Short-chain fatty acids inhibit bovine rumen epithelial cells proliferation via upregulation of cyclin-dependent kinase inhibitors 1A, but not mediated by G protein-coupled receptor 41
Abstract
Short-chain fatty acids (SCFAs) play a critical role in regulation of rumen epithelial growth. The mechanisms underlying the regulatory effects of SCFAs on the proliferation of bovine rumen epithelial cells (BRECs) remain unknown; however, SCFAs can bind to G protein-coupled receptor 41 (GPR41); hence, the regulatory effects of SCFAs on BRECs proliferation may be mediated by GPR41. Here, we investigated the molecular mechanisms underlying the effects of SCFAs and GPR41 on BRECs proliferation. We demonstrated that SCFAs activate the expression of GPR41 and inhibit (p < .05) BRECs proliferation, while the GPR41 knockdown (GPR41KD) BRECs exhibited (p < .05) slow proliferation compared with controls. The treatment of BRECs with 10 mM SCFAs significantly enhanced (p < .05) expression of cyclin-dependent kinase inhibitors 1A (CDKN1A), 2A (CDKN2A) and 2B (CDKN2B) and inhibited (p < .05) their transition from G1 to S phase of the cell cycle, compared with controls. Remarkably, the GPR41KD BRECs treated with SCFAs restored high level of CDKN1A, relative to GPR41KD BRECs, but did not affect (p > .05) the expression of CDKN2A and CDKN2B. The GPR41KD BRECs had significantly reduced (p < .05) cyclin-dependent kinase 4 (CDK4) and cyclin D2 mRNA abundance compared with controls. The GPR41KD BRECs treated with SCFAs significantly decreased (p < .05) CDK4, cyclin D2, CDKN2A and CDKN2B mRNA abundance compared with BRECs treated with SCFAs. Overall, our results demonstrated that downregulation of CDK4 and cyclin D2 likely mediates the inhibitory effects of GPR41KD on BRECs proliferation. Additionally, CDKN1A plays a vital role in mediating the inhibitory effect of SCFAs on the BRECs proliferation, and that these changes are not mediated by GPR41.
1 INTRODUCTION
Short-chain fatty acids (SCFAs) are produced during rumen microbial fermentation of dietary fibre (Bergman, 1990) and are major source of energy for ruminants. Acetate, propionate and butyrate constitute approximately 95% of total SCFAs (Kristensen, Danfaer, & Agergaard, 1998). In addition to providing an energy source for ruminants, SCFAs can enhance leptin production, neutrophil recruitment and expression of antimicrobial peptides (Kles & Chang, 2006; Sina et al., 2009; Xiong et al., 2004). Furthermore, SCFAs regulate cell proliferation, differentiation and apoptosis, along with inflammatory responses and the sympathetic nervous system (Hague, Elder, Hicks, & Paraskeva, 1995; Kimura et al., 2011; Maslowski et al., 2009).
The rumen epithelium exhibits profound changes in calves during its development and maturation from birth to 4 weeks of age (Lyford, 1988). These physical and functional alterations in the rumen epithelium are thought to be caused by SCFAs (Lane, Baldwin, & Jesse, 2000). SCFAs, including acetate, propionate and butyrate, stimulate the growth of rumen epithelium and increase the concentration of blood insulin, leptin production and neutrophil recruitment (Horino, Machlin, Hertelendy, & Kipnis, 1968; Lane & Jesse, 1997; Sina et al., 2009; Xiong et al., 2004); however, the mechanisms underlying the regulatory effects of SCFAs on bovine rumen epithelial cells (BRECs) proliferation have yet to be fully elucidated. Previous studies have demonstrated that continuous intraruminal infusion of physiological SCFAs or dietary supplementation with sodium butyrate can contribute to ruminal epithelium development and maturation in young ruminants (Gorka et al., 2009; Lane & Jesse, 1997), and butyrate plays the predominant role in regulating the growth of ruminal epithelium (Mentschel, Leiser, Mulling, Pfarrer, & Claus, 2001; Tamate, Mcgilliard, Jacobson, & Getty, 1962). Another study demonstrated that rumen fluid inhibits the proliferation of BRECs via upregulation of the cyclin-dependent kinase inhibitors 1A (CDKN1A) and 2A (CDKN2A) (Wang & Jiang, 2010). The individual addition of 6.5 mM acetate, 2.5 mM propionate or 1 mM butyrate did not affect BREC proliferation (Wang & Jiang, 2010). However, SCFAs, including butyrate, can also inhibit the proliferation of numerous cell types in vitro (Qiu, Ma, Yang, Wang, & Jiang, 2017; Wu, Zhou, Hu, & Dong, 2012). This apparent discrepancy between in vivo and in vitro results has led some to hypothesize that the stimulatory effect of SCFAs on ruminal epithelium growth may be indirect in vivo (Davie, 2003; Harmon, 1992).
Human G protein-coupled receptor 41 (GPR41) is a receptor for SCFAs (Brown et al., 2003; Le Poul et al., 2003; Nilsson, Kotarsky, Owman, & Olde, 2003). In addition, the bovine GPR41 gene was identified as being expressed in bovine rumen epithelium (Wang, Gu, Heid, Akers, & Jiang, 2009). Wang et al. (2009) also focused on the potential role of GPR41 in mediating the regulatory effects of SCFAs on rumen epithelium growth in cattle. We hypothesized that the regulatory effects of SCFAs on BREC proliferation may also be mediated by GPR41. Therefore, we investigated the mechanisms underlying the regulatory effects of SCFAs and GPR41 on BRECs proliferation.
2 MATERIALS AND METHODS
2.1 Isolation and cultivation of primary bovine rumen epithelial cells
Bovine used in this study was complied with the guidelines of the Institutional Animal Care and Use Committee of Yang Zhou University. Bovine rumen epithelial tissues from three young Holstein calves were obtained from the Experimental Farm of Yang Zhou University. Primary bovine rumen epithelial cells (BRECs) were obtained as described previously with minor adaptations (Wang & Jiang, 2010). The rumen tissues were quickly excised and repeatedly rinsed using PBS (Sigma-Aldrich, Shanghai, China) containing 500 U/mL penicillin, 500 µg/ml streptomycin, 250 µg/ml gentamicin (Solarbio, Beijing, China) and 12.5 µg/ml amphotericin B (5 × PSGA; Invitrogen, Shanghai, China). Then, the rumen epithelium tissues were separated using blunt dissection and transported to laboratory in DMEM medium containing 5 × PSGA on ice right now. These tissue pieces were repeatedly washed with DMEM/F12 medium (Invitrogen) containing 5 × PSGA until the supernatant was clear. Subsequently, these tissues were minced into ~1 mm3 size and repeatedly washed by centrifugation at 200 × g at 4°C for 1 min until the supernatant was clear. Minced epithelium was placed into the digestion flask containing 30 ml of 0.25% trypsin-0.02% EDTA (Invitrogen). The flask was placed in a slow shaking hot air incubator for 10 min at 37°C. The process was performed for three times, and the digestion solution was discarded. These remaining epithelium tissues were digested again as described above. The process was performed for four times, and the digestion solution was filtered through 74-µm nylon mesh into 50-mL sterile tube containing DMEM/F12 medium supplemented with 10% foetal bovine serum (FBS) and collected by centrifugation at 200 × g at 4°C for 5 min and discard the supernatant. Cells were washed with DMEM/F12 medium containing 5 × PSGA for three times. Cells pellet was resuspended in DMEM/F12 medium containing 10% FBS, 100 U/mL penicillin, 100 µg/ml streptomycin, 1% non-essential amino acids (NEAA), 4 mm/L glutamine, 1 × insulin, transferrin, sodium selenite (10 µg/ml insulin, 5.5 µg/ml transferrin, 0.0067 µg/ml sodium selenite, Invitrogen, Shanghai), 15 ng/ml epidermal growth factor (EGF; PeproTech, Shanghai, China) and seeded in 6-well plate.
2.2 Generation of immortalized BRECs and GPR41 knockdown BRECs
Immortalized BRECs and BRECs with GPR41 knockdown (GPR41KD BRECs) using the CRISPR/Cas9 system were provided by the Institute of Animal Culture Collection and Application, Yangzhou University. Primary BRECs were immortalized using simian virus 40 large T antigen, and 200 µl cell suspension was aliquoted into each well of 96-well plates to perform the cell purification. In this way, five immortalized BRECs clones were successfully established. In addition, these cells are expressed for cytokeratin 18 and SCFAs transporters including monocarboxylate transporter 1 (MCT1), MCT4, Na+/H+ exchanger 1 (NHE1), NHE2 and NHE3 (Supplementary Figure S1 and S2). The GPR41KD BRECs were generated by CRISPR/Cas9 system. The GPR41 targeted gRNA expression oligos were introduced into the vector expressing Cas9 protein and gRNA. The 6 µg plasmids DNA containing each target gRNA sequence (Table 1) were together electrotransfected into BRECs. The transfected cells were cultured for 3 days, and cells were diluted to aliquot into one cell per well. Single cell colony was select to extract the genome DNA. The genomic region surrounding the GPR41 target gRNA site was amplified. The GPR41 amplified forward and reverse primers, respectively, were 5 GGAAGCTGATGGTGCGAGAC 3, 5 AACCTGGAGAAGGGGCAGAA 3. The PCR products were separated in 2% agarose gel electrophoresis, and the 2% agarose gel with target bands were cut out to purify the target bands by Universal DNA Purification Kit. These target bands from cutting agarose gel were cloned into the T vector to analyse the target PCR products sequence.
| Name | gRNA sequence (5’−3’) | PAM sequence |
|---|---|---|
| gRNA1 | CCTTCTTCCTCGGCAATCAC | TGG |
| gRNA2 | GATGGCCCTGGTGATCTTCG | TGG |
| gRNA3 | AAACCTCACCCTCTCGGATC | TGG |
2.3 Cell proliferation assay
The BRECs and GPR41KD BRECs were seeded into 96-well plates at 2,500 cells per well. After 12 hr, the BRECs and GPR41KD BRECs were cultured in DMEM/F12 medium in the presence or absence (control group) of 10 mM SCFAs respectively. The mixture of 10 mM SCFAs contained 6 mM sodium acetate, 2.5 mM sodium propionate and 1.5 mM sodium butyrate (Sigma, Shanghai, China). The 10 mM SCFAs was chosen for the study according to the previous study (Wang & Jiang, 2010). These cells were then cultured for 1, 2, 3, 4, 5 or 6 days (n = 3) respectively. Next, cells were incubated in 100 μl DMEM/F12 supplemented with 10 μl of Cell Counting Kit-8 (CCK-8; Dojindo, Shanghai, China) at 37°C and 5% CO2 for 2 hr. Absorbance at 450 nm was then measured in each well using Multiskan Go microplate reader (Thermo Scientific, Shanghai, China).
2.4 Flow cytometric assay
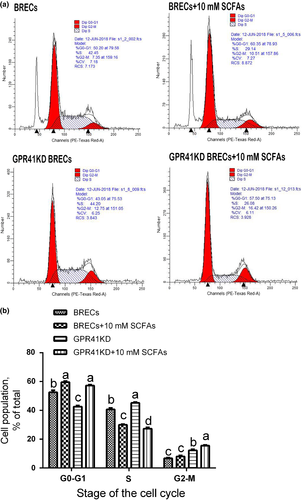
The cells were seeded in 25 cm2 tissue culture plates (1.3 × 105 cells per plate) and incubated in DMEM/F12 medium at 37°C, 5% CO2 for 24 hr. After incubation, these cells were isolated by trypsinization and washed by adding 40 ml PBS. Then, cells were centrifuged at 300 × g for 10 min, and supernatants were carefully aspirated. Cells were then incubated in 5 ml cold 75% ethanol at −20°C for 2 hr. Next, these cells were washed twice, and the supernatants were carefully removed. Thereafter, cells were washed in PBS and then incubated in stain buffer containing 5% horse serum, followed by careful aspiration of supernatants and incubation of cells in 100 µl PI/RNase Staining Buffer (BD Biosciences, Shanghai, China) for 15 min at room temperature. DNA content was determined by flow cytometry (BD Biosciences), and cell cycle phase characteristics were analysed using ModFit LT software (Verity Software House, Topsham, USA).
2.5 RNA Extraction and Real-Time Quantitative PCR
For mRNA expression analysis, cells were seeded in 12-well plates at 5 × 104 cells per well and grown in DMEM/F12 medium at 37°C, 5% CO2. The BRECs and the GPR41KD BRECs were, respectively, cultured in the presence or absence of 10 mM SCFAs for 72 hr, followed by isolation of total RNA using a TRIzol kit (Tiangen, Beijing, China). Reverse transcription (RT) was performed using an RT Kit (Takara, Beijing, China). RT reaction mixtures contained 1 μg total RNA and 1 × PrimeScript RT Master Mix in a final volume of 20 µl and were conducted for 15 min at 37°C. Reverse transcriptase was inactivated by heating to 85°C for 5 s. Quantitative RT-PCR (qRT-PCR) assays were performed using SYBR® Premix Ex TaqTM II Kit (Takara). The qRT-PCR reaction mixture contained 1 × SYBR® Premix Ex TaqTM II, 0.4 μM each forward and reverse primers and 2 μl cDNA templates in a final volume of 20 µl. Reactions were performed as follows: initial denaturation at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and 60°C for 30 s. The primers used are listed in Table 2 and were synthesized by Suzhou Genewiz Biological Co. (Suzhou, China). The relative expression levels of target genes were normalized to those of GAPDH and calculated using the 2−ΔΔCT method.
| Gene | Primer sequence, 5' to 3' | Accession number | Product size (bp) | Source |
|---|---|---|---|---|
| GAPDH | F: GGGTCATCATCTCTGCACCT | NM_001034034 | 176 | Zhou, Akers, & Jiang, 2008 |
| R: GGTCATAAGTCCCTCCACGA | ||||
| CDKN2B | F: ACCCGGAAGTCACCTCAATT | NM_001075894 | 226 | Present study |
| R: GGGGCTCTCTGAATCCTACC | ||||
| CDKN2A | F: CCTCTGAAGTCAAAAGGCGG | XM_010807758 | 121 | Present study |
| R: AAATCCTGACTCGTGGTGGG | ||||
| CDKN1A | F: GCAGACCAGCATGACAGATT | NM_001098958 | 205 | Wang & Jiang, 2010 |
| R: GTATGTACAAGAGGAGGCGT | ||||
| CDKN1B | F: GACCTGCCGCAGATGATTCC | NM_001100346 | 249 | Wang & Jiang, 2010 |
| R: CCATTCTTGGAGTCAGCGAT | ||||
| Cyclin D1 | F: GCACTTCCTCTCCAAGATGC | NM_001046273 | 204 | Wang & Jiang, 2010 |
| R: GTCAGGCGGTGATAGGAGAG | ||||
| GPR41 | F: AACCTCACCCTCTCGGATCT | NM_001145233 | 214 | Wang et al., 2009 |
| R: GCCGAGTCTTGTACCAAAGC | ||||
| HDAC1 | F: CTCCATCCGCCCAGATAACA | NM_001037444 | 124 | Wang et al., 2011 |
| R: CACAGAGCCACCAGTAGACAG | ||||
| HDAC7 | F: GCTTCTCAATAAGGACAAGA | NM_001193141 | 121 | Present study |
| R: ATTAGGATGAACCGTTCTCT | ||||
| HDAC8 | F: GCGAAGATGGAGATGATGAT | NM_001076231 | 168 | Present study |
| R: CAGACCAGTTGATTGCTACT | ||||
| Cyclin E1 | F:TTGACAGGACTGTGAGAAGC | XM_612960 | 229 | Wang & Jiang, 2010 |
| R:TTCAGTACAGGCAGTGGCGA | ||||
| Cyclin E2 | F:CTGCATTCTGAGTTGGAACC | NM_001015665 | 210 | Wang & Jiang, 2010 |
| R:CTTGGAGCTTAGGAGCGTAG | ||||
| CDK2 | F: CTCACTGATCTTGTCTGGTT | NM_001014934 | 170 | Present study |
| R:TAAGCAACGACTAAGAGGAG | ||||
| CDK4 | F ACTCTGGTATCGTGCTCCAGAAG | NM_001037594 | 114 | Totty, Morrell, & Spicer, 2017 |
| R:CAGAAGAGAGGCTTTCGACGAA | ||||
| CDK6 | F:TTCGTGGAAGTTCAGATGTC | NM_001192301 | 165 | Present study |
| R:TGCCTTGTTCATCAATGTCT | ||||
| Cyclin D2 | F CCAGACCTTCATCGCTCTGT | XM_024992177 | 163 | Wang & Jiang, 2010 |
| R:GATCTTTGCCAGGAGATCCA | ||||
| Cyclin D3 | F:TCCAAGCTGCGCGAGACTAC | XM_005223490 | 178 | Wang & Jiang, 2010 |
| R:GAGAGAGCCGGTGCAGAATC |
- Abbreviations: F, forward; R, reverse.
2.6 Statistical analysis
All data are presented as means ± standard error of the results of three independent experiments. The cell proliferation was analysed using two-way analysis of variance, and the others results were evaluated by one-way analysis of variance (ANOVA), followed by determination of the least significant difference (LSD) for post hoc multiple comparisons of treatment means, using SPSS 19.0 software (SPSS Inc.; Chicago, IL, USA). p values < .05 were considered significant. Trends towards significance are discussed for 0.05 < p < .10.
3 RESULTS AND DISCUSSION
To determine whether the inhibitory effects of SCFAs on BREC proliferation are mediated by GPR41, we examined the proliferation status of BRECs with GPR41 expression knocked down using the CRISPR/Cas9 system. The genomic region surrounding GPR41 target gRNA site was amplified, and the 2% agarose gel containing target bands was cut out to purify the target bands. These target bands were separated in 2% agarose gel electrophoresis (Figure 1a). The lane 1 shows the PCR product from the wild-type (WT) BRECs. The lane 2 and lane 3 represent the PCR products from GPR41KD BRECs an allele and GPR41KD BRECs another allele with 142 bp deletion between gRNA1 and gRNA3 region respectively (Figure 1a). Next, the PCR products from cutting agarose gel were performed the sequencing analysis. Sequence analysis showed the 142 bp deletion between gRNA1 and gRNA3 region in GPR41KD BRECs (Figure 1b–d). qRT-PCR analyses confirmed the 50% expression of GPR41 in GPR41KD BRECs, relatively to control group (Figure 1e). In addition, GPR41 protein level was detected with GPR41 polyclonal antibody (Invitrogen, Cat No: PA5-75521) by Western blot. Unfortunately, the GPR41 band was not found. The polyclonal antibody GPR41 antigen is derived from synthetic peptide corresponding to amino acids 61–106 of human GPR41, which may did not cross-react with bovine.

The BRECs treated with 10 mM SCFAs inhibit cells proliferation (p < .001; Figure 2) and activate (p < .05) the expression of GPR41 relative to the control group, while the GPR41KD BRECs with SCFAs completely abolished their proliferation ability (Figure 2). The rumen fluid inhibits the proliferation of BRECs, but, the individual addition of 6.5 mM acetate, 2.5 mM propionate or 1 mM butyrate did not alter the proliferation of BRECs (Wang & Jiang, 2010). These data indicate that the regulatory effects of SCFAs on BRECs proliferation are not mediated by GPR41; nevertheless, the GPR41 receptor does influence proliferation of these cells. Consistent with previous study, butyrate and propionate induce the apoptosis of neutrophils, but not mediated by GPR41 and GPR43 pathway (Aoyama, Kotani, & Usami, 2010).

A previous study demonstrated that BRECs induced by 10% rumen fluid had more cells at the G1/G0 phase and less cells at the G2/M phase compared with BRECs lacking 10% rumen fluid. However, 10% rumen fluid did not alter the number of cells at the S phase (Wang & Jiang, 2010). In comparison with the control group, BRECs treated with SCFAs for 24 hr included a higher proportion of cells in G0-G1 phase (p = .001; Figure 3a and b). Consistent with this observation, BRECs treated with SCFAs for 24 hr included fewer cells in S phase (p < .001). These data suggest that SCFAs can inhibit BRECs proliferation by disruption of progression from G1 to S phase of the cell cycle.
To understand the molecular mechanisms underlying the inhibitory effects of SCFAs and GPR41KD on BRECs proliferation, expression of cell cycle regulators and histone deacetylases (HDACs) was analysed by qRT-PCR. The cyclins D1, D2, D3 and E1 interact with cyclin-dependent kinases, triggering an elevation of cyclin-dependent kinases in G1 phase of the cell cycle (Khleif et al., 1996). The activity of these kinases is required for the progression from G1 to S phase, and their activity during the cell cycle is regulated by cyclin-dependent kinase inhibitors (Sherr, 1996). One such inhibitor, CDKN1A, inhibits the catalytic activity of the CDK2/cyclin E complex in mid- to late G1 phase to block the progression from G1 to S phase (Pestell et al., 1999). Another inhibitor, CDKN2A, inhibits CDK4 and CDK6 activity in early-G1 phase to block progression from G1 to S phase (Khleif et al., 1996). In our study, BRECs treated with SCFAs exhibited (p < .05; Table 3) the enhanced CDKN1A, CDKN2A and CDKN2B mRNA expression compared with the control group, but, the GPR41KD BRECs did not affect the expression of CDKN1A mRNA. Remarkably, the GPR41KD BRECs with SCFAs restored high level of CDKN1A relative to the GPR41KD BRECs (p < .001; Table 3), but did not change the expression of CDKN2A and CDKN2B. In addition, CDKN2A and CDKN2B mRNA expression were attenuated in GPR41KD BRECs. Butyrate induces cell proliferation arrest through modulation of CDKN1A and cyclin expression (Muhlethaler-Mottet et al., 2008; Ocker & Schneider-Stock, 2007). In addition, previous study reported that rumen fluid enhanced the inhibition of BRECs proliferation is associated with CDKN1A and CDKN2A upregulation; however, the individual addition of 6.5 mM acetate, 2.5 mM propionate or 1 mM butyrate did not affect BRECs proliferation (Wang & Jiang, 2010). These data indicate that SCFAs inhibit the proliferation of BRECs through increasing CDKN1A expression, and inhibitory effects of GPR41KD on BRECs proliferation is not related to CDKN1A, CDKN1B, CDKN2A and CDKN2B.
| Symbol | Treatmenta | SEM | p-value | |||
|---|---|---|---|---|---|---|
| Control | SCFAs |
GPR41KD BRECs |
GPR41KD BRECs + SCFAs |
|||
| CDKN1A | 1.00b | 4.60a | 0.72b | 4.30a | 0.55 | <.001 |
| CDKN1B | 1.05 | 0.76 | 0.60 | 0.55 | 0.08 | .086 |
| CDKN2A | 1.03b | 4.29a | 0.78b | 0.79b | 0.45 | <.001 |
|
CDKN2B |
1.02b | 1.89a | 0.39c | 0.67bc | 0.18 |
<.001 |
| Cyclin E1 | 1.00c | 3.89a | 0.71c | 2.58b | 0.39 | <.001 |
| Cyclin E2 | 1.46b | 4.42a | 0.83b | 2.00ab | 0.43 | .044 |
| CDK2 | 1.01b | 1.71a | 0.72b | 1.01b | 0.12 | .001 |
| CDK4 | 1.00a | 0.99a | 0.68b | 0.72b | 0.06 | .02 |
| CDK6 | 1.09b | 2.21a | 0.98b | 1.50b | 0.16 | .003 |
| Cyclin D1 | 1.00d | 3.53b | 1.97c | 4.8a | 0.45 | <.001 |
| Cyclin D2 | 1.00c | 2.33a | 0.47d | 1.92b | 0.08 | <.001 |
| Cyclin D3 | 1.00ab | 2.19a | 0.70b | 2.17a | 0.22 | .024 |
| HDAC1 | 1.01b | 1.36b | 1.41b | 1.98a | 0.12 | .004 |
| HDAC7 | 1.00c | 1.61ab | 1.12bc | 1.90a | 0.13 | .01 |
| HDAC8 | 1.02 | 0.85 | 0.81 | 1.26 | 0.08 | .209 |
- a,b,c,dMeans in the same row with different superscripts differ significantly for treatment effect.
- a The BRECs were cultured in DMEM/F12 medium in the presence or absence (control group) of 10 mM SCFAs. The GPR41KD BRECs were cultured in DMEM/F12 medium in the presence or absence of 10 mM SCFAs.
To determine whether the upregulation of CDKN1A can inhibit the expression of cyclin-dependent kinases, we examined the expression of cell cycle regulators. The BRECs induced by SCFAs significantly enhanced (p < .05; Table 3) the expression of genes involved in cell cycle regulators, CDK2, CDK6, cyclin D1, cyclin D2, cyclin E1 and cyclin E2 compared with control. The GPR41KD BRECs treated with SCFAs did not reduce the expression of CDK2, CDK4, CDK6, cyclin D1, cyclin D2, cyclin D3, cyclin E1 and cyclin E2, relative to the GPR41KD BRECs. These results suggest that cyclin-dependent kinase inhibitors have no inhibitory effects on cyclin-dependent kinases. We presumed that this could depend on species of origin of the cell and cell type-specific. Remarkably, GPR41KD BRECs trigger a decrease (p < .05) in the mRNA level of CDK4 and cyclin D2 relative to the control group. Moreover, the GPR41KD BRECs induced by SCFAs significantly decreased (p < .05) the level of CDK4 and cyclin D2 compared with BRECs treated with SCFAs. These results indicate that inhibitory effects of GPR41KD on BRECs proliferation may be involved in downregulation of CDK4 and cyclin D2 expression. The complex of CDK4/cyclin D is activated, and this in turn leads to phosphorylation of retinoblastoma protein that causes the release of E2F activators to bypass of the G1/S phase checkpoint (Lukas, Bartkova, & Bartek, 1996). The GPR41KD BRECs did not upregulate the expression of CDKN1A compared with control, but decrease the level of CDK4 and cyclin D2, leading to the slow proliferation in GPR41KD BRECs. Remarkably, the downregulation of CDK4 and upregulation of CDKN1A combined in GPR41KD BRECs treated with SCFAs show a strong inhibitory effect on cells proliferation, which leads to the abrogation of their proliferation. These results suggest that CDKN1A plays a vital role in mediating the inhibitory effect of SCFAs on the proliferation of BRECs. In addition, inhibitory effect of GPR41KD on the proliferation of BRECs is likely involved in the inactivation of CDK4/cyclin D2 complex.
The inhibitory effects of SCFAs on HDACs have been investigated (Hinnebusch, Meng, Wu, Archer, & Hodin, 2002), and inhibition of HDACs can suppress cell proliferation and trigger blockade of cell cycle progression (Muhlethaler-Mottet et al., 2008). An in vitro cell culture study showed that butyrate and propionate inhibit neutrophil proliferation by attenuating the HDAC activity (Aoyama et al., 2010). Moreover, transcriptome analysis revealed that addition of SCFAs to BRECs for 24 hr did not lead to profound alterations in the expression of HDAC1, 2, 3, 4, 5, 6, 9 or 10, but enhanced HDAC11 expression (data not shown). Our data revealed that HDAC1 expression was significantly enhanced in GPR41KD BRECs with SCFAs compared with the other groups (p < .05; Table 3). In addition, BRECs with SCFAs exhibited a significant increase in HDAC7 mRNA abundance compared with controls (p < .001), and GPR41KD BRECs with SCFAs have higher expression level of HDAC7, relatively to other groups. Previous study shows that butyrate is more potent in downregulation of HDAC activity, compared with acetate and propionate (Davie, 2003). In addition, butyrate is reported to induce GPR41 activation, thereby inhibiting histone acetylation and cell proliferation (Wu et al., 2012). However, the silenced genes were activated though inhibiting the activity of HDAC, leading to the activation of transcriptional level in cancer cells (Glozak & Seto, 2007). Our data indicate that inhibitory effects of SCFAs and GPR41KD on BRECs proliferation are not involved in the downregulation of HDACs. These contradictory findings made us reconsider the regulatory effect of HDACs on cell proliferation.
4 CONCLUSION
In the present study, we hypothesized that the regulatory effects of SCFAs on the proliferation of BRECs in vitro may be mediated by GPR41. Our results do not support our original hypothesis. Our findings reveal that downregulation of CDK4 and cyclin D2 likely mediates the inhibitory effects of GPR41KD on BRECs proliferation. In addition, CDKN1A plays a vital role in mediating the inhibitory effect of SCFAs on the proliferation of BRECs, and that these changes are not mediated by GPR41.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (No. 31572430), National Key Research and Development Project of China (2017YFD0502104-3), and China Agriculture Research System (CARS-36).
ANIMAL WELFARE STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The authors confirm that they have followed EU standards for the protection of animals used for scientific purposes.