Effects of Heat Waves and Light Deprivation on Giant Kelp Juveniles (Macrocystis pyrifera, Laminariales, Phaeophyceae)
Abstract
Due to climate change, the incidence of marine heat waves (MHWs) has increased, yet their effects on seaweeds are still not well understood. Adult sporophytes of Macrocystis pyrifera, the species forming the iconic giant kelp forests, can be negatively affected by thermal stress and associated environmental factors (e.g., nutrient depletion, light deprivation); however, little is known about the tolerance/vulnerability of juvenile sporophytes. Simultaneously to MHWs, juveniles can be subjected to light limitation for extended periods of time (days–weeks) due to factors causing turbidity, or even because of shading by understory canopy-forming seaweeds. This study evaluated the effects of a simulated MHW (24°C, 7 d) in combination (or not) with light deprivation, on the photosynthetic capacities, nutrient uptake, and tissue composition, as well as oxidative stress descriptors of M. pyrifera juvenile sporophytes (single blade stage, up to 20 cm length). Maximum quantum yield (Fv/Fm) decreased in juveniles under light at 24°C, likely reflecting some damage on the photosynthetic apparatus or dynamic photoinhibition; however, no other sign of physiological alteration was found in this treatment (i.e., pigments, nutrient reserves and uptake, oxidative stress). Photosynthetic capacities were maintained or even enhanced in plants under light deprivation, likely supported by photoacclimation (pigments increment); by contrast, nitrate uptake and internal storage of carbohydrates were strongly reduced, regardless of temperature. This study indicated that light limitation can be more detrimental to juvenile survival, and therefore recruitment success of M. pyrifera forests, than episodic thermal stress from MHWs.
Abbreviations
-
- D
-
- darkness
-
- ETR
-
- electron transport rate
-
- L
-
- Light
-
- MHW
-
- marine heat wave
-
- NPQ
-
- non-photochemical quenching
-
- RLC
-
- rapid light curve
In the framework of global climate change, a rise of seawater temperature (up to 3–4°C) is predicted by the end of this century (IPCC 2013), and thus, there is an increasing concern about the effects of thermal stress on coastal biological communities, such as seaweed beds (Smale et al. 2019). Macroalgae-dominated habitats provide critical ecological and human services worldwide (Hurd et al. 2014). Therefore, it is important to predict their response (tolerance, resilience) to thermal stress and the subsequent impact on species-specific distribution patterns (Andrews et al. 2014, Smale et al. 2019, Straub et al. 2019).
Exposure to high temperatures can lead to “disruptive stress” in seaweeds, resulting from metabolic damage (Davison and Pearson 1996). Both experimental approaches and in situ observations have shown that the physiology of marine macrophytes can be altered by exposure to high temperatures (Bruhn and Gerard 1996, Short et al. 2015, Marín-Guirao et al. 2017). Generally, thermal stress can (i) alter enzymatic-mediated processes and cell membrane composition (Machalek et al. 1996, Los and Murata 2004), (ii) increase or decrease photosynthesis and growth depending on the duration and intensity of heat stress and its interaction with other environmental factors (e.g., light, nutrients, UVB radiation; Kübler and Davison 1993, Bruhn and Gerard 1996, Brown et al. 2014, Xiao et al. 2015, Mabin et al. 2019), and (iii) result in photoinhibition, increased respiration, and changes in pigment composition (Kübler and Davison 1993, Bruhn and Gerard 1996, Koch et al. 2007, Andersen et al. 2013). Heat stress has also been correlated with the production of reactive oxygen species and increased antioxidant capacity of intertidal seaweeds (Collén and Davison 1999, Cruces et al. 2012). Populations near their distribution boundaries will most likely be more affected by thermal stress (Wernberg et al. 2011).
There is evidence that short-term (days) thermal anomalies of high intensity can be more harmful for submerged vegetation than persistent (but moderate) warming (Thompson et al. 2013, Wilson et al. 2015, Guerrero-Meseguer et al. 2017). The exposure to increasing annual maximum temperatures, the influence of marine heat waves (MHWs), or even the influence of episodic/seasonal natural events (e.g., ENSO) strongly affect macroalgae at regional and local scales (Pacheco-Ruíz et al. 2003, Jordà et al. 2012, Schiel and Foster 2015). Even short-term exposure to thermal stress may affect distribution patterns, growth, and recruitment of seaweeds (Andrews et al. 2014, Smale et al. 2019).
As a result of climate change, short-term periods of extreme temperature anomalies, such as longer and more severe summer MHWs, have increased (Schär and Jendritzky 2004, Perkins et al. 2012). MHWs, often of short duration, can reduce reproduction and alter distribution of some seaweeds (Andrews et al. 2014), and can promote mortality of crustose coralline algae and local extinction of kelp communities (Short et al. 2015, Thomsen et al. 2019). The impact of MHWs on kelp forests may depend on the influence of latitudinal patterns of genetic diversity, physiological versatility, and ecological resilience (Wernberg et al. 2018). Although understanding the effects of weather extremes (including MHWs) on submerged vegetation has been highlighted (Wernberg et al. 2012, 2016), seaweed responses to thermal stress, such as physiological plasticity, tolerance, and resilience, remain poorly addressed to date in juvenile stages.
The giant kelp, Macrocystis pyrifera, is a large kelp forming some of the biggest and most productive temperate submerged vegetated ecosystems of the world, with relevant ecological and economic values (e.g., aquaculture, maintenance of water quality, nursery and shelter for invertebrates, fishes, mammals, and other algae; Reed and Brzezinski 2009, Gordon and Cook 2013). The coastline of Baja California, Mexico, is considered among the regions particularly vulnerable to MHWs, due to the high levels of biodiversity, the prevalence of important kelp species at their warm boundaries (such as M. pyrifera), and the influence of anthropogenic impacts (Holbrook et al. 2019, Smale et al. 2019). In Baja California, the influence of the California Current and upwelling events provides the basic abiotic requirements (e.g., lower temperatures, nutrients) for the development of M. pyrifera (Tegner and Dayton 1987). Conversely, thermal anomalies as a consequence of episodic events such as ENSO, MHWs, or prolonged events such as the “Blob” (see Hu et al. 2017) can be correlated with damage or even complete disappearance of Giant Kelp forests, likely due to the cross-correlation of temperature and other environmental factors, such as the deepening of the thermocline and associated reduction of nutrients (North and Zimmerman 1984, Zimmerman and Robertson 1985, Ladah 2003, Schiel and Foster 2015, Arafeh-Dalmau et al. 2019). Normal summer warming causes reduced growth, bleaching and softening of tissues, increased epiphytation and death (North 1987); however, these effects can be ameliorated by sub-thermocline nitrate influx fueled by internal waves and mixing (Zimmerman and Robertson 1985, Ladah et al. 2012). Ocean warming and linked factors such as nutrient limitation can restrict the distribution boundaries of kelp forests, as particularly observed along the coastline of Baja California Peninsula (Ladah et al. 1999, Graham et al. 2007, Arafeh-Dalmau et al. 2019) and other coastal systems.
Despite the well-described negative effects of thermal stress on Macrocystis pyrifera forests (e.g., population dynamics, loss of canopy; North 1987, Ladah et al. 1999, Cavanaugh et al. 2019), knowledge about the physiological mechanisms governing their vulnerability and/or resilience is still limited (Brown et al. 2014), particularly at the juvenile stages. Moreover, M. pyrifera has a complicated alternation of generations in its life history, and juvenile stages such as gametophytes and juvenile sporophytes may exhibit different resistance to environmental stressors (Manley and North 1984, Dean and Jacobsen 1986, Muñoz et al. 2004, Ladah and Zertuche-González 2007, Mabin et al. 2019). Differences in the thermal tolerance of the various life history stages of kelps can be decisive for the survival of a population (Graham 1996, Clarke 2003). Metabolism differs between the ontogenic stages of M. pyrifera (i.e., photosynthesis, light requirements; Dean and Jacobsen 1986, Xu et al. 2015), similar to that indicated for other seaweeds (Xu et al. 2017). Also, juvenile sporophytes of M. pyrifera lack the structural complexity of adults, and consequently, they cannot rely on translocation of resources to cope with stressful conditions (Graham et al. 2007). Therefore, acclimation responses and resistance to thermal stress would be expected to differ between adults and juveniles.
The differential interaction of adults and juveniles with changing environmental factors that can occur simultaneously with thermal stress, such as light availability, can represent another potential source of variation of the responses to heat stress. For instance, increased turbidity due to storm waves, coastal runoff, or even phytoplankton blooms can dramatically reduce incident irradiance (near darkness) for weeks on the benthos (Dean and Jacobsen 1984, Dean 1985, Cabello-Pasini et al. 2002, Schiel and Foster 2015). Phytoplankton blooms commonly resulted for the combination of rising of seawater temperature and upwelled nutrients in spring–summer, and can lead to drastic reductions of irradiance during weeks (less than 0.5 mol photons · m−2 · d−1) in shallow bottoms near giant kelp forests (Dean and Jacobsen 1984). Also overgrowth of canopy-forming seaweeds in later-spring/early-summer at the edges of Macrocystis pyrifera populations, like the invasive Sargassum horneri, Sargassum muticum, and Undaria pinnatifida that have colonized progressively new coastal areas on the Pacific coast of North America, can comprise light availability for the development of juvenile stages and recruitment (Ambrose and Nelson 1982, Schiel and Foster 2015, South et al. 2017). The canopy of M. pyrifera adults and understory canopies of other seaweeds (e.g., kelps species such as Laminaria and Pterygophora) can also shade juveniles of M. pyrifera (North 1994). While adult plants can counterbalance light limitation by both photoacclimation of deeper blades and translocation of resources from apical parts of the fronds reaching the sea surface (Manley and North 1984, Colombo-Pallota et al. 2006), young juveniles must depend on their photosynthetic plasticity to survive. Light deprivation results in lower availability to metabolic energy needed to incorporate and assimilate inorganic carbon, and thus, in a drop in photosynthate production (Hanelt and Lopez-Figueroa 2012). Since the assimilation of inorganic nitrogen into organic compounds requires energy and carbon skeletons from photoassimilates, light limitation can lead to the alteration of the plant N-metabolism, including N-uptake rates (Hurd et al. 2014). Changes in the bio-optical properties of photosynthetic tissues (e.g., absorptance) and pigments content are among the photoacclimation mechanisms which can be activated to optimize light harvesting in seaweeds, including M. pyrifera juveniles (Umanzor et al. 2020). Compared to adult sporophytes, photoacclimation strategies of juveniles are still poorly understood (Mabin et al. 2019, Umanzor et al. 2020).
The purpose of the present study was to evaluate the combined effects of a simulated MHW and light deprivation on juvenile sporophytes of Macrocystis pyrifera. An orthogonal design was used, based on the exposure of juveniles to two temperatures (16 and 24°C) and two light conditions (saturating irradiance ~180 μmol photons · m−2 · s−1, and a light below compensation irradiance for photosynthesis, 5–10 μmol photons · m−2 · s−1). The main hypothesis of this study was that MHWs and light deprivation can induce physiological changes in juvenile sporophytes, and the metabolic integration of these responses can be decisive to their tolerance and/or vulnerability to those combined stressors. Photosynthesis, bio-optical properties, nutritional status, oxidative stress descriptors, and nitrate uptake rates were evaluated. As far as we know, there is no background information about the integration of such physiological descriptors of M. pyrifera juveniles under the combination of disruptive (heat) and limitation (light deprivation) stressful conditions.
Materials and methods
Collection of juvenile sporophytes
In September 2017, juvenile sporophytes (single blade stage, up to 20 cm length) were collected along the edges of a healthy and dense (~0.3 stipes · m−2) Macrocystis pyrifera forest growing at 7–10 m depth in Bahía Todos Santos, Baja California, Mexico (35°54′21.90″ N, 116°45′12.05″ W). Surface seawater temperature in this region ranges between 12 and 20°C, although it can increase up to 4°C above the long-term mean during ENSO and MHWs (Hernández de la Torre et al. 2004, Leising et al. 2015).
During collection, algae were kept in black plastic bags to avoid exposure to excessive sunlight. Within 2 h after collection, juveniles were transported in large coolers filled with seawater to the Marine Botany Lab at the University of Baja California. Juveniles were kept in 1-m3 outdoor tanks until the beginning of the experiment, at averaged values of field temperature and light, 16°C and 6.6 mol photons · m−2 · d−1, respectively. Temperature was adjusted by using chillers, while the tanks were covered with plastic meshes to adjust light to field values. Temperature was recorded at the collection site during the previous weeks using HOBO MX2202 (ONSET, Bourne, MA, USA) data loggers. Light values were obtained by using a spherical 4π underwater quantum sensor attached to a data logger (LI-192 and LI-1500; LI-COR Biosciences, Lincoln, NE, USA), and HOBO MX2202 data loggers calibrated against 2π quantum sensor LI-190R (LI-COR Biosciences).
Experimental setup and design
After a 4 d acclimation period, Macrocystis pyrifera juveniles were transplanted to 10-L plastic transparent containers (nine juveniles per container) filled with filtered (1 μm) UV-irradiated seawater and aerated. Each container was considered as an experimental unit (EU), and all the EUs were placed within large incubators (VWR, model 2015 2015-2), which allowed for the control of light and temperature. Within the incubators, temperature was adjusted to 16 and 24°C, corresponding to the averaged field values at the collection site and maximum values recorded during MHWs, respectively. A control light treatment was also adjusted at 180 μmol photons · m−2 · s−1, photoperiod 12:12 h light:dark; this light corresponded to the averaged maximum irradiances measured at the collection site at midday (from 90 to 300 μmol photons · m−2 · s−1), and can be saturating for the photosynthesis of juvenile sporophytes (Umanzor et al. 2020). Other juveniles were kept in almost darkness (5–10 μmol photons · m−2 · s−1) by covering the EUs with black plastic mesh. This low light condition, which is below compensation irradiance for photosynthesis of juveniles (Umanzor et al. 2020), was selected following Dean and Jacobsen (1984) and Cabello-Pasini et al. (2002) who reported light values close to zero (0–0.5 mol photons · m−2 · d−1) near giant kelp forests in northern Baja California and southern California during prolonged periods (weeks) of phytoplanktonic blooms and storms. Four EUs were used for each treatment (N = 4): 16L, 24L, 16D, 24D (L = light treatment, D = darkness treatment). To adjust the simulated MHW, temperature was gradually increased by 2°C per day, which is within the increment rates measured during these events in the northern Pacific of Baja California (Arafeh-Dalmau et al. 2019). Seawater was replaced every day in each container and water quality was checked by using a submersible multiparameter probe (YSI Pro Plus, USA); nitrate (1–2 μM), salinity (33.5), and pH (7.9–8.1) were maintained almost constant during the experiment. Juveniles were kept under the experimental treatments for 7 d.
Physiological descriptors
All the physiological descriptors were measured for two juveniles per EU (i.e., eight plants), and values per container were averaged to obtain the true experimental replication (n = 4). Reference field values of physiological traits were also measured in juveniles of Macrocystis pyrifera (n = 6) prior to acclimation (Table 1).
| Mean (SE) | Min–max | |
|---|---|---|
| Photochemistry | ||
| Fv/Fm | 0.763 (0.002) | 0.757–0.769 |
| ETRμmol photons · m−2 · s−1 | 7.5 (0.2) | 7.0–8.2 |
| ФPSII | 0.150 (0.002) | 0.140–0.163 |
| NPQ | 0.067 (0.004) | 0.053–0.075 |
| α | 0.166 (0.003) | 0.155-0.176 |
| Bio-optical properties | ||
| A400-700 | 0.629 (0.017) | 0.577–0.667 |
| A680 | 0.779 (0.003) | 0.768–0.785 |
| Pigmentsμg · g FW−1 | ||
| Chl a | 766 (22) | 722–789 |
| Chl c | 139 (18) | 106–168 |
| Carotenoids | 346 (41) | 272–414 |
| Soluble NSCs % DW | 3.55 (0.18) | 3.13–3.87 |
| Nitrogen % DW | 1.85 (0.09) | 1.73–2.03 |
Chlorophyll-a fluorescence
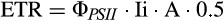 (1)
(1)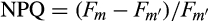 (2)
(2)Blade bio-optical properties
Blade spectral absorption in the PAR range (400–700 nm) was determined by using a Taylor-type integrating sphere (Li-Cor 1800-12, USA) attached via fiber optic cable to a portable spectroradiometer (Fieldspec, ASD, Boulder, CO, USA). A blade section of ~2 cm was placed between two glass slides then attached to the sample holder of the integrating sphere, covering the entire light path. The spectroradiometer recorded Reflectance (R) and Transmittance (T) at 1 nm intervals, by using barium sulfate as the reference material. Absorptance was determined as 1-T-R following Krause and Weis (1991). Absorption not related to pigments was corrected by subtracting the averaged absorbance between 725 and 750 nm.
Pigment content
Approximately 0.02 g of fresh tissue was ground twice with 0.5 mL of 1% aqueous magnesium carbonate, 4 mL of acetone, and 2 mL distilled water; volume was completed with acetone up to 10 mL. Homogenates were kept in the dark at −18°C for 24 h and centrifuged for 5 min at 3,000 rpm. Supernatants were collected in a glass cuvette (1 cm length) and the concentration of chlorophyll a, c and total carotenoids was determined by spectrophotometry using the equations described by Parsons et al. (1984) and Seely et al. (1972).
Total soluble carbohydrates
Soluble carbohydrates were measured using the colorimetric phenol-sulfuric acid method using glucose as standard (Dubois et al. 1956). Blade tissue was dried, ground, and then digested with 2 mL of 0.2 M HCl at 60°C during 3 h, and centrifuged for 5 min at 1,000g. A mixture of 0.025 mL of supernatant, 1 mL of distilled water, and 0.25 mL of 3% phenol was cooled in an ice bath; then, 2.5 mL of concentrated sulfuric acid was added directly into the solution and mixed vigorously. After 30 min, absorbance was read at 490 nm.
Total nitrogen content
Total nitrogen was quantified by elemental analysis. Blade surfaces were rinsed with distilled water and dried at 60°C for 72 h. Samples were then ground into fine powder and encapsulated in tins. Analyses were carried out at UC Davis Stable Isotope Facility using an elemental analyzer (EA) interfaced to a continuous flow isotope ratio mass spectrometer (IRMS).
Nitrate uptake rates
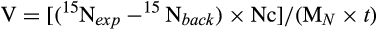 (3)
(3)Lipid peroxidation
Lipid peroxidation was measured following the thiobarbituric acid reactive substances (TBARS) assay described by Hodges et al. (1999) and Correia et al. (2006). Ultrafrozen blade tissues were ground in liquid nitrogen using a mechanical grinder, and homogenized (dilution 1:10) in trichloroacetic acid (TCA, 20%). Tissue homogenates were then centrifuged for 10 min (3,000g, 4°C) and supernatants were mixed with a solution of TCA (20%) and thiobarbituric acid (0.5% v/v). These solutions were heated at 90°C for 30 min, and then centrifuged again (10,000g) for 10 min. The supernatants were extracted and their absorbances (440, 532, and 600 nm) were determined spectrophotometrically. Lipid peroxidation was expressed as equivalents of malonil-dialdehyde (Eq MDA; molar extinction coefficient 155 · mM−1 · cm−1) using the equations in Hodges et al. (1999).
Total phenolic content and antioxidant capacity
Dried and ground blade tissue (0.03 g DW) was extracted in 0.75 mL 80% methanol in darkness for 24 h, and the extract was then centrifuged at 10 rpm for 10 min. The phenolic compounds and antioxidant capacity were quantified in the methanolic supernatant. The phenolic compounds were measured with a modification of the Folin–Ciocalteu assay using gallic acid as standard (Singleton and Rossi 1965). A volume of 0.025 mL of methanolic extract was diluted in 1 mL of distilled water. Then, 0.1 mL of Folin–Ciocalteu reagent and 0.3 mL of distilled water saturated in NaCO3 were added. The mixture was homogenized, heated (40°C for 3 min), and the absorbance determined at 765 nm. The radical scavenging activity of the same methanolic extracts was also determined (Farvin and Jacobsen 2013); the reaction mixture was prepared with 0.1 mL of diluted extract (1:4 with 80% methanol) and 1 mL of DPPH 30 μM dissolved in 90% methanol. Absorbance at 517 nm was measured after 30 min of DPPH addition. The total antioxidant capacity of algal extract was expressed as ascorbic acid equivalents.
Statistical analysis
Multivariate analyses were performed with the normalized data of all biological variables, and a ranked triangular similarity matrix was constructed using Euclidean distances. Then, a two-way PERMANOVA crossed design was performed (9999 permutations) to test the factors of temperature, light, and their interactive effects on the physiological status of juvenile sporophytes. Significant differences in the pairwise posteriori comparisons were checked using Monte Carlo P-values, P (MC), due to the restricted number of possible permutations. SIMPER analysis was applied to explore similarities among treatments (average distance). These statistical procedures were implemented using PRIMER 6 & PERMANOVA+ v.1.0.2 software package (Anderson et al. 2008). Principal components analysis (PCA) was performed using PAST software (Hammer et al. 2001) to display multivariate patterns of treatments, and to explore their relationship with the different biological descriptors.
A two-way analysis of variance (ANOVA) was used to test the effect of temperature and light on each response variable. A post hoc Student Newman–Keuls (SNK; Zar 1984) was performed when significant differences were found (P < 0.05). Prior to the analysis, Shapiro–Wilk and Levene tests were used to check the assumptions of normality and homocedasticity, and data were transformed when necessary. A non-parametric Kruskal–Wallis test was applied if assumptions were not met. All analyses and graphs were done using the statistical package STATISTICA (StatSoft INC, version 8.0).
Results
The initial physiological status was similar in all the studied plants (Table 1), therefore, physiological changes observed at the end of the experimental period were due to the treatment conditions.
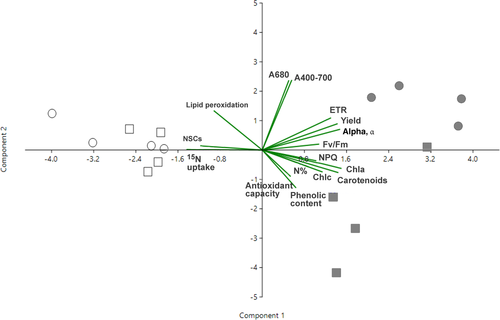
The two-way PERMANOVA indicated that both temperature and light had significant effects on physiological traits of kelp juveniles after the 7 d treatment, and that temperature effects differed between light or dark conditions (i.e., significant interaction Temp. × Light; Table 2). The two-dimensional PCA plot (Fig. 1; Table S1 in the Supporting Information) showed two clear eigenvectors: the component 2, which explained the highest percentage of variance (45.4%) and mainly represented differences due to the light treatments; and the second component 1, which explained the variance of 17.4%, which separates both temperature treatments. The component 2 was highly and positively correlated (correlation coefficient ~0.8) with ETRmax, effective quantum yield, NPQ, α, Fv/Fm, and pigment (Chl a, Chl c, and carotenoids); on the contrary, component 2 was negatively correlated with nitrate uptake rates (−0.8) and soluble carbohydrates content (−0.7), which diminished in juvenile sporophytes subjected to light deprivation. Component 1 was positively correlated (0.85) with absorptance values (A400-700 and A680). SIMPER analysis (Table S2 in the Supporting Information) also indicated that light treatments (16D, 24D vs. 16L, 24L) had higher dissimilarity (average distance = 42.9) and were more separately in the biplot than temperature treatments (16D, 16L vs. 24L, 24D; average distance = 24.3). Moreover, the change in temperature promoted more pronounced changes on the biological traits of Macrocystis pyrifera under light limitation (average distance = 28.5, 16D vs. 24D) than light conditions (16L vs. 24L, average distance = 20.5).
| Main test | SS | MS | Pseudo-F | P (perm) |
|---|---|---|---|---|
| Temp. | 22.489 | 22.489 | 2.9159 | 0.0161 |
| Light | 123.26 | 123.26 | 15.983 | 0.0001 |
| Temp. × Light | 31.698 | 31.698 | 4.11 | 0.0007 |
| Pairwise | (L) Light | (D) Dark | ||
| t | P (MC) | t | P (MC) | |
| 16 × 24 | 1.7677 | 0.0341 | 1.9535 | 0.03 |
| Pairwise | 16°C | 24°C | ||
| t | P (MC) | t | P (MC) | |
| L × D | 3.2902 | 0.0023 | 3.0442 | 0.0024 |
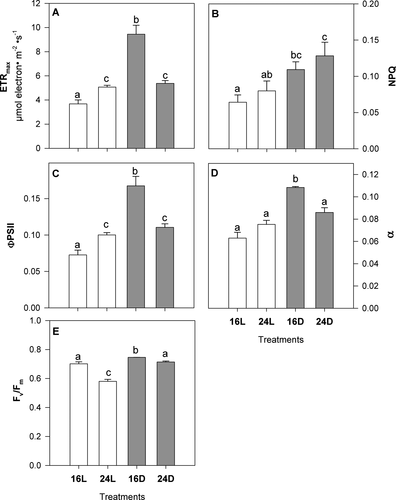
Electron transport rate (ETR), ФPSII, and α values were 2- to 3-fold greater in juveniles exposed to the 16D treatment than control plants (16L; Fig. 2, A, C, and D). Also, these values corresponded to the maximum values measured among treatments. Juveniles at 24L and 24D also showed increased ETR (two-way ANOVA, F1,12 = 9.78, P = 0.0087) and ФPSII (two-way ANOVA, F1,12 = 3.5, P = 0.08) with respect to 16L sporophytes, while higher maximum values were found at 16D. Non-photochemical quenching (NPQ) significantly increased with the increasing temperature in dark conditions (two-way ANOVA, F1,12 = 5.63, P = 0.035), 2-fold higher at 24D compared to 16L (Fig. 2E). Values of Fv/Fm were slightly higher (but significant) in 16D plants with respect to control (two-way ANOVA, F1,12 = 76.74, P < 0.001), while Fv/Fm was reduced by 17% in juveniles exposed to the 24L treatment (Fig. 2E). In general, all photochemical descriptors showed significant effects caused independently by light and temperature.
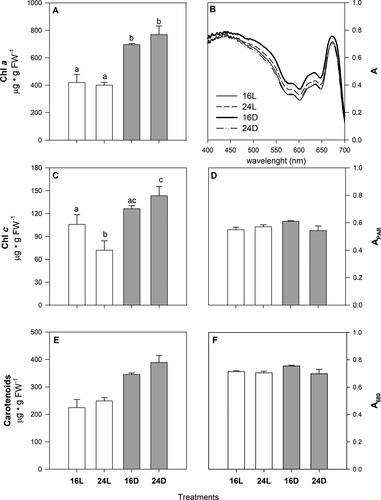
Chlorophyll a (two-way ANOVA, F1,12 = 61.79, P < 0.001) and total carotenoids (Kruskal–Wallis ANOVA, H3,16 = 11.67; P = 0.087) increased 2-fold in juveniles exposed to darkness relative to the controls (Fig. 3, A and E), regardless of the temperature level. A slight (but significant) increase in Chl c was detected in plants at 24D with respect to the control treatment, and it was significantly reduced in 24L plants (Fig. 3C; two-way ANOVA, F1,12 = 29.79, P < 0.001). Blade absorptance (A400-700, A680; Fig. 3, B, D, and F) increased by ~10% in juveniles at 16D compared to the other treatments, including the control; however, these differences were only near significant for A680 (two-way ANOVA, F1,12 = 4.91, P = 0.057).
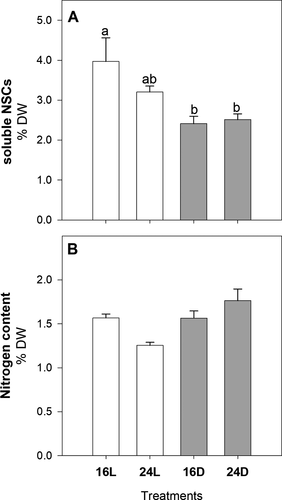
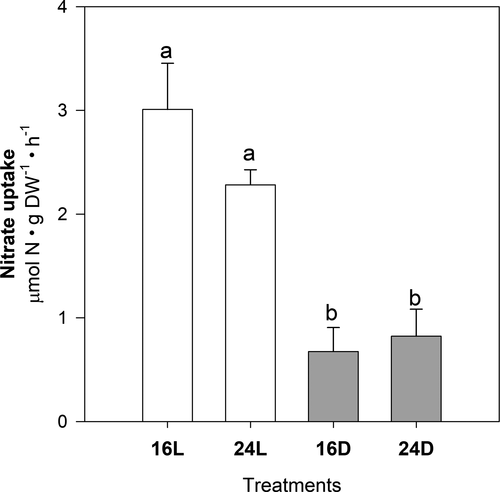
Light condition (regardless of temperature) had significant effects on soluble carbohydrates, nitrogen concentration, and nitrate uptake rates (Figs. 4 and 5). Soluble carbohydrates were about 40% lower in juveniles growing under dark conditions (16D, 24D; Fig. 4A; two-way ANOVA, F1,12 = 11.76, P = 0.004), with the opposite pattern found for N content (Fig. 4B; two-way ANOVA, F1,12 = 7.24, P = 0.019). Similar to carbohydrate concentration, nitrate uptake rates measured in juveniles from 16D and 24D were up to 7.7 times lower than in illuminated juveniles (Fig. 5; two-way ANOVA, F1,12 = 73.03, P < 0.001).
Changes in total phenolic content, antioxidant capacity, and lipid peroxidation were not statistically significant among treatments (Table 3); uniquely the effect of light was close to significance (two-way ANOVA, F1,12 = 3.82, P = 0.074) in the case of lipid peroxidation.
| Experimental treatments | ||||
|---|---|---|---|---|
| 16L | 24L | 16D | 24D | |
| Total phenolic content(Eq. mg GA · g DW−1) | 3.215 (0.936) | 5.035 (0.441) | 4.232 (0.783) | 5.136 (0.832) |
| Antioxidant capacity(Eq. mg AA · g DW−1) | 0.778 (0.058) | 0.878 (0.065) | 0.946 (0.112) | 0.909 (0.078) |
| Lipid peroxidation(Eq. nmol MDA · g FW−1) | 21.22 (1.20) | 17.48 (2.92) | 16.99 (1.25) | 16.35 (2.81) |
The two-way ANOVA (Table 4) revealed that effects of temperature significantly differed between light and dark treatments for all photochemical descriptors, except for NPQ.
| Temperature | Light | T × L | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | MS | F | P | MS | F | P | MS | F | P | |
| Photochemistry | ||||||||||
| Fv/Fm | 1 | 0.02 | 57.19 | <0.001 | 0.03 | 76.74 | <0.001 | 0.01 | 19.48 | <0.001 |
| ETR | 1 | 7.21 | 9.79 | 0.009 | 36.92 | 50.15 | <0.001 | 29.92 | 40.64 | <0.001 |
| ФPSII | 1 | 0.0008 | 3.50 | 0.086 | 0.01 | 44.59 | <0.001 | 0.01 | 28.74 | <0.001 |
| NPQ | 1 | 0.0001 | 0.0001 | 0.998 | 0.01 | 5.64 | 0.035 | 0.00 | 0.31 | 0.586 |
| Bio-optics | ||||||||||
| APAR | 1 | 0.002 | 2.25 | 0.172 | 0.0004 | 0.39 | 0.550 | 0.005 | 4.38 | 0.070 |
| A680 | 1 | 0.004 | 4.91 | 0.051 | 0.0004 | 0.43 | 0.53 | 0.001 | 1.11 | 0.32 |
| Pigments | ||||||||||
| Chl a | 1 | 2947.46 | 0.44 | 0.522 | 417958 | 61.79 | <0.001 | 8535.64 | 1.26 | 0.283 |
| Chl c | 1 | 265.39 | 0.95 | 0.350 | 8366.58 | 29.79 | <0.001 | 2642.52 | 9.41 | 0.010 |
| Nutrient content | ||||||||||
| Soluble NSCs | 1 | 0.44 | 1.03 | 0.331 | 5.05 | 11.76 | 0.005 | 0.75 | 1.75 | 0.211 |
| Nitrogen | 1 | 0.01 | 0.11 | 0.743 | 0.50 | 7.25 | 0.020 | 0.09 | 1.36 | 0.266 |
| Nitrate uptake | 1 | 0.57 | 1.64 | 0.225 | 25.40 | 73.03 | 0.0001 | 1.04 | 2.99 | 0.109 |
| Lipid peroxidation | 1 | 1176.52 | 1.13 | 0.310 | 3992.76 | 3.82 | 0.074 | 458.80 | 0.44 | 0.520 |
| Total phenols | 1 | 7.42 | 3.12 | 0.103 | 1.25 | 0.53 | 0.482 | 0.84 | 0.35 | 0.564 |
| Antioxidant capacity | 1 | 0.00 | 0.15 | 0.704 | 0.04 | 1.51 | 0.242 | 0.02 | 0.71 | 0.415 |
- a Total carotenoids (df = 3; H = 11.67; P = 0.087).
- b Photosynthetic efficiency-Alpha (df = 3; H = 12.79; P = 0.005).
Discussion
Giant kelp populations can be negatively impacted by thermal stress and/or its associated environmental factors, for example, nutrient limitation (North 1987). Both seasonal-dependent temperature increments and thermal anomalies associated with ENSO and MHWs can cause deterioration and loss of populations (Edwards and Estes 2006, Graham et al. 2007). Available knowledge about the effects of thermal stress, in combination or not with other factors, is typically restricted to adult sporophytes of Macrocystis pyrifera, while little is known about the resistance/vulnerability of juvenile stages. This study represents one of the few works (see Mabin et al. 2019) which assess the physiological plasticity of juvenile sporophytes. Juvenile stages, including microscopic phases, can be decisive for the population resilience after extreme events causing loss of kelp forest, such as MHWs (Cavanaugh et al. 2019).
This study examined the responses of juvenile sporophytes to a simulated MHW in the laboratory, similar in duration and intensity (7 d, 24°C) to those that occur in the Mexican North Pacific during summer and early-autumn (Hernández de la Torre et al. 2004, Leising et al. 2015, Arafeh-Dalmau et al. 2019). These responses were compared to plants maintained under control temperature (16°C). Also, some sporophytes were also subjected to light values below compensation irradiance for photosynthesis (5–10 μmol photons · m−2 · s−1) to explore potential interactive effects between the experimental factors. Generally, our results indicated that both high temperature and light deprivation caused significant biological changes in juvenile sporophytes (Fig. 1, Table 2). A notable reduction in Fv/Fm (until values below 0.6) was detected in juvenile sporophytes when exposed to a high temperature and control saturating light (180 μmol photons · m−2 · s−1; i.e., 24L treatment; Fig. 2E), but no other evidence of physiological damage associated with thermal stress was found. Although juvenile sporophytes maintained photosynthetic functionality under light deprivation, regardless the temperature level, this imposed experimental condition induced stronger physiological changes than thermal stress, including photoacclimation responses and nutrient physiology alteration (Fig. 1, Table 2).
Maximum quantum yield (Fv/Fm) decreased drastically in illuminated juveniles exposed to heat stress. Recently, Mabin et al. (2019) documented a similar reduction of Fv/Fm when juvenile sporophytes of Macrocystis pyrifera were exposed to experimental temperatures of 22°C, although the extent depended on the combination of temperature with light and nutrient (nitrate) availability. In addition, thermal stress has been correlated with decreasing Fv/Fm in other seaweeds, likely associated with the enhancement of energy dissipation mechanisms (e.g., NPQ) or the impairment of PSII and related downstream metabolic processes (e.g., carbon fixation; Davison and Davison 1987, Sharkey 2005, Fernández-Marín et al. 2011, Pereira et al. 2015, Mabin et al. 2019). In adult M. pyrifera sporophytes, quantum efficiency was also shown to be negatively affected by light overexposure in surface blades (Cabello-Pasini et al. 2000, Colombo-Pallotta et al. 2006). Super-optimal temperature also appears to enhance photoinhibition in Saccharina latissima when exposed to saturating irradiances (Bruhn and Gerard 1996). This effect might be responsible for the decline in populations of this species along the south coast of Norway during high temperatures (Andersen et al. 2013). In our study, despite the reduction in Fv/Fm, juveniles at 24L showed similar or higher values of ETRmax, photosynthetic efficiency-α, and ФPSII than juveniles growing at optimum conditions of temperature and light, 16L. Although these responses seem contradictory, these might be explained by the activation of alternative electron sinks which, under diminished photosynthetic capacities, help to dissipate excess photons and avoid over-reduction of electron transport carriers (Niyogi 2000). One of these mechanisms is the thermal dissipation of energy operated by the xanthophylls cycle, expressed as an increase in NPQ. This photoprotective mechanism has been demonstrated in adults and juveniles of M. pyrifera mainly in the framework of light acclimation (Colombo-Pallotta et al. 2006, García-Mendoza and Colombo-Pallotta 2007, Umanzor et al. 2020). Because our results did not show a significant increase in NPQ in 24L juveniles, other mechanisms could likely act as safety valves for photosynthesis (e.g., photoreduction of oxygen by PS I or the water–water cycle; Niyogi 2000). Contrary to that found in 24L, Fv/Fm was not reduced in juveniles maintained in darkness and higher temperatures (i.e., 24D). Also, values of ETRmax, quantum yield, and α were greater in 24D plants than those at optimum conditions (16L). This indicated that light can critically contribute to the damage of the photosynthetic apparatus in combination with temperature. Because of the absence of photonic energy in 24D, photodamage cannot occur and costly photo repair processes (e.g., D1 protein replacement) are unnecessary (Demmig-Adams and Adams 2006, Raven 2011). Previous works indicated that factors associated with higher temperatures (such as nutrient depletion, pathogenic biota, or epibionts), instead of thermal stress itself, can be responsible for seaweed deterioration. Moreover, M. pyrifera can maintain or even enhance its physiological performance (e.g., photosynthesis, nutrient uptake) when exposed solely to increasing temperatures (Clendenning 1971, Dean and Jacobsen 1984, Arnold and Manley 1985, North 1994). The maximum values of ETRmax, α, and ФPSII corresponded to juveniles exposed to 16°C and darkness, and indicated the optimum status of their photosynthetic machinery. Particularly, the increase in photosynthetic efficiency, α, is a typical photoacclimative response which helps to cope with light scarcity in M. pyrifera and other seaweeds (Fairhead and Chesire 2004, Sampath-Wiley et al. 2008). Higher α can reflect an increase in the number and/or size of photosynthetic units (Colombo-Pallotta et al. 2006, Falkowski and Raven 2007). Our results are also consistent with the general increase in pigment concentration (Chl a, Chl c, and carotenoids) in juveniles kept in darkness, regardless of temperature, as a widely recognized response within the photoacclimation strategies of this and other seaweeds (Schiel and Foster 2015, Mabin et al. 2019). This increase in blade pigmentation was uniquely reflected in a slight rise in leaf absorptance in 16D juveniles, but not in 24D, likely due to a pigment packaging phenomenon (Enríquez et al. 1994). We did not find evidence of pigment degradation in juveniles at 24L, which reinforced the idea that tissue bleaching can be a consequence of environmental factors related to higher temperatures (e.g., nutrient depletion) than from thermal stress itself (Gerard 1984, North 1994, Schiel and Foster 2015).
Values of nitrogen and soluble non-structural carbohydrates content were similar to the levels reported previously for juvenile and adults of Macrocystis pyrifera (North and Zimmerman 1984, Davison and Davison 1987, Kopczak 1994). Also, experimental sporophytes showed values of N-content close 1.5% DW (or even higher) than the assumed critical level of 1% DW known to represent N-limitation to support plant growth (Gerard 1982, Zimmerman and Kremer 1984). Internal concentration of nitrogen (free amino acids, proteins, internal nitrate) and soluble sugars (e.g., mannitol) can vary among M. pyrifera tissues and are strongly influenced by seasonal-dependent factors, such as temperature, light, and nutrient availability (Wheeler and Srivastava 1984, North 1994). In our study, carbohydrate content decayed in dark treatments (16D, 24D). Under light limitation, the reduction of sugars is caused by metabolic carbon imbalance reflecting both photoassimilate synthesis reduction and/or their consumption by respiratory processes (Hurd et al. 2014). An interesting example of a similar (but much more extreme) condition can be observed in Arctic kelps, which maintain growth during the Arctic winter (i.e., 6 months of darkness) at the expense of the use of carbohydrates previously accumulated during the warmer season when sea ice breaks up (Young et al. 2007). Nitrogen content slightly increased in juveniles kept under light deprivation, following the opposite pattern of sugars. This inverse correlation has been documented in M. pyrifera, primarily related to nitrate availability and the use of internal carbon for its assimilation (Zimmerman and Kremer 1986, North 1994).
Nitrate uptake rates measured in experimental juveniles (1–3 μmol N · g DW−1 · h−1) were within the range measured for adult blades of Macrocystis pyrifera, both in situ and in the laboratory (Gerard 1982, Druehl 1984, Kopczak 1994). Kopczak (1994) showed that rates can be near saturation (i.e., near to Vmax) at the concentrations used in this study (15 μM), which is similar to the nitrate measured in kelp stands during upwelling (Reed et al. 2011). Nitrate uptake rates were strongly reduced in juveniles subjected to light-limiting conditions, regardless of temperature, similar to that found for carbohydrate content. There is contrasting literature on the effect of light on nitrate uptake in M. pyrifera. For example, Gerard (1982) and Wheeler (1982) reported that light limitation can suppress nitrate uptake, while the opposite trend was reported for this and other seaweeds (Wheeler and Srivastava 1984, Philips and Hurd 2003, Young et al. 2007). Furthermore, Kopczak (1994) did not find any correlation between irradiance and nitrate uptake. In our study, the decrease of nitrate uptake can be linked to the lack of metabolic energy and carbon skeletons required to incorporate and assimilate this nutrient, and the activity and/or synthesis of involved enzymes (e.g., nitrate reductase; Berges 1997, Hurd et al. 2014). Therefore, nutrition of juvenile sporophytes can be comprised in nature under limiting irradiance as nitrate is the nitrogen form most acquired by giant kelp, although urea and ammonium associated with biota excretion and sediment efflux, respectively, can contribute to the plant N budget (Brzezinski et al. 2013, Schiel and Foster 2015). Besides light, the incorporation of nitrate by M. pyrifera (and other species) can also be modulated by both the surrounding environment and internal physiological and morphological factors (e.g., tissue type, blade morphology, N-status; North 1994).
Diminished nitrate uptake rates in juveniles under darkness were not reflected in a decrease in tissue N-content. This apparent discrepancy may be explained by the metabolic decoupling between nitrate incorporation and the consumption of internal non-structural N, due to a potential decrease in plant growth. Nitrogen uptake and utilization can be temporally uncoupled due to light availability, as in the strategies developed by Arctic kelps among seasons, or even other seaweeds during diurnal cycles (Wiencke and Amsler 2012). There are other examples of this temporal partitioning between N-acquisition, internal N-metabolic processes, and Macrocystis pyrifera growth. This species can maintain growth for days to weeks at the expense of internal non-structural N compounds (including nitrate) accumulated during “luxury consumption” when N concentration is high in the surrounding water (Chapman and Craigie 1977, Schiel and Foster 2015).
Our results indicated that higher temperatures did not trigger stimulation of non-enzymatic antioxidative capacities and oxidative damage (i.e., lipid peroxidation) in juvenile sporophytes. This suggested that the generation of reactive oxygen species (ROS) was restricted by the activation of photoprotective mechanism. For instance, the decrease in Fv/Fm and Chl c in 24L sporophytes can reflect a “dynamic photoinhibition” strategy, either to reduce light harvesting or to induce photochemical quench (Franklin and Forster 1998). The production of ROS is typically associated with disruptive environmental stressors, including temperature and light overexposure (Collén and Davison 1999, Bischof and Rautenberger 2012, Cruces et al. 2012). In intertidal seaweeds, for example, exposure to extreme temperatures and light lead to the over-energization (or over-reduction) of the photosynthetic apparatus at the thylakoid level, and the production of ROS by the activation of alternative electron sinks (Niyogi 2000, Bischof and Rautenberger 2012). To avoid oxidative damage, seaweeds are able to activate a variety of antioxidant mechanisms to scavenge ROS species, including antioxidant enzymes (e.g., catalase, superoxide dismutase) and compounds (e.g., amino acids, carotenoids, phenols such as phlorotannins; Bischof and Rautenberger 2012, Cruces et al. 2012). However, and reinforcing the neutral response of antioxidant response in our study, total phenols did not change in plants subjected to thermal stress.
In conclusion, our results supported that light limitation can be more harmful to juvenile sporophytes of Macrocystis pyrifera than high temperatures similar to that experienced by this species in Baja California during MHWs. Although light deprivation can induce some photoacclimation responses, it can lead to severe reduction in plant carbon balance and diminished capacities to acquire nitrate from the surrounding seawater. These effects can obviously comprise growth and survival of juvenile sporophytes, and thus regeneration and recruitment of giant kelp forest. Simultaneously to MHWs, several causes can lead to a reduction of incident light on M. pyrifera juveniles, including hydrodynamic forces causing resuspension and turbidity, phytoplanktonic blooms, and the overgrowth of canopy-forming seaweeds. The effects of light deprivation on juvenile stages (up to 20 cm length) by the presence of invasive canopy-forming species are particularly interesting, because some introduced species are spreading prolifically in coastal areas on the Pacific coast of North America, including Baja California. For instance, when the invasive brown algae Undaria pinnatifida and Sargassum spp. reach their maximum size in spring–summer, they can overshadow M. pyrifera juveniles growing at the external edges of the understory (Schiel and Foster 2015, South et al. 2017). The particular interaction between M. pyrifera and invasive species is an issue of special concern (Raffo et al. 2009, Schiel and Foster 2015), since warming trends of seawater (tropicalization of temperate systems) can promote the survival and spread of exotic seaweeds in temperate regions (Bartsch et al. 2012). Finally, although juveniles did not exhibit apparent vulnerability under simulated MHWs in this study, it must be highlighted that more specific research is needed to explore the implication of other external (e.g., duration and intensity of heat stress, incidence of fluctuating temperature) and internal (e.g., physiological status, ecotypic/genotypic divergences) limiting factors on their response.
This work was funded by NPTC-PRODEP project granted to JMSG, as well as by the companies PROMAC (Productos Marinos de las Californias) and Blue Evolution under the project “Ulva sp cultivation in land-based ponds” to JAZG. A doctoral CONACYT (Consejo Nacional de Ciencia y Tecnología) grant was awarded to MSB. A postdoctoral PRODEP grant was awarded to MDBT. Authors are deeply grateful for all the support and insights provided by the people of Marine Botany Lab at UABC: Laura Karina Rangel-Mendoza, Schery Umanzor, Víctor Macías-Carranza.




