Chloroplast Dual Divergent Promoter Plasmid for Heterologous Protein Expression in Tetraselmis suecica (Chlorophyceae, Chlorodendrales)
Abstract
The eukaryotic green microalga Tetraselmis suecica is commonly used for aquaculture purposes because of its high stress tolerance and ease of culture in a wide spectrum of environments; they are therefore suitable candidates for biotechnology applications. To date, no data are available regarding chloroplast transformation vectors based on specific endogenous promoters and homologous targeting regions. We report on the identification of Tetraselmis suecica genes encoding the ribulose bisphosphate carboxylase/oxygenase large subunit protein, the photosystem II D1 protein and the ATP synthase CF1-beta subunit protein together with their untranslated regions (5′UTR, 3′UTR). The full-length ORFs of the putative genes with their regulatory sequences were obtained. We were also able to identify the downstream 3′ end of the large subunit ribosomal RNA gene (23S) along with the 5S RNA end-to-end with the psbA gene on the complementary strand. The intergenic region between these genes appears to be a good target site for the integration of target proteins. Moreover, we identified a back-to-back promoter region among the rbcL and atpB genes. To assess the bidirectionality activities of both promoters, a dual reporter vector was constructed for Tetraselmis suecica transformation containing the cat and TurboGFP genes driven by the 5′rbcL/5′atpB divergent promoter. The vector included the 23S-5S and psbA nucleotide sequences as flanking regions. These flanking regions provided suitable insertion sites within the chloroplast genome for cassette integration via homologous recombination. Simultaneous expression of the chloramphenicol-resistant conferring gene and the gene coding for TurboGFP driven by 5′rbcL/5′atpB showed a potent natural bidirectional promoter as a reliable genetic tool.
Abbreviations
-
- LFR
-
- left flanking region
-
- ORF
-
- open reading frame
-
- RAGE
-
- random amplification of genomic ends
-
- RFR
-
- right flanking region
-
- tGFP
-
- turbo Green fluorescent protein
-
- UTR
-
- untranslated region
Macroalgal genetic engineering has gained increasing attention because it has many unique advantages over conventional expression systems with regard to the production of recombinant proteins for industrial, pharmaceutical, or other value-added uses (Ng et al. 2017, Kwon et al. 2018). Microalgal systems offer not only an economic alternative and high potential for scale-up but also the possibility to exploit two different ways for achieving expression, either in the nuclear or in the chloroplast genome (Doron et al. 2016). Chloroplast transformation has some advantages when compared with the approach using the nuclear genome: (i) high level of gene expression, (ii) devoid of gene silencing and other epigenetic mechanisms, (iii) higher precise insertion sites than nuclear expression, (iv) ideal bioreactor for toxic proteins, (iv) multigene expression arranged in artificial operons, (v) reduced gene dispersal in the environment due to maternal inheritance, and (vi) post-translational modification by disulfide bridge formation and phosphorylation (Adem et al. 2017, Kwon et al. 2018).
The stable transformation of chloroplasts in the freshwater chlorophyte Chlamydomonas reinhardtii was first demonstrated in the late 1980s (Boynton et al. 1988). Since then, many recombinant proteins have been expressed in C. reinhardtii chloroplast (Dyo and Purton 2018). Although the number of algal species transformed with the chloroplast approach remains low, data report that the following species have been transformed: Euglena gracilis (Doetsch et al 2001), Porphyridium sp. (Lapidot et al. 2002), Dunaliella salina (Tan et al. 2005), Haematococcus pluvialis (Gutiérrez et al. 2012), Parietochloris incisa (Grundman et al. 2012), Dunaliella tertiolecta (Georgianna et al. 2013), Phaeodactylum tricornutum (Xie et al. 2014), Platymonas subcordiformis (Cui et al. 2014), and Nannochloropsis oceanica (Gan et al. 2018).
Tetraselmis suecica is a unicellular green marine microalga belonging to the class Chlorophyceae, which are broadly used in aquaculture for the feeding of molluscs and crustacean larvae (Chini-Zittelli et al. 2006) and as a probiotic in fish farming (Irianto and Austin 2002). Tetraselmis suecica is rich in vitamin E, carotenoids, chlorophyll, and tocopherols (Sathasivam and Ki 2019) and has been proposed as a food supplement in human and animal diets (Carballo-Cárdenas et al. 2003). Additionally, its pigment extract has been patented for its ability to enhance dermal pigmentation, reduce psoriasis lesions, and increase hair growth (Carballo-Cárdenas et al. 2003). Furthermore, recent studies reported that Tetraselmis extract reduces oxidative stress in human cells due to the presence of high levels of carotenoids (Sansone et al. 2017). Tetraselmis suecica has also been used for biofuel production (Montero et al. 2011, Patidar et al. 2018). To date, only two chloroplast genomes have been reported: Tetraselmis sp. CCMP 881 (Turmel et al. 2016) and Tetraselmis desikacharyi (Li et al. 2019). However, plastome genetic engineering tools are not yet available for T. suecica; therefore, the development of genetic tools for this microalga will be a feasible strategy to enlarge and generate new biotechnological opportunities for this alga.
Accordingly, the first step toward understanding how to drive heterologous gene expression in the chloroplast of Tetraselmis suecica requires the identification and characterization of efficient promoters and untranslated regions (UTRs). As chloroplast transformation occurs by homologous recombination (Blowers et al. 1989), two flanking regions are required for introducing foreign genes into the plastome guided by site-specific integration events, resulting in uniform transgene expression among transgenic lines, eliminating the position effect (Taunt et al. 2017).
The identification of strong promoters was focused on genes with a high rate of transcription, such as those encoding ribulose-1,5-bisphosphate carboxylase/oxygenase (rbcL) and the ATP synthase subunit beta. We used the Rapid Amplification of Genomic Ends (RAGE) method to obtain those promoters (Cormack and Somssich 1997). Additionally, the identification of flanking regions was performed through partial characterization of the chloroplast genome by the amplification of long DNA fragments.
In this work, we report the isolation, cloning and complete nucleotide sequences of the rbcL, psbA, atpB, and atpE genes of Tetraselmis suecica as well as the 3′ end of the large subunit ribosomal RNA (23S) and the 5S RNA genes. Interestingly, the identification of the rbcL promoter provides us with an arrangement of divergent and overlapping atpB promoter that will become an efficient tool for driving the coexpression of multiple genes. Thus, we have taken this intrinsic characteristic from T. suecica to design the coexpression plasmid pSM657, which includes the cat and TurboGFP reporter genes, to validate the transcriptional activity of the chloroplast dual divergent promoter. The 23S-5S and psbA sequences were the flanking regions used to integrate the expression cassette.
This study is a leading step for forthcoming investigations regarding genetic engineering in the chloroplast of the green microalga Tetraselmis suecica.
Materials and Methods
Strains and culture conditions
Tetraselmis suecica CCMP 904 cells were axenically grown in modified liquid Guillard's f/2 medium (Guillard 1975) comprising 50% sterile-filtered natural seawater and 50% sterile-distilled water. Cultures were incubated at 22–25°C with continuous illumination of 30 μmol photons · m−2 · s−1 provided by white fluorescent tubes and were manually shaken once a day. The microalgae were harvested when they attained 2 × 105 cells · mL−1, as determined by manually counting the cells using a hemocytometer. Agar f/2 plates containing 200 μg · mL−1 chloramphenicol were used for selection. The procedure used for total DNA isolation has been described previously by Gutiérrez et al. (2012). All recombinant DNA manipulations were performed in accordance with standard techniques (Sambrook and Russel 2001). All enzymes were provided by commercial suppliers, and reactions were carried out under recommended conditions. Escherichia coli TOP10 (Invitrogen, Carlsbad, CA, USA) was used for plasmid propagation.
Antibiotic sensitivity assay
Antibiotic concentrations for Tetraselmis suecica were defined by plating a serially diluted culture on solid f/2 media containing different concentrations of kanamycin (Sigma-Aldrich, St. Louis, MO, USA), spectinomycin (Sigma-Aldrich), and chloramphenicol (Sigma-Aldrich). We assayed at antibiotic concentrations ranging from 100 to 800 μg · mL−1. The plates were incubated at the growth conditions mentioned above. Each treatment was performed in triplicate.
Rapid Amplification of Genomic Ends (RAGE)
RAGE degenerate primers were designed by comparative analysis of the psbA and rbcL genes among Chlamydomonas reinhardtii (GenBank accession number BK000554), Chlorella vulgaris (GenBank accession number AB001684), Dunaliella salina (GenBank accession number AY531529), Tetraselmis marina (GenBank accession number U30284.1), Tetraselmis aff. maculata (GenBank accession number U30284), Mesostigma viride (GenBank accession number AF166114), Euglena gracilis (GenBank accession number X70810), and Nephroselmis olivacea (GenBank accession number AF137379) with CLUSTALW (http://www.ebi.ac.uk/clustalw/). Primers directed against conserved regions of the ribosomal operon were also designed for the above-mentioned species. Gene-specific primers from Tetraselmis suecica were designed upon obtaining specific sequence data. Primer sequences are shown in Table 1.
| Primer | Sequence 5′ to 3′ |
|---|---|
| TH231 | GTTAAGCTTAATTTTTGATTGGAGTCAAAAG |
| TH144 | TAAATTTGATTAAAAAAGATTGTCCGATC |
| JPC1 | GTAACTAGTGTTGATACTTTATAATCTTTCAGTCTCTAA |
| JPC2 | GTTTCTAGAAATTGGCTTTGTTGGTCAACGATTA |
| JPC3 | GTAGGTACCACTGTAGTCTGGAATTGGGTTTGGGTTC |
| JPC6 | GTTATCGATTTAGTTCAATTTATATCCCCCTTCCTC |
| JPC7 | GTTGCGGCCGCCCTAAGCGTTTACAGATGGAGCTTCTACTGA |
| JPC8 | GTAGAGCTCCCTTTGGTTCGAATCCAGGCAGG |
| TH235 | GTAAAGCTTATGGAGAAAAAAATCACTGGATAT |
| JPC4 | GTTACTAGTATCAAATTACGCCCCGCCCTGC |
| TH101 | GTTGGTACCAAGCTGAAACTGGAAATAA |
| TH260 | GTTAAGCTTTCAAACCATATTGAGACAGTT |
| TH395 | GGTGAATTCATGTTGGAGAGCGACGAG |
| TH416 | GTAACCGGTACCTTATTCTTCACC |
| TH129 | GTTAAGCTTATGGAGAAAAAAATCACTGGA |
| TH146 | GGCCAGTTGTAGGTATTTGGTTTAC |
| TH143 | GAAACAATAAGATCAATAAATCCATGGTCT |
| TH419 | GTTCGAGTCCGGTTGGGTCTA |
| TH2 | GRTCTTTWACACCDGCYTTRAAKCC |
| TH4 | GYTCTTCWGGWGGWACWCCWGGTTG |
| TH18 | GTTTTCGTTTCAGTTTGTGGAGCCAT |
| TH16 | GCCCGAAAATGATCTAGCGTCTTTG |
| TH7 | GCTGCATGYGAAGTYTGGAARGAA |
| 16SR | GTCTGATCGTCCTCTCAAACCATA |
| Oligod(T)-anchor | ACCACGCGTATCGATGTCGATTTTTTTTTTTTTTTTT |
| PCR-anchor | GACCACGCGTATCGATGTCGAC |
A PCR-based method for amplification of genomic DNA was performed by digesting 5 μg of Tetraselmis suecica genomic DNA overnight at 37°C with 30 units of restriction endonucleases in a final volume of 50 µL, in accordance with the protocol described by Cormack and Somssich (1997). The restriction endonucleases HindIII, BamHI, and EcoRI (Promega, Madison, WI, USA) were used in a mixture or individually. After digestion, the reactions were heat inactivated at 65°C for 10 min. Then, samples were extracted once with an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1) and precipitated with 2.5 vol of 95% ethanol, followed by a 70% ethanol wash. The 500 ng digested DNA was used for a polyadenylation reaction, boiled for 5 min, and then quick-cooled on ice. The sample was incubated with 0.5 mM dATP in 20 µL of terminal transferase (TdT) buffer (Promega) containing 50 units of TdT at 37°C for 90 min. Heating the sample at 72°C for 5 min stopped the reaction.
The first PCR round was performed using 100 ng of the polyadenylated total genomic DNA as template, 1 µM of gene-specific primer-1, 1 µM oligod(T)-anchor primer, 200 µM dNTP's, and 2.5 units of Taq DNA polymerase (Promega) in 50 µL total volume. The PCR reaction was carried out under standard conditions. The amplification program was performed in a thermocycler (Eppendorf, Hamburg, Germany). One µL from the first PCR round was used as template for a second PCR round (nested) in a volume of 50 µL under the same conditions, except that 1 µM of gene-specific primer-2 and 1 µM of PCR-anchor primer were used.
The fragments were cloned using the TOPO TA Cloning Kit (Invitrogen). Finally, the clones were submitted to Macrogen (Korea) for sequencing from both ends. Novel sequences were analyzed with Internet tools to predict promoters and secondary structure sequences (http://www.fruitfly.org, http://www.softberry.com and http://www.genebee.msu.su).
Amplification of long target DNA sequences
Tetraselmis suecica total genomic DNA was amplified using primers designed against conserved regions of the Chlorophyta ribosomal operon in combination with primers against the T. suecica chloroplast genes described in this study. The long-PCR reaction was performed in a final volume of 50 µL containing 100 ng of template DNA, 2.5 units of TaKaRa LA Taq DNA polymerase (TaKaRa Bio Inc, Shiga, Japan), 1.6 mM dNTPs, 2.5 mM MgCl2, and 0.2 µM of each primer set. The PCR conditions were as follows: an initial denaturation step at 94°C for 1 min, followed by 30 cycles of 94°C for 10 s, 60°C for 30 s, and 68°C for 15 min and an extra final elongation step at 72°C for 10 min. PCR products were observed by 1% agarose gel electrophoresis, cloned and submitted for sequencing from both ends.
Designing the plasmid vectors
The dual divergent promotor 5′rbcL/5′atpB was directly amplified from Tetraselmis suecica with primers TH231/TH144 containing the restrictions sites HindIII and EcoRI, respectively. For the amplification of the 3′ rbcL, the primer pair JPC1/JPC2 with SpeI and XbaI restriction sites, respectively, were used. Left and right flanking regions were amplified with the primers set JPC3/JPC6 and JPC7/JPC8 (KpnI, ClaI, NotI, and SacI, respectively). The mentioned sequences were cloned into the plasmid pBluescript II KS+ (Fermentas International Inc., Burlington, ON, Canada), creating the vector pTSue. The cat gene (chloramphenicol resistance) was amplified from the pDNR-LIB (TaKaRa Bio Inc.) with the specific primers TH235/JPC4, which included the restriction sites HindIII and SpeI, respectively, and cloned into pSM303 digested with the same enzymes, generating the pSMcm. The 3′ atpB was amplified with primers TH101/TH260, cloned into pSMcm and digested with KpnI and HindIII.
This new plasmid named pSM547 was digested with HindIII. The fragment containing the cat gene and 3′atpB was subcloned into the plasmid pTsue, creating the vector pSM570. Finally, as a reporter gene, we used the fluorescent protein TurboGFP (tGFP). The tGFP protein matures rapidly and is highly soluble (Evdokimov et al. 2006), and the specific variant used here was codon-optimized for Chlamydomonas reinhardtii chloroplast codon bias (http://www.kazusa.or.jp/codon/) using OPTIMIZER (http://genomes.urv.is/CAIcal/). The generated sequence was then manually cured using CAIcal (http://genomes.urv.is/CAIcal/) for avoiding too many repetitions of single codons while maintaining a high Codon Adaption Index (CAI; Sharp and Li 1987). Quickfold was then used for eliminating potential secondary structures (http://mfold.rna.albany.edu/?q=DINAMelt/Quickfold). The final coding sequence was submitted to GeneScript (Piscataway, NJ, USA) for chemical synthesis flanked with suitable restriction sites for cloning (EcoRI and KpnI).
The primers TH395 and TH416 carrying the restrictions sites EcoRI and AgeI, respectively, were used to amplify the tGFP optimized sequence. The amplicon was cloned with the same mentioned restriction sites into pSM635. Afterward, the new vector, pSM637, was digested with EcoRV and AgeI enzymes and subcloned into the vector pSM570, providing the new vector pSM657 for Tetraselmis suecica transformation (Fig. 1).
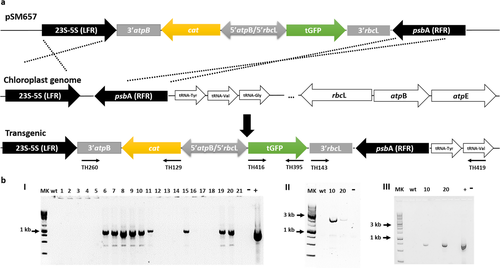
Chloroplast transformation of Tetraselmis suecica
Cultures of Tetraselmis suecica cells were grown in modified liquid f/2 medium under the conditions described above. Cells were harvested by centrifugation at 2,700g for 10 min at 4°C. The cell pellet was resuspended in f/2 medium to a density of 105 cells · mL−1. From these cell suspensions, 200 µL was layered onto the central area of the f/2 agar plate 24 h prior to use. Gold carrier particles with an average diameter of 550 nm were mixed with plasmid DNA in accordance with the manufacturer's instructions (Seashell Technology, La Jolla, CA, USA). The Biolistic PDS-1000⁄He system (BioRad, Hercules, CA, USA) was used for transformation. Plates were bombarded with 0.5 µg of DNA at 1,350 psi from a distance of 7.0 cm a total of three times at different angles per plate. After transformation, the plates containing T. suecica cells were regenerated for 48 h at 22–25°C, with moderate illumination. Upon regeneration, the cells were transferred to fresh f/2 medium agar plates containing 100 µg · mL−1 chloramphenicol and incubated under the same conditions as described above. The colonies were counted 30 days later to calculate the transformation efficiency, and then each colony was propagated on f/2 medium agar plates supplemented with 100 µg · mL−1 chloramphenicol. An empty vector (plasmid DNA lacking the expression cassette) was used as a negative control.
PCR for site-specific transgene integration into the chloroplast genome
First, the cat gene from transformed microalgal cells was confirmed using the specific primer set TH260/TH129 (Table 1, Fig. 1a), corresponding to the cat/3′atpB sequence. Clones were replicated every 2 weeks for 5 months. Afterward, wild-type and transgenic microalgal clones were harvested at the late logarithmic phase of growth by centrifugation at 2,000g for 15 min. Genomic DNA was extracted by the above-mentioned protocol. Clones were tested for stability of the site-specific transgene integration into the chloroplast genome by PCR using the specific primer TH143 against the 3′rbcL and the primer TH419, which hybridizes to the tRNAVAL sequence outside the right flanking region of psbA used in the vector (Table 1, Fig. 1a).
RT-PCR Analysis
The RNA isolation was carried out from wild-type and transformed cells using the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) in accordance with the manufacturer's instructions. The final pellet was dissolved in RNase-free water and stored at −80°C. Total RNA quality and quantity were assessed by spectrophotometry (NanoDrop Technologies, Inc., Wilmington, NC, USA). A total of 500 ng RNA was reverse transcribed into cDNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). The resulting cDNA( VOLUME was then used as the template to run PCR with the specific primer pair TH395/TH416 against tGFP, using Q5® High-Fidelity DNA Polymerase (NEB, Ipswich, MA, USA). The reaction products were analyzed by 1% agarose gel electrophoresis. The gel image was captured with ChemiDoc Imaging System (BioRad).
Flow cytometry analysis
Flow cytometric analyses of Tetraselmis suecica were performed on a CytoFlex flow cytometer (Beckman-Coulter, Brea, CA, USA) using a blue LED laser emitting at 488 nm. The capillary and nozzle of the flow cell had an inner diameter of 420 μm × 180 μm. Before analysis, 105 cell · mL−1 transformed and untransformed microalgal cells were centrifuged at 3,000 g for 10 min at 4°C and washed three times with 1× PBS buffer (Thermo Fisher Scientific). Afterward, the emission of the tGFP gene was detected in the FL1 channel (wavelength 490–550 nm), and the FITC spectrum was obtained by CytExpert version 1.2.11.0 (Beckman-Coulter, Indianapolis, IN, USA). The forward scatter signal, side scatter signal, and fluorescence intensities were measured simultaneously. The voltages and gains of the photomultiplier tubes were optimized when used. Fluorescence beads (CytoFLEX Daily QC Fluorospheres; Beckman-Coulter) were used for optical alignment of the instrument.
Confocal scanning microscopy (CSM)
Wild-type Tetraselmis suecica cells and transformed clones were grown in f/2 medium to late log phase in a rotary shaker. Image acquisition was performed with a Leica TCSSP5 II Confocal Microscope and samples observed under a 63×, 1.2 NA water immersion objective (Leica Microsystems Inc., Mannheim, Germany). Microalgae cells were observed by detecting chlorophyll autofluorescence. The following filters were used to image autofluorescence of photosynthetic pigments: excitation 470 nm and emission 630 nm. Transformed clones carrying tGFP were observed using: excitation 480 nm and emission 500 nm. Images were captured using Leica software. Acquisition with multiple channels was done by sequential scanning. For compensate the bleaching, the brightness of sequential images was adjusted using pinhole – open wider >1 Airy Unit (AU) at a range between 450 and 750+ of master grain. The excitation was bunched into brief intense pulses.
Results
Identification of the rbcL, atpB, and atpE genes of Tetraselmis suecica
Regarding the characterization of the 5′UTR and 3′UTR regions of the rbcL gene, we used HindIII digested and polyadenosine tailed genomic fragments as template for PCR amplifications. As a mean to doing the genome-walking method RAGE, multiple-sequence alignments targeted to rbcL gene were conducted. Two degenerate primers were designed and positioned approximately at 132 bp (TH2) and 33 bp (TH4) downstream of the gene coding region. No detectable distinct bands were present from the first PCR-RAGE reaction with primers TH2/oligod(T)-anchor. A visible fragment of 250 bp was obtained from the nested PCR-RAGE with primers TH4/PCR-anchor (data not shown). This fragment was cloned and submitted to sequence analysis. Based on the sequence results, we confirmed that corresponds to a portion of the rbcL-5′UTR. Therefore, two new gene-specific primers were designed against this new sequence to get an extended rbcL-5′UTR. These primers were targeted to the start of the Tetraselmis suecica rbcL ORF (TH18) and the other one at 70 bp upstream of rbcL-5′UTR (TH16). As before, no distinct bands were visible from the first RAGE-PCR (TH18/oligod(T)-anchor). Whereas two visible fragments (650 bp and 350 bp) were achieved with the second round (nested) RAGE-PCR (TH16/PCR-anchor; data not shown). Both fragments were cloned and submitted to sequence analysis. The 350 bp fragment was entirely included within the 650 bp fragment, corroborating that we were positioned at the T. suecica rbcL promoter. Surprisingly, the 5′ end of the rbcL promoter matched the beginning of the atpB chloroplast coding gene but positioned in the opposite direction. The analysis suggested a back-to-back promoter region. The rbcL and atpB genes are divergently transcribed within the T. suecica chloroplast genome from complementary DNA strands.
The full-length ribulose bisphosphate carboxylase/oxygenase large subunit sequence was identified with the primer set TH231/JPC2. The resulted PCR product (1,436 bp) was cloned and fully sequenced on both strands. This fragment reveals a potential ORF encoding a protein of 475 amino acid residues.
Previous studies of chloroplast promoters related to the transcription of photosynthetic genes showed that they contain hexanucleotide sequences upstream of the transcription start site resembling bacterial −10 (TATAAT) and −35 (TTGACA) consensus motifs (Klein et al. 1992, Shevelev and Hübscher 2002). In general, nucleotide sequences outside of coding regions tend to be less conserved among organisms but still include consensus motifs that could be identified. The promoter prediction analysis confirmed a putative promoter region 131 nt upstream of the rbcL ORF. The −10 element contains the consensus motif TAAAAT and the −35 consensus sequence corresponds to TTTTAA (Fig. 2).

The same approach was used to find the 3′ rbcL regulatory sequence. Upon nested PCR with the gene-specific primer TH7, positioned at 57 bp upstream of the stop codon of the rbcL gene, we got fragments of 200, 300, and 650 bp. The 200 bp fragment was comprised entirely within the 300 bp and 650 bp fragments, and the same happened with the 300 bp fragment within the 650 bp fragment.
A region of a strong stem-loop structure was identified in the rbcL-3′UTR, a large inverted repeat positioned 54 nt downstream the stop codon (AAATATAAATTTCTAGGC…. [8 nt]…. GCCTAGAAATTTATATTT).
Toward the identification of the full atpB gene, a PCR was carried out with the gene-specific primer TH144 in combination with a conserved primer against the 16S ribosomal operon (16SR). The primers 16SR/TH144 yielded a product of ~3 kb, which was cloned and fully sequenced on both strands. This fragment encodes two proteins, the ATP synthase CF1-beta subunit and the ATP synthase CF1-epsilon subunit, with 484 and 132 amino acid residues, respectively.
The atpE protein lies at the 3′ end of the atpB gene. These two proteins are highly conserved and are encoded as part of a bicistronic operon. These kind of arrangement exist in a number of higher plants and algal species, including tobacco, the green alga Tetraselmis sp. CCMP 881, Nephroselmis olivacea and Chlorella vulgaris, the unicellular Euglena gracilis, and the red alga Porphyra purpurea. Using BLAST searches, high similarities were found between the atpB and atpE genes of T. suecica and other chlorophyta microalgae. The atpB 5′-flanking region showed a putative promoter 145 nt upstream of the ATG codon comprising the same consensus sequence identified for the −10 box of the rbcL putative promoter region (Fig. 2). With respect to the −35 box, we predicted the sequence motif TTCATA.
Identification of flanking regions for Tetraselmis suecica
Long RAGE PCR allows the amplification of products much larger than those achieved with conventional Taq polymerases. The amplification of long DNA fragments was desirable for finding chloroplast genome sequences available as flanking regions. We amplified the Tetraselmis suecica DNA with universal primers (chloroplast ribosomal operon) in combination with specific primers targeting to the genes identified in this study. A single major product was obtained with primers JPC3 and TH231 (data not shown) directed against the large subunit ribosomal RNA (23S) gene and the 5′rbcL, respectively. Sequencing results shown the full-length psbA gene downstream the 3′ end of the 23S RNA and 5S RNA but at the complementary strand. The psbA gene encodes a protein of 354 amino acid residues.
Interestingly, the sequencing analysis results provided the putative psbA promoter. It includes a −10 (TAAAAT) consensus motif located 143 nt upstream the psbA translation initiation codon ATG (Fig. 2).
5′rbcL/5′atpB dual divergent promoter
In the present study, we propose the 5′rbcL/5′atpB dual divergent promoter from Tetraselmis suecica as an important tool for chloroplast transformation. First, the antibiotic sensitivity pattern of the T. suecica cells was precisely evaluated. Among the tested antibiotics, chloramphenicol is the one chosen as selection marker (Table 2). T. suecica chloroplast transformation was achieved with the pSM657 vector, which includes the dual divergent promoter 5′rbcL/5′atpB for driving the expression of the tGFP and cat genes. In addition, the 23S-5S and psbA sequences were included as flanking regions (left and right, respectively) for carrying out the transformation via homologous recombination (Fig. 1a). The transformation frequency was 2.7 × 102 transformants/µDNA, based on the 400 colonies of microalgae cells grown on 100 µg · mL−1 chloramphenicol 30 d post-transformation. The successful stable integration, transcription, and expression of the cat and tGFP genes were confirmed by PCR, RT-PCR, flow cytometry analysis, and confocal microscopy. First of all, the cat gene was validated from 21 transformed clones with primers TH260/TH129 (953 bp), resulting positive 9 out of 21 clones (Fig. 1b, I). Two selected transgenic microalgal clones were analyzed to confirm integration of the transgenes into the chloroplast genome. Specific primers TH419/TH143 amplified a 2.5 kb product validating the integration of the expression cassette (Fig. 1b, II).
| Concentration (μg · mL−1) | Sterile-filtered natural seawater | |||
|---|---|---|---|---|
| 100% | 50% | 25% | ||
| 0 | + | + | + | |
| Spectinomycin | 100 | + | + | − |
| 200 | + | + | − | |
| 300 | + | + | − | |
| 400 | + | +/− | − | |
| 800 | + | − | − | |
| Chloramphenicol | 100 | +/− | − | − |
| 200 | − | − | − | |
| 300 | − | − | − | |
| 400 | − | − | − | |
| 800 | − | − | − | |
| Kanamycin | 100 | + | + | + |
| 200 | + | + | + | |
| 300 | + | + | + | |
| 400 | + | + | + | |
| 800 | + | + | +/− | |
- −, Sensitive; +/−, relatively sensitive; +, resistance.
Four months after transformation, transcriptional activity and expression of tGFP were determined by RT-PCR, flow cytometry, and confocal microscopy (Figs. 1b, III, 3 and 4). Primer pair TH395/TH416 was used to validate the presence of the transcriptionally active tGFP gene (Fig. 1b, III). Both Tetraselmis suecica transformed clones (10 and 20) driven by the endogenous 5′rbcL promoter and 3′rbcL terminator were transcriptionally actives.
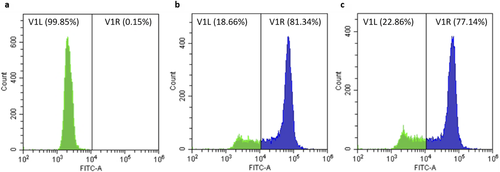
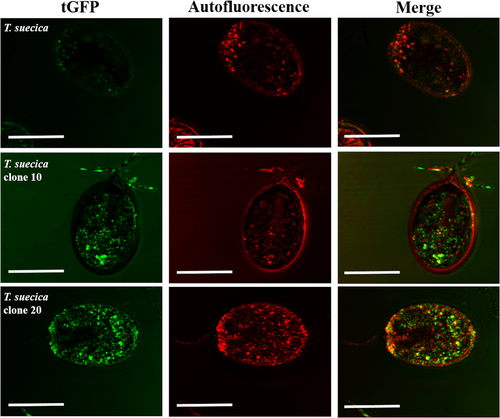
Regarding the expression of the tGFP by flow cytometry, both clones showed a high fluorescence intensity (Fig 3, b and c, respectively); meanwhile, Tetraselmis suecica wild-type cells just showed autofluorescence (Fig 3a). Additionally, the fluorescence signal visualization of the tGFP was also observed under confocal microscopy. As shown in Figure 4, strong tGFP signals were detected for both transgenic clones, while the untransformed T. suecica showed only background noise due to the autofluorescence of chlorophylls.
Nucleotide sequence accession number
The DNA gene sequences described in this study were submitted to the GenBank nucleotide database under the following accession numbers: ABA02339 for rbcL, ABA02341 for psbA, ABA02340 for atpB, and ABB04096 for atpE. The BLAST2 program (Altschul et al. 1997) was used for searching sequence homologies.
Discussion
Currently, a variety of organisms are being used for the production of recombinant proteins; among them, land plants have received considerable attention due to the high demand of biomolecules (Lomonossoff and D'Aoust 2016). However, throughout the years, microalgae have been recognized for their potential as an ideal host for recombinant protein expression, where large-scale and reduced material costs are important (Specht et al. 2010, Taunt et al. 2017, Dyo and Purton 2018). Genetic manipulation of chloroplasts seems to be a key strategy for the production of recombinant proteins and has also led to growing interest in using metabolic genetic engineering for developing strains, which can increase the production of high-value secondary metabolites for commercial applications. Nonetheless, chloroplast genetic engineering is limited to a few microalgae because of the lack of genome information (Gangl et al. 2015). The availability of endogenous promoters and regulatory mRNA untranslated regions (UTRs) is essential for developing expression vectors, key factors for transgene expression levels (Specht et al. 2010). Noncoding regions in the DNA such as promoters play a role in regulating transcription, and considering that most regulation in plastids is post-transcriptional, the UTRs in mRNA are crucial to regulating translation efficiency and mRNA stability (Marín-Navarro et al. 2007, Rasala et al. 2011).
In this study, we identified and characterized the rbcL and psbA genes and the bicistronic operon atpB-atpE from Tetraselmis suecica based on the transcriptional analysis of Chlamydomonas reinhardtii (Blowers et al. 1990). It has already been demonstrated that the promoters and UTRs of these genes could be used to generate high expression levels of heterologous proteins in the chloroplast (Mayfield et al. 2003). Previous studies have shown that chloroplast promoters resemble bacterial σ70 promoters (Rasala et al. 2011). The motif analysis of the described genes has the −10 element that is well conserved among bacterial σ70 promoters (Fig. 2).
Transgene cassettes are delivered to the chloroplast by particle bombardment and integrated into the plastome through homologous recombination (Boynton et al. 1988). Therefore, chloroplast plasmids are designed with regions of homology on both sides of the transgene cassette directing gene cassette integration by homologous recombination. We have identified a 23S-5S rRNA sequence and the psbA gene lying on the complementary strand for use as flanking regions for directing the expression cassette to the desired insertion sites. These regions have no special features other than that they are homologous to the chosen target site and can be directed without modifying the sequence regions. The optimal sizes of these flanking regions are generally 1 kb, and we have achieved a similar size with the sequences used. Moreover, the chloroplast genomes of algae and land plants generally have two identical copies of a large inverted repeat (IR) sequence, which usually include coding regions of rRNA and tRNA genes and some ORFs (Turmel et al. 2017). We assume that the flanking regions chosen, 23S-5S and psbA, are in the inverted repeat regions (IR) of the Tetraselmis suecica plastid genome; this is desirable because the integration in one IR is followed by the phenomenon of copy correction, which duplicates the introduced transgene into the other IR as well (Beeramganti et al. 2012).
Natural bidirectional promoters and divergent transcription have been characterized in all model organisms (Vogl et al. 2018). Transcription at a bidirectional promoter involves pairs of protein-coding transcripts. Nonetheless, this type of arrangement offers an attractive approach for designing selection-expression vectors to constitutively express a protein of interest and a reporter protein arising from divergent transcription (Velten and Schell 1985).
Bidirectional promoters are extremely useful for efficient gene coexpression. However, despite the potential of bidirectional promoters in plant research, studies on bidirectional promoters in plants have lagged behind those in microorganisms, animals, and humans (Liu et al. 2018). In microalgae, metabolic engineering of multigene expression through a bidirectional promoter has been utilized in Nannochloropsis species (Poliner et al. 2018). Here, we have demonstrated a divergent dual promoter for multigene expression engineering of Tetraselmis suecica as a transgenic strategy to allow the compact assembly of multiple genes.
We tested the 5′rbcL/5′atpB bidirectional promoter driving the coexpression of chloramphenicol and TurboGFP genes in the chloroplast of Tetraselmis suecica. Marker and fluorescent proteins have become essential tools for designing microalgal constructs. Because of the natural chloroplast autofluorescence by the presence of chlorophyll and other accessory pigments, the selection of the fluorescent protein is critical for visualizing its expression (Kristoffersen et al. 2016, Di Lena et al. 2019).
Flow cytometry is able to simultaneously measure autofluorescence and fluorescence of single cells, and regardless of the autofluorescence, we were able to distinguish between the intensities emitted by wild-type Tetraselmis suecica and transformed T. suecica cell populations growing in the presence of chloramphenicol medium (Fig. 3).
The tGFP used as a reporter gene in Tetraselmis suecica clones have achieved high levels of fluorescence, suggesting a successful codon optimization for such gene. Codon usage is the most important determinant of transgene expression efficiency in Chlamydomonas reinhardtii (Barahimipour et al. 2015) along with the availability of strong promoters and UTRs (Doron et al. 2016). Confocal microscopy images revealed that tGFP expression is strongly detected without any interference chlorophyll autofluorescence (Fig 4).
As previously mentioned, algae are presently emerging as an alternative system for the production of recombinant proteins. Chlamydomonas reinhardtti is the microalga most widely studied for a variety of biotechnological applications, including vaccine antigens, antibodies, and some industrial enzymes. However, Tetraselmis suecica could be as well visualized as good model for molecular manipulation and metabolic engineering. T. suecica will expand the biotechnological applications being used par example as an improved animal feed.
Conclusions
Currently, we have provided useful tools for the efficient optimization of gene expression in Tetraselmis suecica, opening up new possibilities for its biotechnological uses.
This study has established 5′rbcL/5′atpB as a potent natural bidirectional promoter of Tetraselmis suecica, raising its potential application in future gene manipulation attempts as biofactories or metabolic genetic engineering by efficiently and specifically driving multiple target genes.
This research was supported by a Postdoctoral fellowship PUCV to Carla L. Gutiérrez.
Authors’ Contributions
C. L. Gutiérrez designed the final expression vector with tGFP, the flow cytometry, and confocal microscopy assays. C. Muñoz did the preliminary cloning design expression vector. M. San Martín did the gene codon optimization, RT-PCR, and carry out the transformation process by biolistic. J. P. Cadoret designed preliminary experiments. V. Henríquez directed the project and wrote the manuscript with support from C. L. Gutiérrez. All the authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Statement of Informed Consent, Human/Animal Rights
No conflicts, informed consent, human or animal rights applicable.




