CO2 Enrichment Stimulates Dissolved Organic Carbon Release in Coral Reef Macroalgae
Abstract
Dissolved organic carbon (DOC) released by macroalgae is important in the context of coral reef degradation as it contributes to coral mortality by promoting bacterial metabolism on the coral surface. Using experimental carbon dioxide (CO2) manipulations in outdoor flow-through tanks, we found that seawater CO2 enrichment enhances daily net DOC release in a range of macroalgal species in the Great Barrier Reef (Australia). There was, however, large variability in DOC release among species, light and dark conditions, and CO2 exposure times. Under light conditions, DOC release in the red macroalga Amansia was 15 times higher under high CO2 conditions compared to ambient CO2, however, CO2 enhancement did not affect DOC production in the other species. Results from the night incubations were more consistent as three of the four species (Amansia, Lobophora, and Sargassum) enhanced DOC release when enriched with CO2. DOC fluxes shifted from production in the 1-d incubations to consumption in the 19-d experiment under light conditions, suggesting an important role of bacteria in DOC balances. The results suggest that rising CO2 (and ocean acidification) will continue to intensify space competition in favor of the macroalgae, potentially exacerbating reef degradation and ecological phase shifts from coral to macroalgal dominance.
Abbreviations
-
- CCM
-
- carbon concentration mechanisms
-
- DIC
-
- dissolved inorganic carbon
-
- DOC
-
- dissolved organic carbon
-
- GBR
-
- Great Barrier Reef
-
- HIRS
-
- Heron Island Research Station
-
- IPCC
-
- Intergovernmental Panel on Climate Change
-
- NBS
-
- US National Bureau of Standards
-
- NIST
-
- National Institute of Standards and Technology
-
- PTFE
-
- polytetrafluoroethylene
-
- RCP
-
- Representative Concentration Pathway
Coral reefs are under considerable threat due to human activities and are in serious decline (Smith et al. 2016, Hughes et al. 2017). A major threat to reefs is the global problem of ocean acidification. On one hand, ocean acidification reduces calcification rates of corals and other calcifying organisms potentially compromising the ability of reefs to maintain positive growth rates (Hoegh-Guldberg et al. 2007), and on the other hand, ocean acidification may strengthen space competition between corals and macroalgae in favor of the algae, further exacerbating ecosystem transitions from reefs dominated by corals and coralline algae to reefs dominated by fleshy macroalgae (Diaz-Pulido et al. 2011, Fabricius et al. 2011). However, the mechanisms by which space competition is enhanced under elevated CO2 are not clear. Therefore, addressing the effects of increased CO2 on DOC fluxes from macroalgae will provide insights into the ecological consequences of ocean acidification and macroalgal blooms in coral reefs.
Dissolved inorganic carbon (DIC; including carbon dioxide [CO2] and bicarbonate [HCO3−]) in seawater is converted into organic carbon via photosynthesis by primary producers such as macroalgae (or seaweeds). The organic carbon fixed by algal photosynthesis can be invested in new biomass formation (i.e., tissue growth), lost by respiration, accumulated in storage compounds, and can be released as dissolved or particulate organic carbon (DOC and POC, respectively) to the surrounding water (Carr et al. 1997, Hurd et al. 2014, Iniguez et al. 2016). DOC is consumed and respired by heterotrophic organisms (e.g., bacteria) and as a product of this metabolism, the organic carbon is then returned to the environment in the form of CO2. Sponges are also important in recycling DOC in benthic environments via the “sponge loop” (De Goeij et al. 2013). The balance between DOC production (mainly by algae) and consumption will determine the direction and magnitude of DOC fluxes in macroalgal communities (Haas et al. 2010b). A significant fraction of the anthropogenic CO2 emissions to the atmosphere dissolves in the surface ocean causing a re-equilibration of the seawater inorganic C system and an increase in DIC and H+. These changes cause a decline in pH (i.e., ocean acidification; IPCC 2014), which may not only affect calcifying organisms (Kroeker et al. 2013) but may also enhance macroalgal photosynthesis (Zou et al. 2011) and growth rates (Diaz-Pulido et al. 2007, Koch et al. 2013, Johnson et al. 2014, Hofmann et al. 2015). Furthermore, elevated CO2 has been shown to enhance DOC release by planktonic microalgae and Arctic macroalgae by increasing CO2 cellular uptake and/or altering the cellular carbon budget (Arrigo 2007, Riebesell et al. 2007, Iniguez et al. 2016, 2017). The release of DOC by macroalgae has been proposed as a mechanism to protect the photosynthetic apparatus from an overload of products that cannot be used in growth or stored, thus maintaining the metabolic integrity of the cell (Gordillo et al. 2001). Under nutrient-limiting and high CO2 conditions, the release of DOC by algae is proposed to act as a valve balancing C:N ratios (Gordillo et al. 2001), although it has also been suggested that some unicellular algae and cyanobacteria may leak excess CO2 from intracellular pools as a potential mechanism to keep carbon uptake pathways energetically efficient (Raven and Beardall 2016). However, the role elevated CO2 on DOC fluxes by tropical benthic macroalgae has not been investigated yet.
DOC release by benthic primary producers has gained considerable attention due to the role of DOC in the marine carbon cycle (Barrón et al. 2014, Watanabe and Kuwae 2015) and as a potential driver of coral reef decline (Kline et al. 2006, Smith et al. 2006, Haas et al. 2009, Pawlik et al. 2016). Estimates of net DOC fluxes on coral reefs show that rates of DOC release by macroalgae are higher than those from corals (Wild et al. 2010). It has been shown that DOC concentrations underneath macroalgal canopies are around 30% higher than those above their canopies in inshore reefs of the Great Barrier Reef (Hauri et al. 2010). On the other hand, a recent study showed a negative correlation between macroalgal cover and DOC concentrations in reefs (Haas et al. 2016). Sponges preferentially assimilate algal-derived over coral-derived DOC, however, the algal-derived DOC assimilated by the sponges is released as particulate sponge detritus at a higher rate compared to coral-derived DOC (Rix et al. 2017). These studies suggest that DOC released by macroalgae play important roles in the carbon balance on tropical reefs. Furthermore, DOC release by macroalgae has been identified as a mechanism by which macroalgae damage corals when competing for space, via enhanced bacterial metabolism on the coral surface and promoting coral tissue mortality via hypoxic conditions (Smith et al. 2006, Haas et al. 2010a, Barott and Rohwer 2012, Nelson et al. 2013). These studies reveal the important role of macroalgal DOC in coral reef biogeochemistry and ecology. Despite this importance, it remains unclear whether elevated CO2 may enhance DOC release by benthic macroalgae, further contributing to coral reef decline.
Release of DOC by macroalgae depends on light availability (Haas et al. 2010b, Mueller et al. 2014) as photosynthesis is the process responsible for the uptake of inorganic carbon. Under light conditions, DOC released by macroalgae may contribute up to 39% and 27% of the gross primary production of isolated individuals (Khailov and Burlakova 1969, Abdullah and Fredriksen 2004, Haas et al. 2013) and in situ macroalgal communities (Barrón et al. 2014), respectively. Net DOC release, however, also occurs under dark conditions (Brylinsky 1977, Haas et al. 2013, Barrón et al. 2014), suggesting other mechanisms of DOC release at night-time, in addition to those from recent photosynthesis (e.g., release of stored carbon). Consequently, elucidation of the effect of elevated CO2 on DOC release requires quantification of DOC fluxes both during the day and during night.
In this study, we tested whether seawater CO2 enrichment affects net DOC fluxes of coral reef macroalgae and whether these fluxes vary under light and dark conditions using an outdoor experimental system in the Great Barrier Reef, Australia (GBR). Since the duration of exposure to CO2 may affect macroalgal DOC fluxes due to potential down-regulation processes, as shown for macroalgal photosynthesis (Zou et al. 2011), we repeated the experiments using short-term (1 d) and long-term (19 d) CO2 exposure times. Because elevated concentrations of dissolved CO2 in seawater affect the energetic and carbon budgets of the algal cells (Iniguez et al. 2016), we hypothesize that CO2 enrichment will enhance net DOC release rates and that this outcome will be sustained in the long-term experiment. Lastly, since different macroalgal species have varied strategies for inorganic carbon acquisition (Raven and Beardall 2003, Cornwall et al. 2015, Diaz-Pulido et al. 2016), we hypothesize that the responses of DOC fluxes to varying CO2 concentrations will differ among macroalgal species. To do this we used a range of macroalgal taxa from different phyla: Chlorophyta (green, Chlorodesmis fastigiata), Ochrophyta (Phaeophyceae, brown, Lobophora sp. and Sargassum sp.), and Rhodophyta (red, Amansia glomerata).
Materials and Methods
General approach and species collection
To test whether elevated CO2 affects macroalgal DOC fluxes, we incubated thalli of macroalgae under varying CO2 levels and measured the changes in DOC concentrations in an outdoor flow-through aquarium system in Heron Island Research Station (HIRS, southern Great Barrier Reef [GBR], Australia, 23°26′32.3″ S, 151°54′45.9″ E). We exposed the macroalgae to two CO2 exposure times, 1 d (short term) and 19 d (long term), and for each exposure time, macroalgae were incubated during 3–4 hours under light and dark conditions to estimate the effect of CO2 on light and dark net DOC fluxes, as well as daily fluxes (see details below).
We collected thalli of the fleshy macroalgae Chlorodesmis fastigiata, Lobophora cf. variegata, and Sargassum sp. from the shallow (2–3 m depth) reef flat, and Amansia glomerata from the upper reef slope (4–6 m) of Heron Island reef (Coral Gardens). Whole individuals (including their holdfasts) were carefully removed from the reef and special care was taken to avoid tissue damage which could have resulted in the release of intracellular DOC. Experimental specimens were all visibly clean of epiphytes at the time of collection and during the experimental period. These taxa were selected because they are common and may be locally abundant in the study area (Bender et al. 2012), have been implicated in coral mortality and reef decline (Rasher and Hay 2010), and used in previous ocean acidification experiments (Diaz-Pulido et al. 2011). Following collection, macroalgae were transported to the HIRS outdoor flow-through holding tanks (with similar light and temperature conditions as the experimental tanks) and acclimated for 48 h prior to experimentation. The short-term experiment was conducted with the four seaweed species mentioned above, while the long-term (19 d) experiment was conducted with Lobophora and Chlorodesmis. Both experiments were carried out during the Austral autumn (April 2013). Dissolved inorganic nitrogen and phosphate concentrations measured in tank experiments in the outdoor seawater facilities in HIRS varied between 0.49–0.52 NH4 μmol · L−1 and 0.13–0.26 PO4 μmol · L−1, respectively (Bender-Champ et al. 2017).
Seawater CO2 manipulations
Seawater CO2 concentrations were manipulated following protocols previously described (Anthony et al. 2008, Diaz-Pulido et al. 2011). In summary, pure (analytic grade) CO2 was bubbled into 200 L mixing sumps that were continuously fed with water from the reef flat. CO2 levels were controlled by a computerized aquarium system (Aquatronica; AEB Technologies, Cavriago, Italy) connected to solenoid valves, which regulated the amount of CO2 injected into the sumps based on pH measurements (InPro4501VP electrodes; Mettler-Toledo, Urdorf, Switzerland). Seawater temperature (measured with an Aquatronica temperature sensor ACQ001S connected to the Aquatronica control aquarium system) and pH measurements were recorded every 30 min during the course of the experiments and calibration was linearly performed using three NIST-certified (i.e., NBS) pH buffers (Mettler Toledo). We used three seawater CO2 concentrations following projections by the Intergovernmental Panel on Climate Change (IPCC) for the RCP8.5 CO2 stabilization scenario (IPCC 2014). The levels included: ambient control (present ambient CO2 level without addition of CO2: 380 ppm [average ±SE pH: 8.02 ± 0.01]); medium CO2 level to represent projected values for year 2,050: 540 ppm (pH 7.87 ± 0.02); and high CO2 level representing concentrations projected for 2,100: 936 ppm (pH 7.67 ± 0.02; Table 1). For the long-term experiment, macroalgae were exposed to the three CO2 levels, but due to logistic limitations, only two CO2 levels (ambient and high) were applied in the short-term incubations. There was one mixing sump per CO2 level, each containing a pump for water circulation. Ambient and CO2-treated seawater was continuously pumped from the sumps to 20 L experimental plastic tanks placed on an experimental table at a flow rate of 1.8 L · min−1. Seawater temperature in the tanks averaged 24.8 ± 0.04°C (mean ± SE) during the duration of the experiment. Tanks had a small powerhead for water circulation. Shading screens were placed over the experimental area to reduce light intensity and heating, approximating field conditions. Irradiance at 13:30 h averaged 356 ± 78 (SE) μmol photons · m−2 · s−1 (n = 8) and light measurements were obtained using a LI-1400 data logger, LI-192SA sensor (LI-COR, Lincoln, NE, USA). Three replicate 20 L tanks (n = 3) were used for each combination of CO2, macroalgae, and experimental duration treatment levels, each tank containing one individual macroalgal thallus which was placed at the bottom of each tank following the 48 h acclimation period. Species of macroalgae were not intermixed in each tank but kept separate. Tanks were cleaned regularly with a cloth to remove build-up of filamentous algae and sediments.
| CO2 Level | pH | pCO2 (μatm) | pCO2 (μatm)-CO2Sys | Temp (°C) | TA (μmol · kg−1) | TCO2 (mmol · kg SW−1) | HCO3− (μmol · kg−1) | CO32− (μmol · kg−1) | Ω Arag |
|---|---|---|---|---|---|---|---|---|---|
| Ambient | 8.02 ± 0.03 | 380 | 419.57 ± 31.63 | 23.77 ± 0.18 | 2,274.40 ± 9.42 | 1,993.40 ± 17.77 | 1,780.13 ± 27.72 | 200.93 ± 11.92 | 3.18 ± 0.19 |
| Medium | 7.95 ± 0.01 | 540 | 499.18 ± 18.02 | 23.77 ± 0.18 | 2,271.40 ± 9.69 | 2,031.83 ± 5.27 | 1,842.90 ± 7.27 | 174.30 ± 5.53 | 2.76 ± 0.09 |
| High | 7.72 ± 0.02 | 936 | 949.20 ± 58.07 | 23.77 ± 0.18 | 2,275.49 ± 11.10 | 2,142.13 ± 15.81 | 2,003.53 ± 18.62 | 110.78 ± 5.84 | 1.75 ± 0.09 |
- Values are means (n = 12, ±SE). pH, pHNBS; pCO2, carbon dioxide partial pressure, target value; pCO2, CO2Sys: calculated partial pressure of CO2 determined based on total alkalinity and pH (both calculated using a Mettler-Toledo T50 automated titrator) measurements using Microsoft Excel CO2SYS version 2.1 (Lewis and Wallace 1998). Temp, seawater temperature; TA, total alkalinity; TCO2, total dissolved inorganic carbon-DIC; HCO3−, bicarbonate; CO32−, carbonate; Ω Arag, saturation state of seawater with respect to aragonite. CO2SYS calculations used temperature = 23.77°C, salinity = 34.46, and assumed pressure, Total P, and Total Si = 0.
Sampling and fluxes of dissolved organic carbon (DOC)
Short-term experiment (1 day)
Following the 48 h acclimation period macroalgae were exposed to the different CO2 levels in the experimental tanks for 24 h. At the end of that time, macroalgae were incubated inside sealed plastic, polyethylene ziplock bags placed inside the experimental tanks for a period of 3–4 h in their respective CO2 treatments.
Long-term experiment (19 day)
A different set of individuals were used for the long-term experiment, which involved exposure of macroalgae to the different CO2 levels in the experimental tanks for 19 d. At the end of this period, following the same protocol as per the short-term experiment, macroalgae were incubated inside plastic bags inside the experimental tanks for 3–4 h in their respective CO2 treatments.
For both the short-term and long-term experiments, the incubation plastic bags had a sampling port for water sample collection (as described earlier; Barrón and Duarte 2009). Each bag was rinsed three times and filled with 1–2 L of fresh seawater from the specific sumps from each CO2 treatment level. Macroalgae were placed inside the bags and isolated from the surrounding water (of the experimental tank) by the zipped closed system. There was only one bag per tank. In addition to the three replicate bags (tanks) per macroalgal species and CO2 level, three bags filled only with seawater from each CO2 level were incubated in the same way as per the macroalgae and used as control for the incubations (i.e., plankton contribution). Because we were unable to use filtered or sterilized seawater for the incubations or remove microbial epiphytic communities from the experimental macroalgae, DOC release rates estimated here must be considered net DOC fluxes. The use of ziplock bags for assessing DOC release is common practice in DOC work (e.g., Barrón et al. 2014), however, due to reduced water flow in the bags and potential issues with boundary layers, macroalgal metabolism (e.g., Carpenter et al. 1991) and DOC release may be reduced, therefore our results may be conservative estimates. Although we did not measure carbonate chemistry parameters inside the ziplock bags during the incubation period, the high water volume/algal mass ratio minimized any potential influence of algae on seawater pH during incubation (cf. Cohen et al. 2017).
Macroalgae and incubation controls were incubated for 3–4 h under light and dark conditions. Water samples were collected through the sampling port using acid-washed (4% HCl) syringes rinsed with water from the inside of the bag at the beginning of each incubation to determine initial DOC concentration. The light incubation began at 13:00–14:00 h and finished just before sunset (irradiance at 13:30 h was 356 [±78 SE] μmol photons · m−2 · s−1]), while the night incubation started right after sunset and finished 4 h later. Water samples were filtered through PTFE 0.45 μm syringe filters which were tested for DOC leaching using MilliQ water. DOC leakage was insignificant (<0.1 mg C · L−1 [or 8.33 μmol DOC · L−1]) similar to filters tested by Khan and Subramania-Pillai (2007). The first volume of filtered water was used to rinse the filter and the vials. Twenty milliliter of water was collected in acid-washed glass vials and Teflon cups and four drops of 85% orthophosphoric acid were added to preserve the samples for further analyses.
DOC samples were analyzed using a high-temperature catalytic oxidation technique in a TOC-VCSH Shimadzu and a Thermalox. The instruments were calibrated using potassium hydrogen phthalate. DOC standards (Consensus Reference Materials, CRM) of deep seawater reference water (DSR, Florida Strait at 700 m, 41–44 μmol DOC · L−1, provided by D. A. Hansell and W. Chen, University of Miami, FL, USA) were used to estimate the accuracy of the measurements. CRM standards were tested for DOC concentrations by the Griffith University laboratories (Smart Water Research Centre) and levels were 44 (±3 SE) μmol DOC · L−1 during the analytical period. At the end of the incubation, the volume of seawater for each bag was measured for further calculations. To factor in the contribution of the plankton from the DOC fluxes, we subtracted the average changes in DOC concentrations (over the hours of the incubation) of the incubation controls (without macroalgae) from the changes in DOC concentration over the incubation period for each macroalga. Net DOC release rates were based on the rate of change in concentration between initial and final samples and were normalized to time of the incubation, specific volume, and macroalgal biomass. Daily DOC fluxes (in μmol C · g−1 · d−1) were calculated combining daylight and nightlight hours multiplied by hourly DOC fluxes under light and dark conditions, respectively, using the following equation: Daily DOC (in μmol C · g−1 · d−1) = DOC release (in μmol C · g−1 · h−1) under light × 11.233 h + DOC release (in μmol C · g−1 · h−1) under dark × 12.767 h. Positive rates of net DOC production indicate DOC release, while negative values indicate net DOC uptake in the system. At the end of the experiment, macroalgae were dried at 60°C for 24 h and the dry weight was estimated using a precision scale. Average macroalgal dry weight (±SE) for Chlorodesmis, Lobophora, Amansia, and Sargassum was 1.79 ± 0.32 g, 0.18 ± 0.01 g, 2.44 ± 0.44 g, and 4.18 ± 0.23 g, respectively. Initial DOC concentrations in control incubations (i.e., plankton contribution) averaged 84.4 ± 9 SE μmol C · L−1, values slightly higher than those reported in the GBR (e.g., Heron Is.: mean 57 μmol C · L−1 [Diaz-Pulido et al. 2011], and other reefs: mean 66 μmol C · L−1 [Schaffelke et al. 2012]).
Carbonate chemistry analyses
Water samples for carbonate chemistry analyses were collected in duplicate from the experimental sumps every 4 h during a 24 h period. Total alkalinity was estimated using a T50 Titrator (Mettler-Toledo; Dickson et al. 2003), calibrated using “Reference materials for CO2 measurements” obtained from Prof Dickson laboratory, University of California, Scripps. The titrator pH sensor was calibrated using 4.01 and 7.0 pH NIST-certified buffers. pH and temperature values of the water samples were used to constrain the carbonate chemistry parameters using CO2SYS software (Lewis and Wallace 1998). Salinity of 34.46 was used for the calculations (Table 1).
Statistical analyses
To test the effects of CO2 treatments and macroalgal species on DOC fluxes, we used a 2-way ANOVA, with treatments (CO2 and macroalgal species) as fixed factors, and tanks (macroalgal thalli) as replicates (n = 3), followed by post hoc Tukey tests. This analysis was used for the short- and long-term CO2 exposure time experiments, and for the light, dark, and daily data sets. When the CO2 × species interaction term was significant, one-way ANOVAs (or t-test) were conducted within each treatment combination (i.e., the means of one factor were compared separately at each level of the other factor; Underwood 1997). To investigate whether DOC release rates varied between light and dark incubations across the different CO2 levels, we conducted a one-way repeated-measure ANOVA for each macroalgal species and each CO2 exposure time (short and long term). The repeated factor was the light condition: light and darkness. To examine whether the time of exposure (short term vs. long term) to elevated CO2 affected the rates of macroalgal DOC release among macroalgal species, a three-way ANOVA was conducted (fixed factors: CO2, species, and experimental duration), and when significant interactions occurred between factors, two-way ANOVA were carried out. This analysis was repeated for the daily fluxes, and light and dark conditions. We acknowledge the relatively low sample size, but inclusion of several experimental factors in the design and logistic limitations did not allow using higher sample sizes. Data were tested for normality and homogeneity of variances using a Shapiro–Wilk test and Cochran's test, respectively. Minor transgressions to data normality were accepted given ANOVA robustness to non-normal data (Underwood 1997).
Results
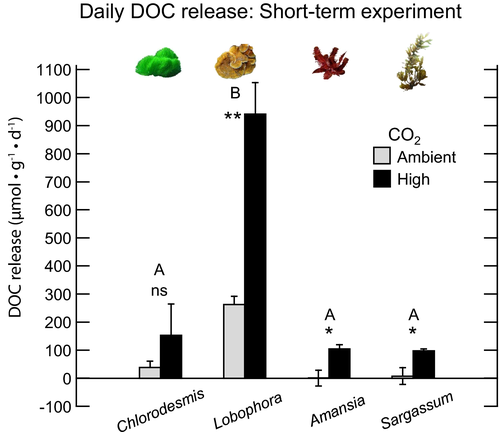
Effect of CO2 and macroalgal species on DOC fluxes in the short-term (1-d) experiment
Daily rates (DOC light +DOC night)
The effect of CO2 enrichment on daily DOC release rates depended on the species of macroalgae examined (significant interaction between treatments [Algae × CO2] in the two-way ANOVA: F3,16 = 20.3, P < 0.001; Table 2; Fig. 1). Since the Algae × CO2 interaction was significant, we subsequently conducted one-way ANOVAs within treatment combinations. Elevated CO2 concentrations significantly increased the daily DOC release rate for the macroalgae Lobophora (one-way ANOVA: F1,4 = 59.29, P = 0.002), Sargassum (one-way ANOVA: F1,4 = 15.07, P = 0.018), and Amansia (one-way ANOVA: F1,4 = 18.05, P = 0.013). A similar trend was observed for Chlorodesmis, but the difference was not significant (one-way ANOVA: F1,4 = 1.78, P = 0.253). Daily DOC rates were significantly higher for the brown macroalgae Lobophora than for the other macroalgal species under both CO2 levels (ambient, one-way ANOVA: F3,8 = 34.91, P < 0.001; Tukey HSD: P < 0.001; high, one-way ANOVA: F3,8 = 46.78, P < 0.001; Tukey HSD: P < 0.001, Table 2). Since the daily DOC release is the difference between rates of DOC release under light and dark conditions, and light and dark release rates were significantly different (see below for two-way repeated-measure ANOVA), here we present the results by light and dark conditions separately.
| Source of variation | df | MS | F | P | Tukey's test/Conclusion |
|---|---|---|---|---|---|
| Daily | |||||
| Algae (A) | 3 | 432,060.3 | 70.6 | Ambient: <0.001 | Lob>Chl=Sar=Ama |
| High: <0.001 | Lob>Chl=Sar=Ama | ||||
| CO2 | 1 | 365,074.3 | 59.7 | Chl: 0.253 | ns |
| Lob: 0.002 | High>Ambient | ||||
| Sar: 0.018 | High>Ambient | ||||
| Ama: 0.013 | High>Ambient | ||||
| A × CO2 | 3 | 124,299.3 | 20.3 | <0.001 | |
| Error | 16 | 6,116.7 | |||
| Light | |||||
| Algae (A) | 3 | 112.5 | 5.7 | 0.008 | Lob>Sar=Ama; Lob=Chl; Chl=Ama=Sar |
| CO2 | 1 | 47.1 | 2.4 | 0.142 | ns |
| A × CO2 | 3 | 8.7 | 0.4 | 0.728 | ns |
| Error | 16 | 19.8 | |||
| Dark | |||||
| Algae (A) | 3 | 1,899.2 | 87.9 | Ambient: 0.001 | Lob>Chl=Sar=Ama |
| High: <0.001 | Lob>Chl=Sar=Ama | ||||
| CO2 | 1 | 1,704.7 | 78.9 | Chl: 0.149 | ns |
| Lob: 0.001 | High>Ambient | ||||
| Sar: 0.054 | High>Ambient | ||||
| Ama: 0.007 | High>Ambient | ||||
| A × CO2 | 3 | 904.5 | 41.9 | <0.001 | |
| Error | 16 | 21.6 | |||
- Analyses are presented by daily rates, and light and dark incubations. Lob, Lobophora; Chl, Chlorodesmis; Sar, Sargassum; Ama, Amansia; ns, not significant.
Incubations under light conditions
Under light conditions, the net DOC release varied among macroalgal species (two-way ANOVA: F3,16 = 5.69, P = 0.008), with significantly higher values observed for Lobophora than for Amansia (Tukey HSD: P = 0.022) and Sargassum (Tukey HSD: P = 0.011). Although the main effect of CO2 on net DOC release rates was not significant in the short-term incubation experiment (two-way ANOVA: F1,16 = 2.38, P = 0.142; Fig. 2a, Table 2), one-way ANOVAs for each individual macroalgal species showed that CO2 did significantly increase DOC release in the red macroalga Amansia (from −0.22 [±0.93 SE] μmol C · g−1 · h−1 to 3.08 [±0.53 SE] μmol C · g−1 · h−1, increase that is 15 times higher than the ambient, control CO2 conditions; one-way ANOVA: F1,4 = 9.49, P = 0.037; Fig. 2a).
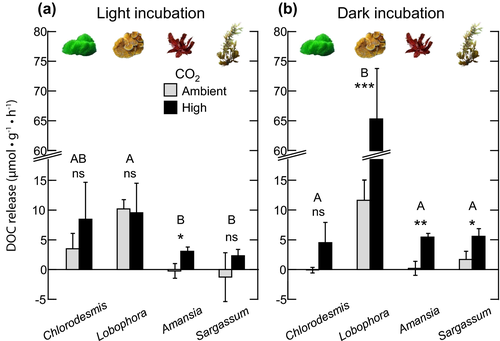
Incubations under dark conditions
Under dark conditions, elevated CO2 enhanced DOC release, but this effect depended on the species of macroalga examined (significant interaction Algae*CO2, two-way ANOVA: F3,16 = 41.88, P < 0.001; Table 2, Fig. 2b). Net DOC release increased from 11.6 μmol C · g−1 · h−1 to 65.3 μmol C · g−1 · h−1 and from 1.7 μmol C · g−1 · h−1 to 5.6 C · g−1 · h−1 in the high CO2 level compared to ambient CO2 in the brown macroalgae Lobophora and Sargassum, respectively. For the red alga Amansia, the effect of CO2 on net DOC release was stronger than for the other macroalgae, rising from 0.2 (±0.9 SE) μmol C · g−1 · h−1 under ambient CO2 to 5.5 (±0.5 SE) μmol C g−1 · h−1 under high CO2, increase that is 24 times higher than the ambient, control CO2 condition (one-way ANOVA: F1,4 = 26.59, P = 0.007; Table 2, Fig. 2b). DOC release in the green macroalga Chlorodesmis was not significantly different across CO2 levels, despite being higher under elevated CO2. DOC release also varied among macroalgal species under both CO2 treatment levels (ambient, one-way ANOVA: F3,8 = 14.11, P = 0.001; high, one-way ANOVA: F3,8 = 74.04, P < 0.001; Table 2, Fig. 2b). Lobophora DOC release was significantly higher than that of the other species, for both ambient and elevated CO2 levels (Fig. 2b).
Rates of macroalgal DOC release differed between light and dark conditions across the different CO2 levels. DOC rates were higher under dark compared to light conditions for two of the four species examined (one-way repeated-measures ANOVA: Lobophora: F1,4 = 42.44, P = 0.003; Amansia: F1,4 = 40.76, P = 0.003; Fig. 2)), but only under elevated CO2. Lobophora DOC release under dark was up to 6 times higher relative to light incubations, suggesting that under elevated CO2, stored organic carbon compounds are more rapidly metabolized and the DOC subsequently released into the water column than under ambient conditions.
Effect of CO2 and macroalgal species on DOC fluxes in the long-term (19-d) experiment
Daily rates
The macroalgal DOC release in the long-term exposure experiment varied across CO2 and macroalgal species treatments (overall results of the two-way ANOVA are shown in Table 3 and Fig. 3). Since there was considerable variability in responses across the two macroalgal species, we conducted one-way ANOVAs by macroalgal species. For Lobophora, higher DOC release rates occurred under the high CO2 treatment level (one-way ANOVA: F2,6 = 6.64, P = 0.030), while for Chlorodesmis, the difference across CO2 levels was not significant. Daily net DOC release was significantly higher in Chlorodesmis compared to Lobophora (two-way ANOVA: F1,12 = 13.87, P = 0.003; Table 3, Fig. 3).
| Source of variation | df | MS | F | P | Tukey's test/conclusion |
|---|---|---|---|---|---|
| Daily | |||||
| Algae (A) | 1 | 1,277,212.6 | 13.9 | 0.003 | Chl>Lob |
| CO2 | 2 | 407,281.1 | 4.4 | 0.036 | High>Medium |
| A × CO2 | 2 | 203,330.9 | 2.2 | 0.153 | ns |
| Error | 12 | 92,118.9 | |||
| Light | |||||
| Alga (A) | 1 | 24,693.8 | 26.8 | <0.001 | Chl>Lob |
| CO2 | 2 | 1,319.3 | 1.4 | 0.277 | ns |
| A × CO2 | 2 | 837.2 | 0.9 | 0.429 | ns |
| Error | 12 | 921.4 | |||
| Dark | |||||
| Algae (A) | 1 | 2,474.3 | 6.4 | Ambient: 0.652 | ns |
| Medium: 0.312 | ns | ||||
| High: 0.020 | Lob>Chl | ||||
| CO2 | 2 | 331.2 | 0.9 | Chl: 0.298 | ns |
| Lob: 0.001 | High>Med=Ambient | ||||
| A × CO2 | 2 | 2,675.9 | 6.9 | 0.010 | |
| Error | 12 | 386.8 | |||
- Analyses are presented by daily rates, and light and dark incubations. MS, mean square; Lob, Lobophora; Chl, Chlorodesmis; ns, not significant.
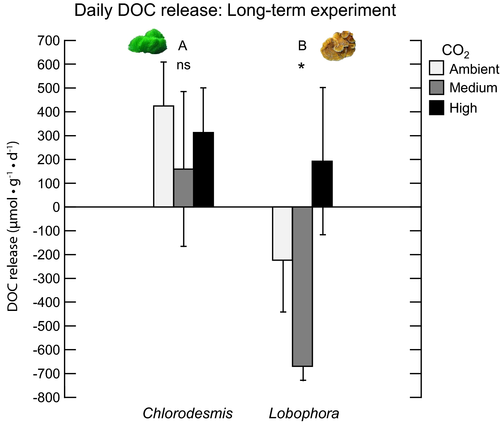
Incubations under light conditions
In the long-term experiment, the overall two-way ANOVA showed that CO2 manipulations did not affect net DOC release (two-way ANOVA: F2,12 = 1.43, P = 0.277; Table 3, Fig. 4a), however, there was a significant difference in the direction of DOC release between the two macroalgae species examined (two-way ANOVA: F1,12 = 26.8, P = <0.001; Table 3, Fig. 4a). Consistent with the short-term experiment, Chlorodesmis showed net DOC release (positive values) under all CO2 levels under light conditions. However, in contrast to the short-term experiment, Lobophora had negative net DOC release rates (i.e., uptake) across all CO2 levels under light incubations (Fig. 4a).
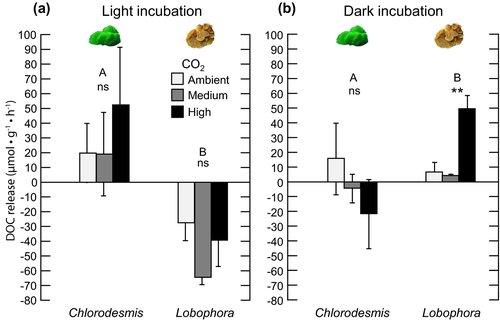
Incubations under dark conditions
In contrast to the light incubations, trials under darkness showed that the effect of CO2 on net DOC release depended on the macroalgal species examined (significant treatment interaction, two-way ANOVA: F2,12 = 9.92, P = 0.010; Table 3, Fig. 4b). High CO2 significantly increased DOC release rates in Lobophora relative to ambient and medium CO2 (one-way ANOVA: F2,6 = 28.28, P = 0.001; Table 3). DOC increased by 648% under high CO2 compared to ambient CO2 (Fig. 4b). Conversely, for Chlorodesmis, elevated CO2 levels did not significantly affect DOC under dark conditions (one-way ANOVA: F2,6 = 1.49, P = 0.298, Table 3), although there was a trend toward negative values (increased uptake) with increased levels of CO2 (Fig. 4b).
The light treatment (light vs. dark) had a significant effect on net DOC fluxes in Lobophora at all CO2 treatment levels (one-way repeated measures ANOVA, F1,6 = 418.29, P < 0.001; Fig. 4), with higher values under dark compared to light incubations. There was a clear shift from net DOC uptake under light conditions to net DOC release under darkness.
Effect of CO2 exposure time on net DOC fluxes
A three-way ANOVA was conducted to explore whether the duration of the experiment (i.e., exposure time to elevated CO2: short term vs. long term), macroalgal species, and CO2 manipulations altered DOC release rates. The analyses showed complex interactions between all factors across the two types of light incubations (light and dark conditions) and daily rates (Table S1 in the Supporting Information). These complex interactions, however, were governed by the type of algal species, that is, the effects of experimental duration and CO2 enrichment on DOC release rates largely depended on the macroalgal species considered (significant interactions between Algae × Exposure time for daily rates [three-way ANOVA: F1,16 = 23.21, P < 0.001] and light incubation [three-way ANOVA, F1,16 = 15.2, P = 0.001]; Table S1). Under light conditions, DOC release by Lobophora was much higher in the short-term than the long-term experiment (two-way ANOVA: F1,8 = 26.46, P = 0.001; Table S1), shifting from production (positive DOC values) under short-term exposure (Fig. 2a) to uptake (negative DOC values) under the long-term experiment (Fig. 4a). A similar trend of declining DOC release rates was measured for Lobophora daily DOC balance (two-way ANOVA: F1,8 = 16.98, P = 0.003, Table S1) and under dark conditions (although only marginally significant, two-way ANOVA: F1,8 = 3.66, P = 0.092; Table S1). Experimental duration had the opposite effect on Chlorodesmis, as the daily DOC rates were higher in the long- than in the short-term exposure experiment (two-way ANOVA: F1,8 = 6.37, P = 0.036; Table S1). Under the light and dark conditions, however, there was no clear effect of experimental duration on net DOC production for Chlorodesmis. The experimental duration did not alter the type of positive response of DOC to CO2 manipulations in Lobophora, as the direction of the response to CO2 was consistent in both short- and long-term exposure treatments for light and dark incubations, and daily rates. However, the shift from net DOC release to net DOC uptake in Lobophora in the long-term experiment (particularly under light conditions and daily rates) suggests an important role of the exposure duration on macroalgal DOC dynamics.
Discussion
Understanding the impacts of climate change on marine ecosystems requires knowledge not only of their direct effects on the status of the organisms (e.g., growth, health, etc.) but also on the functioning of key ecosystem processes. Global environmental problems such as ocean acidification may favor macroalgal-derived DOC production (e.g., as suggested for phytoplankton [Arrigo 2007] and Arctic seaweeds [Iniguez et al. 2016]) potentially influencing the carbon cycle (De Goeij et al. 2013, Rix et al. 2017) and contributing to coral decline by strengthening macroalgal overgrowth of corals. Hence, elucidating the responses of DOC release by macroalgae to elevated CO2 is critical to assess the potential impacts of ocean acidification on reef ecosystems. Our results are first to suggest that CO2 enrichment enhances rates of DOC release in coral reef macroalgae (particularly at night), and that this trend is highly dependent on the species of macroalgae examined. These results may have implications for understanding the potential ramifications of elevated CO2 on carbon balances and coral health, and provide vital information about the drivers of DOC release in reefs, particularly those dominated by macroalgae.
Effects of CO2 on macroalgal DOC release
The enrichment of seawater CO2 increased the daily DOC production in most experimental macroalgae, particularly in the short-term experiment, suggesting an important role of CO2 supply in altering algal carbon metabolism. DOC release in the red macroalga Amansia increased by 15-fold when incubated under high CO2 and light conditions, and most macroalgae increased DOC release during darkness in response to elevated CO2. The increase in DOC release in the red macroalga during the day may be related to a direct effect of CO2 on the algal carbon balance with the excess of carbon formation exuded rapidly as DOC, potentially to release the excess of fixed carbon that cannot be converted into biomass (i.e., algal growth) or stored, potentially protecting algal cells from an overload of carbon products (Iniguez et al. 2016). Although we did not quantify macroalgal photosynthetic rates, the rapid response of DOC to the 1-day CO2 enrichment (Fig. 2a) suggests a direct use of CO2, possibly through direct diffusion of CO2 from the surrounding environment to the site of photosynthesis. It is well known that some red macroalgae, particularly from deeper environments (Cornwall et al. 2015), take up CO2 from the surrounding environment by simply using the gradient of CO2 concentration without requiring the activity of energy-demanding carbon concentration mechanisms (CCM; Kübler et al. 1999). Amansia is a species found at 4–8 m depth in our study site and it is likely that this trait favors the direct CO2 uptake in this macroalga compared to the shallower species. A recent study with two Arctic seaweeds showed a significant increase in DOC in response to CO2 enrichment, but found no effects on carbon fixation or gross photosynthesis (Iniguez et al. 2016), while a study with the tropical alga Amansia showed no effects of elevated CO2 on growth (Ho and Carpenter 2017). Therefore, it seems that for some macroalgal species, elevated CO2 may not enhance growth or photosynthetic rates, but is likely to enhance other metabolic processes such as DOC release, particularly when nutrients are limiting (Gordillo et al. 2001), suggesting significant shifts in cellular carbon balances, as proposed by Iniguez et al. (2016, and references therein). The rapid increase in DOC release by Amansia suggests this macroalga is a rapid responder to elevated CO2.
The brown macroalgae Lobophora and Sargassum also increased daily DOC production (mainly due to darkness production) in response to CO2 enrichment. Several studies have shown that elevated CO2 enhances brown macroalgal photosynthesis and growth (Zou et al. 2011, Johnson et al. 2014, Hofmann et al. 2015), suggesting some brown macroalgae are carbon limited under current ambient DIC concentrations. CCM are present in a number of species of brown macroalgae (Raven et al. 2012), including members of the family Sargassaceae as well as Lobophora variegata (Zou et al. 2003, Enríquez and Rodríguez-Román 2006), therefore, it would be expected that rates of photosynthesis and growth in these species to be affected only marginally under ocean acidification (as discussed by Cornwall et al. 2015, Diaz-Pulido et al. 2016). As for Amansia, we did not assess photosynthesis, yet, our experiment showed that brown macroalgae responded to CO2 enrichment by releasing high amounts of DOC (compared to incubations at ambient CO2), suggesting changes to carbon budgets and potential plasticity in the use of carbon sources in algal metabolism. Enhanced DOC release due to CO2 enrichment in our experimental red and brown macroalgae may also be a stress response caused by the rapid increase in CO2 in the short-term experiment, as has been observed in experiments increasing irradiance levels (Iniguez et al. 2017); a gradual increase in CO2 in future experiments over time may help to elucidate the generality of this response. However, increased DOC release in response to elevated CO2 is in line with phytoplankton (Arrigo 2007) and Arctic macroalgae experiments (Riebesell et al. 2007, 2013, Torstensson et al. 2015, Iniguez et al. 2016). In Riebesell's experiments (Riebesell et al. 2007, 2013) increased CO2 availability enhanced carbon uptake and C:N ratios by phytoplankton, but this uptake did not increase phytoplankton biomass because carbon was exuded as DOC at elevated CO2 levels. Increased macroalgal DOC in response to CO2 enrichment may have important implications for understanding the ecological dynamics and biogeochemical cycles as CO2 increases in the future.
Role of light availability in DOC release
Light availability played an important role in regulating DOC release rates in most macroalgae studied. The rates of net DOC release were higher under dark relative to light conditions in Lobophora and Amansia in the short-term, high CO2 experiment (repeated-measure ANOVAs). This trend of higher DOC under darkness is surprising since most studies have shown higher DOC production under light settings (Haas et al. 2010b, Mueller et al. 2014) given DOC release is related to light availability and photosynthetic activity. A likely explanation is that the organic carbon produced photosynthetically is metabolized mainly into carbohydrates (Haas and Wild 2010), which can be stored in the algal tissue, and subsequently released as DOC, for instance, during the night. Abdullah and Fredriksen (2004) studied net DOC fluxes of the kelp Laminaria hyperborea during an annual cycle and found that DOC production was higher during the night than during daylight, but only during the growing season. During this period of rapid growth in the kelp, the new photosynthetic organic carbon produced is invested in tissue growth during daylight, while the organic carbon is released as DOC to the surrounding environment during the night (Abdullah and Fredriksen 2004). Our experiment was conducted during the Australian autumn, a season of active macroalgal growth in the Great Barrier Reef (Price 1989, Diaz-Pulido and McCook 2005), and this could explain the higher net DOC fluxes in dark incubations. Elevated CO2 may have increased carbon storage capacity in the seaweeds (as documented for some Arctic species; Iniguez et al. 2016) and organic carbon was subsequently mobilized and released. Recent studies on cyanobacterial mats from Caribbean reefs showed high release of DOC during the night, and attributed this to anaerobic metabolism and degradation processes (Brocke et al. 2015). Although the health of our experimental macroalgae was not quantitatively assessed, they appeared healthy (no thallus decay recorded in any treatment) and we believe that our short incubation time (3–4 h) minimized potential organic matter degradation but this is still a potential explanation. Further studies are needed to determine the magnitude and longevity of DOC storage (and their quality) under CO2-enriched conditions, and to elucidate the importance of carbon storage in seaweeds and its relation to net DOC production under future CO2 scenarios.
Effect of CO2 exposure time on net DOC fluxes
A number of aspects can be highlighted from the experiment examining the role of duration of CO2 exposure in DOC release rates. First, the increased rates of DOC release (both daily and under dark) in Lobophora in response to CO2 enrichment was consistent in both short-term (1-d) and long-term (19-d) exposure experiments. While Lobophora (and/or the bacterial-associated community) takes up DOC in the light, the large dark release compensates for this, which leads to an overall increase in the daily DOC release. Increased DOC release under high CO2 implies that the direction of the DOC response to CO2 is independent of the period of exposure (i.e., the positive effect of CO2 enrichment on DOC release observed in the short-term assay persists over a long period of time), suggesting low down-regulation potential of DOC release under high CO2, as initially hypothesized and in line with photosynthetic responses in a brown seaweed (Zou et al. 2011). Second, although both exposure times showed similar directional response to CO2, the long-term duration experiment induced a shift in DOC fluxes toward net DOC uptake (i.e., negative flux) under light conditions in Lobophora. There are two potential reasons for this shift, which need not be mutually exclusive: a) Proliferation of epiphytic bacteria on the macroalgal thallus, which may rapidly consume the produced macroalgal DOC (Barott et al. 2011, Haas et al. 2011, Nelson et al. 2013). A possible tank/culturing effect is also likely, although tanks were cleaned regularly throughout the experimental period and DOC incubations were conducted within ziplock bags. b) Uptake of DOC by macroalgae which may have contributed to depletion of external DOC. Macroalgae are known to take up dissolved organic nutrients from the surrounding environment (Schaffelke 1999, Tyler et al. 2005, Vonk et al. 2008, Haas et al. 2013), while some microalgae can use glucose as carbon source (Morales-Sánchez et al. 2013), suggesting that macroalgae may also have the ability of DOC uptake. In contrast, for Chlorodesmis, DOC fluxes where positive in both duration assays, and the higher daily DOC rates observed in the long-term compared to the short-term exposure experiment may be related to reduced growth of epiphytic bacteria induced by the strong allelopathy documented for this alga (Rasher and Hay 2010, Bender et al. 2012). This higher DOC release in Chlorodesmis in the long-term exposure does not support a tank/culturing effect mentioned above. These results demonstrate that the effect of CO2 and the duration of exposure to CO2 on DOC release rates show complex interactions that strongly depend on the nature of the macroalgal species and further studies are needed to elucidate the cause of DOC shifts toward consumption under the long-term exposure treatment.
Ecological and biogeochemical implications
Our study may have implications for understanding competitive interactions between macroalgae and corals in the context of ocean acidification. While it has been documented that fleshy macroalgae, in particular brown macroalgae, can proliferate in natural habitats exposed to CO2 seeps (Hall-Spencer et al. 2008, Enochs et al. 2015, Linares et al. 2015) and outcompete reef-building corals in ocean acidification experiments (Diaz-Pulido et al. 2011), little is known about the mechanisms mediating these interactions under elevated CO2. Emerging evidence indicates that ocean acidification may enhance the potency of macroalgal allelopathy to reef corals (Del Monaco et al. 2017), although other mechanisms may also play a role in driving coral–macroalgal interactions under high CO2 (Del Monaco et al. 2017). DOC released by macroalgae may promote coral bleaching and death, via enhancing bacterial growth and respiration on the coral surface creating anoxic conditions, particularly at night time, causing opportunistic shifts in the coral-associated bacterial community and increased virulence gene expression, and altering microbial nitrogen fixation in corals (Smith et al. 2006, Vega Thurber et al. 2009, 2012, Barott and Rohwer 2012, Radecker et al. 2015). Here, we demonstrate that CO2 enrichment enhances macroalgal net DOC release especially during night-time and it is likely that increased macroalgal DOC may be contributing to macroalgal-induced coral mortality under elevated CO2 scenarios. This situation will aggravate other negative effects of elevated CO2 on reef ecosystems, such as reduced coral and coralline algae growth rates, reef accretion, and enhanced reef bioerosion (Hoegh-Guldberg et al. 2007). The consequences of rising CO2 on DOC release and competitive interactions, however, will depend not only on the macroalgal species involved (as the effect of CO2 on DOC is species specific; Figs. 1-4) but also on nutrient supply (Zaneveld et al. 2016), sedimentation (Gowan et al. 2014), reef hydrodynamics (Brown and Carpenter 2013, Jorissen et al. 2016), herbivory (Jompa and McCook 2002), and coral mortality (Diaz-Pulido et al. 2009), processes that affect macroalgal growth and biomass accumulation, and consequently may alter the competitive outcomes between corals and macroalgae (McCook et al. 2001, Roff and Mumby 2012). These processes and their interactions with macroalgal DOC (e.g., Pawlik et al. 2016) need to be considered to better understand the consequences of CO2 enrichment on coral algal dynamics.
Our results may also have ramifications for carbon budget dynamics in reef systems under ocean acidification. Macroalgal communities and seagrass meadows are known to export DOC to the open ocean (Barrón and Duarte 2009, Wada and Hama 2013, Barrón et al. 2014). Therefore, the increase in macroalgal DOC release in response to rising CO2 (Figs. 1-4) may suggest that reefs dominated by fleshy macroalgae will contribute to the increase in carbon exports to the adjacent open ocean (Bauer et al. 2013). On the other hand, some macroalgal dominated back reef environments show lower DOC concentrations relative to the adjacent open ocean, suggesting these systems actively remove DOC from reef waters (Nelson et al. 2011, Leichter et al. 2013), likely due to increased microbial metabolism (Haas et al. 2016). The balance between DOC sources (e.g., by macroalgae) and losses (e.g., by bacteria and sponges; De Goeij et al. 2013, Pawlik et al. 2016) will determine the contribution of macroalgal communities to the DOC pool in adjacent reef waters as ocean acidification intensifies into the future, however, this balance will largely depend on the nature of the macroalgal community. The increase in macroalgae-derived DOC shown here, and its documented effects on corals, as well as the potential changes in the carbon balance due to ocean acidification are likely to influence the ecology and functioning of coral reefs.
We thank Carlos del Monaco, Pat Gartrell, Alexandra Ordoñez, and Bonnie Lewis for help in the field and laboratory and Brian Fry for constructive criticism. This research was supported by an Australian Research Council Discovery grant (DP120101778) awarded to GD-P.




