributed. Within each group, the differences between PMNGCF counts of the stimulated gingival crevice and the control site were compared by using the signed-rank test. The total PMNGCF counts of the stimulated gingival crevice and the control site were compared among all groups using the Kruskal-Wallis H test. All other data were assumed to be normally distributed, and differences were tested using a t test for independent or dependent samples. Potential cofounders were delineated and excluded (age, gender, BMI, FPG, HbA1c, erythrocyte sedimentation rate, and PMBPB).
3 RESULTS
3.1 PMNGCF counts
In all groups, the numbers of PMNGCF that migrated into the casein-containing sulcus were elevated compared with those associated with the placebo. The highest PMNGCF counts in stimulated and placebo-stimulated sulci (median: 32751 cells), as well as the greatest difference between test and control teeth (median: 15363), were detected in the T2DM + CP group; overall, the highest level of inflammation was found in this group. Furthermore, the total PMNGCF count in this group (median: 21228) was higher than that in the CP group (median: 4744) (Figure 1A).
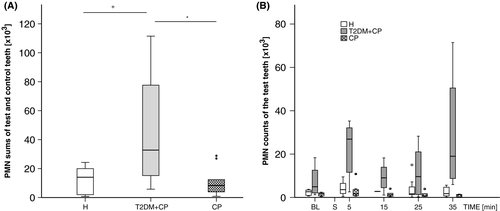
In Figure 1B, a kinetic PMNGCF sampling approach is depicted. After an initial (BL) evaluation, either a PBS(−/−) placebo or casein stimulus was applied to the randomly assigned control and test teeth, respectively (S). Beginning at 5 minutes, PMNGCF sampling took place following a 10-minutes interval. As indicated, an significant increase in cell counts was observed for up to 35 minutes. Hence, to include whole cellular accumulation over time, it was decided to sample at 35 minutes.
An antibody against fluorescein isothiocyanate-labeled protein tyrosine phosphatase receptor type C (CD45) was used to distinguish the numbers of harvested leukocytes from erythrocytes, platelets, and local periodontal cells (Figure 2).
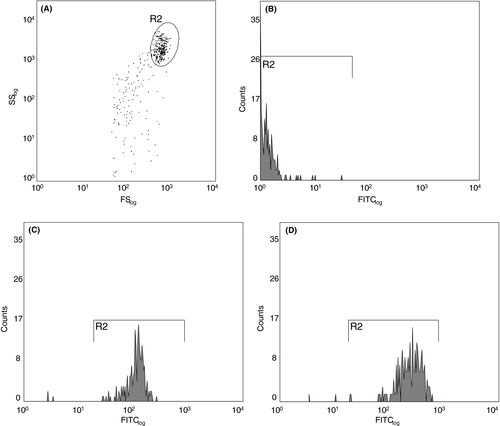
While the isotype control (unlabeled PMNGCF) in FITC-(CD45) labeled GCF exhibited little fluorescence, PMN were detectable with PBS(−/−) and elevated after casein stimulation.
As shown in Figure 3A, the median counts of CD45+ cells from stimulated samples were highest in the T2DM + CP group. Casein always induced an increased CD45+ count (P = .019). However, no statistical differences were found between the H and CP groups (P = .041).
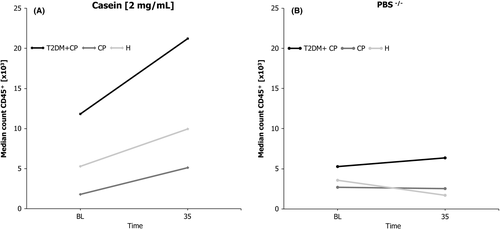
In this setup, PMNGCF were sampled at BL and 35 minutes. At the latter examination, the number of PMNGCF associated with casein stimulation always exceeded those associated with the PBS(−/−) placebo. The maximum casein-dependent activation of leukocytes in vivo was not as fast as soluble chemoattractant stimulations ex vivo are known to act,26 and it peaked at 35 minutes.
Figure 3B indicates that the median counts of CD45+ leukocytes remained constant or showed a tendency to decline (at 35 minutes) after stimulation with the PBS(−/−) placebo.
3.2 Demographic and metabolic parameters derived from peripheral blood
Plasma glucose level, HbA1c values, and BMI are shown in Table 1. Three participants (two individuals with T2DM + CP and one with CP) showed slightly elevated erythrocyte sedimentation rates (data not shown). The T2DM + CP patients exhibited nonsignificantly elevated PMNPB counts compared with CP and H patients. The highest counts of peripheral PMNPB CD11b+ were detected in T2DM + CP patients, followed by H patients. Differences between the H and CP group (P = .051, t test) and the T2DM + CP and CP group (P = .01) were found.
| Diagnose | Plasma glucose (mg/dL) | HbA1c (%) | HbA1c (mmol/mol) | BMI | PMNPB (giga/L) | PMNPBCD11bpos. (count) |
|---|---|---|---|---|---|---|
| H | 91 ± 17 | 5.3 ± 0.3 | 34 ± 3.3 | 25.8 ± 3.5 | 3.96 ± 0.69 | 31.4 ± 14.7 |
| T2DM + CP | 156 ± 37 | 7.5 ± 1.4 | 58 ± 15.3 | 31.8 ± 6.7 | 4.01 ± 1.05 | 73.5 ± 33.1* |
| CP | 91 ± 13 | 5.5 ± 0.3 | 37 ± 3.3 | 24.6 ± 5.9 | 3.08 ± 0.82 | 37.0 ± 22.0 |
- Abbreviations: BMI, body mass index; CP, chronic periodontitis; H, healthy controls; SD, standard deviation; T2DM + CP, type 2 diabetes mellitus and chronic periodontitis.
- * P = .01 CP vs H; P = .051 H vs T2DM + CP (t test).
3.3 Dental parameters
There were no differences between groups regarding the total number of teeth, the PPDs of the test and control teeth, and the mean probing depth and the CAL of the whole dentition, except for the H group (P = .01, t test). The results of dental parameters are shown in Table 2.
| Periodontal parameter | Diagnose | Minimum | Maximum | Median | Mean ± SD | Statistical significance |
|---|---|---|---|---|---|---|
| Number of teeth | H | 22 | 32 | 28 | 27.8 ± 2.7 | n. s. |
| T2DM + CP | 17 | 30 | 25.5 | 25.0 ± 4.1 | ||
| CP | 21 | 28 | 26 | 25.4 ± 2.8 | ||
| Periodontal probing depth (mm) | H | 0.2 | 4.0 | 1.8 | 1.86 ± 0.68 | **T2DM + CP, CP |
| T2DM + CP | 0.6 | 9.0 | 2.4 | 2.69 ± 1.13 | **H, n. s. CP | |
| CP | 0.4 | 9.0 | 2.0 | 2.50 ± 1.09 | **H. n. s. T2DM + CP | |
| Clinical attachment loss (mm) | H | 0.2 | 10.0 | 2.0 | 2.06 ± 0.80 | n. s. T2DM + CP, CP |
| T2DM + CP | 0.6 | 13.0 | 3.0 | 3.02 ± 1.43 | n. s. H, CP | |
| CP | 0.4 | 11.0 | 3.0 | 2.84 ± 1.29 | n. s. H. T2DM + CP |
| Test teeth | Control teeth | |||||
|---|---|---|---|---|---|---|
| Diagnose | Median | Mean ± SD | Median | Mean ± SD | ||
| Periodontal probing depth of test and control teeth (mm) | H | 1.8 | 1.3 ± 0.4 | 1.8 | 1.7 ± 0.3 | n. s. |
| T2DM + CP | 2.3 | 2.3 ± 0.2 | 2.2 | 2.3 ± 0.4 | ||
| CP | 2.2 | 2.1 ± 0.2 | 2.3 | 2.2 ± 0.4 | ||
| Oral hygiene index | Diagnose | Day | Median | Mean ± SD | Statistical significance |
|---|---|---|---|---|---|
| PBI | H | −14 | 0.09 | 0.12 ± 0.11 |
for each cohort: P = .001 over time n. s. at day 0 in between groups |
| BL | 0.00 | 0.02 ± 0.06 | |||
| T2DM + CP | −14 | 0.22 | 0.74 ± 0.97 | ||
| BL | 0.09 | 0.25 ± 0.39 | |||
| CP | −14 | 0.16 | 0.14 ± 0.09 | ||
| BL | 0.00 | 0.03 ± 0.05 | |||
| mPLI | H | −14 | 1.15 | 1.43 ± 0.94 |
for each cohort: P = .001 over time n. s. at day 0 in between groups |
| BL | 0.79 | 1.00 ± 0.46 | |||
| T2DM + CP | −14 | 2.07 | 2.28 ± 1.09 | ||
| BL | 1.34 | 1.42 ± 0.55 | |||
| CP | −14 | 1.44 | 1.51 ± 0.57 | ||
| BL | 0.85 | 0.95 ± 0.45 |
- Abbreviations: CP, chronic periodontitis; H, healthy controls; mPLI, modified plaque index; n. s., no statistically significant difference (Kruskal-Wallis test); n. s., no statistically significant difference (t test); PBI, papillary bleeding index; SD, standard deviation; T2DM + CP, type 2 diabetes mellitus and chronic periodontitis.
- ** P = .01 (t test).
3.4 Oral hygiene phase
Periodontal pockets were excluded from the test teeth. The gingival inflammation was successfully reduced, as indicated by the PBI and mPLI. Oral hygiene indices at different time points are shown in Table 2.
Hygiene parameters improved during the pretest phase for each cohort over time. At the beginning and end of the intensive oral hygiene regimen, before the local inflammation test, patients with T2DM + CP had the highest oral hygiene values, and the controls had the lowest; however, there was no significant difference between the groups. None of the participants exhibited clinical signs of inflammation around the test or control teeth (PBI = 0; in addition, no swelling or redness).
4 DISCUSSION
Inflammation is a bi-directional mechanism associated with T2DM and CP. A topical cellular inflammatory response was demonstrated to be inducible in the absence of gingival/periodontal symptoms, as substantiated by this work. Considering the site specificity of gingivitis and periodontitis, the utmost care was taken to avoid the effects of infection/inflammation, particularly at the sample sites (PPD < 3 mm, BOP absent). Since participants received a stringent oral hygiene regimen, the clinical influence of gingivitis was eliminated. Periodontal pockets were excluded because of their potential to harbor abundances of antigens and immune cells. At these locations, compensatory strategies such as prerinsing would fail to calibrate the assay for BL, cross-sectional, or kinetic assessments.
The counts of PMNGCF induced by the inflammatory stimulus casein were elevated in patients newly diagnosed with T2DM + CP. Systemically and orally healthy (H) individuals as well as those with CP (severe, generalized; CP) participated as controls. A T2DM control group without CP is desirable; however, patients diagnosed with T2DM according to ICD10 code E11.9(-1) all exhibited chronic periodontitis or were edentulous.
Hitherto, our null hypothesis stated that standardized inflammatory stimulation would lead to lower PMNGCF numbers in T2DM + CP patients. Additionally, higher counts were expected in both control groups: those who had CP but were systemically healthy as well as those who were systemically/orally healthy (H). Based on the results presented and careful statistical evaluation, the null hypothesis was rejected.
In the present study, T2DM patients received initial nutritional supervision and dietary control during their hospital stay. Nevertheless, the cohort of T2DM + CP patients exhibited elevated FPG levels at the time of sampling, supporting the hypothesis that hyperglycemia increases the inflammatory state, resembling topically elevated leukocyte recruitment. The mean FPG of diabetes mellitus patients with periodontitis was 156 mg/dL at BL, which was almost double that of other groups (91 mg/dL). Early reports indicated that a reduction in carbohydrate intake decreased clinical symptoms of gingival inflammation.27
In the present study, considering the elevated PMNGCF counts, both stimulated and unstimulated, the hypothesis of carbohydrate attenuation of gingival hyperinflammation28, 29 at the level of inflammatory immune cells and according to the papillary bleeding indices was supported.
Papillary bleeding was absent from any test/control tooth; independently, the overall local inflammatory burden was highly diverse between the diabetic and nondiabetic cohorts. However, at the date of sampling, T2DM + CP patients exhibited a mean PBI value of 0.25 (whole dentition). In comparison, the CP patients and the healthy controls had 8.3- and 12.5-fold lower values, respectively.
Topical inflammation with all pathophysiological adaptations likely resembles an attractive link for the movement of primary immune cells or augmented numbers of PMNGCF. The latter, a dominant population in inflamed periodontal tissues, fosters collateral tissue destruction when confronted with a challenging biofilm.30 In the gingival crevice, where bacteria flourish, antigens continuously activate PMN, leading to elevated levels of bactericidal molecules. These modulations are considered responsible for the hallmark of disease progression: tissue destruction in periodontitis31 and inflammation capable of aggravating metabolic dysregulation.14 Additionally, enhanced PMNGCF counts in T2DM subjects give rationale to the hypothesis of tissue damage by host cells.
Controversially, decreased peripheral chemotaxis (PMNPB) was found in patients with aggressive periodontitis; however, studies focusing on CP showed no alteration26, 32, 33 or elevated PMNPB chemotaxis without the influence of T2DM.
Assays for the evaluation of inflammatory reactions and chemotaxis by human immune cells were performed in vitro using isolated peripheral leukocytes with the Boyden chamber,34 agarose gel, and modified agarose gel methods.35 Depending on the setup and methodological details (eg, stimulus gradient, migration time, depth, filter width, quality), these methods assess chemokinesis30, 36 rather than local inflammation in vivo.37 The contrasting results of studies investigating peripheral leukocyte chemotaxis in patients with T2DM and CP are divergent,26 most likely because PMNPB in existing cohorts with a long history of diabetes were assayed.
However, we assessed newly diagnosed, untreated patients with severe T2DM (cf. ICD10-code E11.9(-1)). Comorbidities associated with duration of diabetes (eg, micro-/macroangiopathies) were excluded via medical examination.
A method of testing the PMN response in the gingival crevice was introduced by Golub,22 but it exposes cells to mechanical stress. Manual and optical counts have further disadvantages. In contrast, the method presented here can be considered cell preserving21 because mechanical, thermal, and osmotic cellular stress is minimal. Controversially, PMNGCF counts were lower in rodents with diabetes than in healthy rodents after casein application.23 These authors later implemented the assay in humans with gingivitis and periodontitis and in one patient with T2DM.24 To date, the aforementioned study is the only one that has analyzed local chemotaxis in GCF from a T2DM + CP patient. However, the subject in that report differed from the diabetes cohort presented in the current study because the medical diagnosis had been long established. Moreover, the sample size presented in our study was considerably higher.
In the present study, the PMN response to standardized inflammatory stimulation was tested locally and in vivo; PMN was not isolated ex vivo. These conditions as well as the state of PMN activation differ essentially from previous studies. Our method aimed to mimic local inflammation in the gingival crevice.
These observations showed elevated gingival crevicular PMN presence with and without stimulation, indicating a topical hyperinflammatory reaction most likely due to diabetes mellitus. The results were validated by a noninvasive in vivo chemotaxis assay. Inflammation, as suggested,15 may augment severity and progression bilaterally.
Severe symptoms and enhanced local inflammatory reactions mandate vigilant therapy, that is, systemic and oral care of patients with diabetes mellitus and periodontitis. Specialized, supportive treatments, and adapted intervals of supervision should be intensified to eliminate infections and inflammation.
ACKNOWLEDGEMENTS
S. K. Sonnenschein received a scholarship from the dental chamber (Landeszahnärztekammer Hessen, Germany) to conduct her doctoral thesis.
CONFLICT OF INTEREST
At any time, none of the following authors, Jens M. Herrmann, Sabine E. Groeger, Borros Arneth, Nils Ewald, and Joerg Meyle, nor their respective departments or institutes received payments or services from a third party (government, commercial, private foundation, etc) for any aspects of the submitted work. No relationships or activities that could be perceived as influencing or appear as potentially influencing the submitted work are declared. Source and intramural funding: Department of Periodontology (Director: Prof. Dr J. Meyle) Justus-Liebig University, Giessen, Germany.




