iMS-Bmal1−/− mice show evident signs of sarcopenia that are counteracted by exercise and melatonin therapies
José Fernández-Martínez and Yolanda Ramírez-Casas contributed equally to this study.
Abstract
Sarcopenia is an age-related disease characterized by a reduction in muscle mass, strength, and function and, therefore, a deterioration in skeletal muscle health and frailty. Although the cause of sarcopenia is still unknown and, thus, there is no treatment, increasing evidence suggests that chronodisruption, particularly alterations in Bmal1 clock gene, can lead to those deficits culminating in sarcopenia. To gain insight into the cause and mechanism of sarcopenia and the protective effect of a therapeutic intervention with exercise and/or melatonin, the gastrocnemius muscles of male and female skeletal muscle-specific and inducible Bmal1 knockout mice (iMS-Bmal1−/−) were examined by phenotypic tests and light and electron microscopy. Our results revealed a disruption of the normal activity/rest rhythm, a drop in skeletal muscle function and mass, and increased frailty in male and female iMS-Bmal1−/− animals compared to controls. A reduction in muscle fiber size and increased collagenous tissue were also detected, accompanied by reduced mitochondrial oxidative capacity and a compensatory shift towards a more oxidative fiber type. Electron microscopy further supports mitochondrial impairment in mutant mice. Melatonin and exercise ameliorated the damage caused by loss of Bmal1 in mutant mice, except for mitochondrial damage, which was worsened by the latter. Thus, iMS-Bmal1−/− mice let us to identify Bmal1 deficiency as the responsible for the appearance of sarcopenia in the gastrocnemius muscle. Moreover, the results support the exercise and melatonin as therapeutic tools to counteract sarcopenia, by a mechanism that does not require the presence of Bmal1.
1 INTRODUCTION
Sarcopenia is an age-related disease characterized by the loss of muscle mass, strength, and function that leads to functional failure, frailty, worse quality of life, and mortality.1 Although the mechanisms of sarcopenia are not fully defined, evidence exist of a correlation between biological clock disruption and aging,2 which may underlie muscle atrophy, inflammaging, oxidative stress, and mitochondrial impairment, processes related to sarcopenia.3 Clock genes and proteins are also expressed in the skeletal muscle,4 where BMAL1, a clock protein that connects the clock with the immune system, participates in muscle regulation and repair, and improves the mitochondrial function in the cell.2, 5, 6 In turn, Bmal1 deletion produces a reduction in muscle strength and oxidative capacity, alters bone and cartilage development, enhances collagen deposition, and deteriorates myofiber formation in mice.7-10 Because Bmal1 decreased with age,6 chronodisruption in the peripheral muscle clock may be linked to sarcopenia.
There is no current treatment for sarcopenia, being exercise and increased protein intake are the main ways to delay the onset of the disease.11 Melatonin, N-acetyl-5-methoxytryptamin (aMT), a pineal hormone,12 is also produced in extrapineal tissues including the skeletal muscle.13 Besides the chronobiotic functions of melatonin, which depend on its pineal production, melatonin has also important antioxidative and anti-inflammatory properties, while boosts mitochondrial ATP formation.14-16 These beneficial effects of melatonin have been proved in multiple experimental conditions including acute and chronic inflammation, aging, and sarcopenia.6, 14, 17-19 We showed normal aging morphological and functional features of sarcopenia in the gastrocnemius muscle (GM) of mice at 12 and 24 months old.20 Here, melatonin treatment totally reversed the sarcopenic changes.19
We then hypothesized that age-related loss of Bmal1 in skeletal muscle may trigger changes that ultimately lead to sarcopenia. We also hypothesized that exercise together with melatonin may counteract sarcopenia. Besides incessant research on Bmal1 in aging-related diseases,21 Bmal1 mutation was not included in a recent review on genetic models of sarcopenia.22 Thus, we considered it worthwhile to study a skeletal muscle-specific and inducible Bmal1 knockout murine model (iMS-Bmal1−/−), to assess phenotypic, structural, and molecular changes in the GM of these mice after deleting Bmal1, and to analyze the benefit that a therapeutic intervention with exercise and/or melatonin may have on this muscle.
2 MATERIALS AND METHODS
2.1 Generation of the inducible skeletal muscle-specific Bmal1 knockout mouse model
Inducible skeletal muscle-specific Bmal1 knockout mice (iMS-Bmal1−/−) were generated as described in Hodge et al.23 Mice Cre LoxP recombination was activated with tamoxifen. Controls were treated with vehicle (Figure 1). Recombination specificity was confirmed by PCR and western blot23 (Supporting Information: Figures S1 and S2). Details are available in Supporting Information.

Procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (CETS #123), and the Spanish law for animal experimentation (R.D. 53/2013). The protocol was approved by the Andalusian's Ethical Committee (#29/05/2020/069).
2.2 Treatments
Animals were separated by sex and divided into the following five groups (n = 36 animals per group [18 males and 18 females]; Figure 1): (a) iMS-Bmal1flox/flox treated with vehicle (control), (b) iMS-Bmal1flox/flox treated with tamoxifen (TMX) to generate iMS-Bmal1−/− (conditional Bmal1 knockout), (c) iMS-Bmal1−/− treated with exercise (TMX + E), (d) iMS-Bmal1−/− treated with melatonin (TMX + aMT), and (e) iMS-Bmal1−/− treated with exercise plus melatonin (TMX + E + aMT).
Exercise consisted of 2 weeks on a horizontal treadmill (5 days/week, 30 min/day, up to a speed of 30 cm/s) plus 2 days of recovery required for reducing animal stress, according to previous reports,24 starting at 12 weeks of age.
Melatonin was administered at a dose of 10 mg/kg/day in the food pellets 25 for 2 weeks starting when the animals reached 12 weeks, according to previous studies showing the effectiveness of this dose in preventing age-dependent sarcopenia.19, 26, 27 Additional details are available in supplementary information.
2.3 Circadian rhythm of locomotor activity
A cohort of mice was used for the circadian behavioral analysis, following a normalized Basic Protocol.28 Wheel-running activity data from a total of 12 mice (6 males and 6 females) of each experimental group were collected and analyzed using the ClockLab program (Actimetrics). Details are available in Supporting Information.
2.4 Physical activity assessment
The spontaneous locomotor activity of the experimental animals was evaluated in an open-field test as previously described.19 Endurance and fatigue were assessed by using a treadmill test as previously described.19 Details are available in Supporting Information.
2.5 Frailty index calculation
A frailty index (FI) that correlates with the human index has been proposed and validated in animals including mice and primates.29 Accordingly, and using the clinical criteria for humans, we calculated the FI from the data of zoometric and muscular physical activity analysis as previously described.19 Details are available in Supporting Information.
2.6 Tissue preparation
After phenotyping studies, animals were weighted, anesthetized by intraperitoneal injection of equithesin (1 mL/kg), and killed to obtain the GM for histological and transmission electron microscopy (TEM) studies.20 Details are available in Supporting Information.
2.7 Skeletal muscle histology and histochemistry
Frozen muscle sections were stained with hematoxylin and eosin (H&E) stain for general histological examination, Van Gieson (VG) stain for differentiation of connective tissue and muscle fibers, and succinate dehydrogenase (SDH) stain for detection of mitochondrial oxidative capacity of muscle fibers. Details are available in Supporting Information.
2.8 Immunofluorescence for fiber type identification
Cryosections of GM were stained with monoclonal antibodies specific for the different myosin heavy chain (MyHC) isoforms, following the protocol described in Dyar et al.30 Details are available in Supporting Information.
2.9 TEM analysis
Samples were processed according to the previously described protocol19 and examined by a Carl Zeiss Libra 120 Plus (EELS) transmission electron microscope. Details are available in Supporting Information.
2.10 Statistical analysis
Data are expressed as mean ± SEM of n = 6 animals of each sex per group (for circadian rhythm assessment of locomotor activity), n = 12 animals of each sex per group (for muscular physical activity and FI assessment), and n = 4 animals of each sex per group (for image examination). All statistical analyses were carried out using GraphPad Prism 8 software (GraphPad Software). Two-way and three-way analysis of variance (ANOVA) were used for statistical comparisons and significance was taken as p < .05.
3 RESULTS
3.1 Lack of Bmal1 in skeletal muscle induces a phase advance in the running activity of mice, with exercise and mainly melatonin preventing the loss of rhythm
During the day (08:00 to 20:00 h), the activity of male and female mice was unmodified by TMX or interventions with exercise and/or melatonin. A significant reduction in the activity, however, occurred during the night (20:00 to 08:00 h) in both sexes of TMX mice, which was counteracted with melatonin but not with exercise (Figure 2A). Actograms (Figure 2C), activity profiles (Figure 2D), and periods (Figure 2F) in LD conditions were quite similar in male and female TMX mice compared to controls, with no influence of treatments. Female mice had higher activity than males at night in the control group (Figure 2A). These changes could also be perceived in actograms (Figure 2C) and activity profiles (Figure 2D). Circadian period in LD conditions showed no variability between sexes, and it was not dependent on an interaction between time of day, sex, or treatment after three-way ANOVA analysis (Figure 2F).
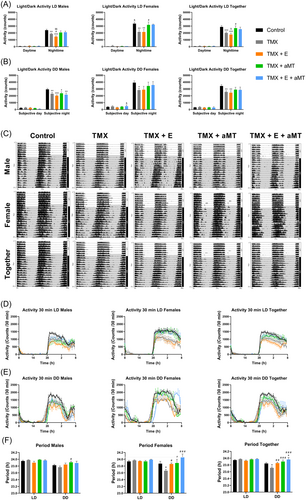
Subsequently, mice were placed in DD conditions for 14 days to define circadian locomotor measurements in free-running conditions. The behavior of mice was like that in the LD studies. Here, the lack of Bmal1 did not alter the activity of both male and female animals in the subjective day (08:00 to 20:00 h), but it significantly declined their activity in the subjective night (20:00 to 08:00 h), compared to controls. Exercise and/or melatonin also unaffected the locomotor activity during the subjective day but, in a similar way to LD studies, melatonin tended to counteract the loss of activity in TMX mice at night (Figure 2B). Actograms of male and female TMX animals in DD conditions exhibited an earlier and more pronounced advance in the onset of activity (phase advance) compared with controls, especially in females. Melatonin, but not exercise, fully restored the normal activity/rest rhythm in both sexes (Figure 2C,E). These variations in the circadian activity rhythms were confirmed by changes in the circadian period in DD conditions, which was significantly shorter in female TMX mice compared to controls. Exercise and/or melatonin increased the free-running period in males and mainly in females (Figure 2F). Again, female mice presented higher locomotor activity than males at subjective night and, in the subjective day, only significant in the TMX + E + aMT group (Figure 2B,C,E). Circadian period in DD conditions showed no significant differences between sexes, and again it was not dependent on an interaction between time of day, sex, or treatment after three-way ANOVA analysis (Figure 2F; see Supporting Information: Table S2).
3.2 Lack of Bmal1 in skeletal muscle prompts a decay in mice's physical activity that correlates with a drop in the FI, which is normalized with melatonin and exercise treatments
Male and female TMX mice showed a significant decrease in the total distance and mean speed, and a significant increase in the resting time compared to their corresponding controls in the open field test. Exercise and/or melatonin restored total distance, mean speed, and resting time changes caused by the skeletal muscle-specific loss of Bmal1 in both sexes, whereas the combination of both treatments showed greater recovery. There was a tendency to increase the total distance and mean speed and diminish the resting time in female animals compared to males, being significant in the TMX group (Figure 3A). Typically acquired maps of locomotor activity are enclosed (Figure 3B). In the treadmill test, male, and to a lesser extent female TMX animals traveled a shorter distance and resisted the exhaustion for a shorter time than controls. No significant effect of exercise was noted in the activity of male and female mice. However, these changes were reversed in males and females of the TMX + aMT and TMX + E + aMT groups, suggesting a main effect of melatonin. No significant difference in activity was found between sexes (Figure 3C).
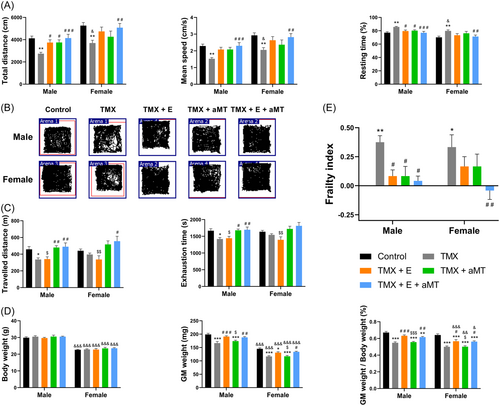
Figure 3E and Supporting Information: Table S1 show the FI calculated from the physical activity and zoometric data (Figure 3D) for each experimental group. The loss of Bmal1 in skeletal muscle enhanced the FI, and interventions with exercise and melatonin separately normalized FI recovering the physical impairment of male and female TMX mice. Exercise plus melatonin treatment further reduced the frailty status of male and female TMX animals, although synergistic effect was detected only in females. There were no differences in FI between sexes (see Supporting Information: Table S2).
3.3 Lack of Bmal1 in skeletal muscle induces a reduction in SDH activity and changes in fiber types of the GM, which were normalized after melatonin and exercise
We examined SDH activity and fiber type composition in the deep and superficial regions of GM, because of the presence of two distinct areas with different mitochondrial oxidative capacities in the latter.31 The absence of Bmal1 significantly reduced SDH activity in the deep region of GM in males and females compared to controls, without changes in the superficial GM. Melatonin administration further boosted SDH activity in deep and superficial GM in both sexes. Exercise improved the mitochondrial oxidative capacity in deep and superficial GM areas of males, but not females (Figure 4A,B).
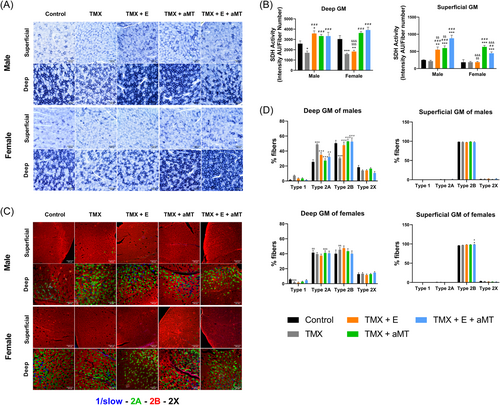
The fiber type profile of male and female TMX mice was comparable to controls, with the deep GM predominantly composed of type 2A and 2B fibers, few type 1 and 2X fibers, and the superficial GM composed almost entirely of type 2B fibers. Quantitative analysis revealed, however, a significant increase in type 2A fibers and a reduction of type 2B fibers in the deep GM area of TMX males, with no changes in females. Interventions with exercise and/or melatonin restored the normal percentage of type 2A and 2B fibers in the deep GM area of males. In other cases, treatments did not modify the fiber type profile (Figure 4C,D). As shown in Figure 4D, quantitative analysis disclosed small variations in muscle fiber composition between sexes in some experimental groups (see Supporting Information: Table S2).
3.4 Lack of Bmal1 in skeletal muscle reveals histological and morphometrical changes in GM that are counteracted by melatonin and exercise
GM of male and female control mice showed a normal structure of the muscle. Skeletal muscle fibers of both male and female animals lacking Bmal1 in skeletal muscle revealed some changes including atrophy of the muscle fibers, and a significant increase in collagen content. Muscles, however, were correctly arranged and nuclei were peripherally located. Interventions with exercise and/or melatonin had a protective effect on the muscle fibers of male and female TMX mice. Both therapies preserved the normal architecture of the fibers, restoring the normal size of the fibers and reducing, in part, the accumulation of collagenous tissue. No variation was found in the structure of the muscle fibers between sexes. Analysis of collagen content revealed no changes between sexes, except in the TMX + aMT group, which presented a higher collagen accumulation in female animals (Figure 5A,C,D).
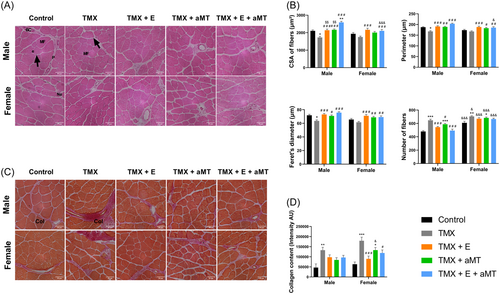
Morphometrically, the loss of Bmal1 in skeletal muscle induced a significant decrease in the individual muscle fiber CSA, perimeter, and Feret's diameter in male TMX mice and a slight reduction of these parameters in TMX females, compared to their corresponding controls. An increase in the number of muscle fibers was, however, observed in both male and female TMX animals compared with controls. Exercise and/or melatonin enhanced muscle fiber CSA, perimeter, and Feret's diameter in male and female mice, and declined the number of fibers in male muscles, with no significant effect on fibers number in females. Female mice presented higher number of muscle fibers than males. Individual muscle fiber CSA, perimeter, and Feret's diameter were similar in both sexes, only with a significant reduction in the CSA and perimeter with the combined treatment of exercise and melatonin in females relative to males (Figure 5B) (see Supporting Information: Table S2).
3.5 Lack of Bmal1 in skeletal muscle provokes ultrastructural alterations in the intermyofibrillar mitochondria of the GM, which were reversed with melatonin treatment, but not with exercise
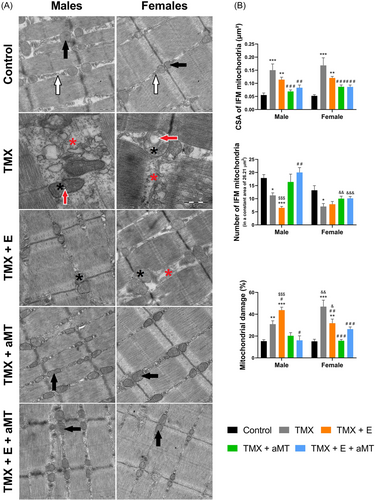
TEM of the gastrocnemius myofibrils (Figure 6A) and intermyofibrillar mitochondria (IFM, Figure 7A) in male and female control mice revealed the normal ultrastructural skeletal muscle architecture. Lacking Bmal1 however, induced disorganization of the sarcoplasmic reticulum (red asterisks) and destruction of the IFM cristae (black asterisks), where some IFM exhibited various-sized vacuoles (red arrows). Exercise therapy had few impacts on preserving myofibrils orientation, IFM damage, and sarcoplasmic reticulum disorganization. However, these alterations have been interestingly avoided with melatonin administration.
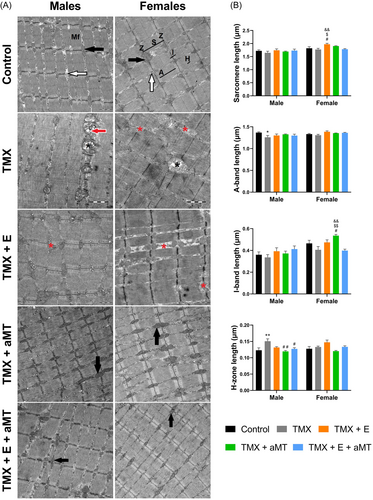
Morphometrically, male mice reported nonsignificant changes in the sarcomere length among the experimental groups. However, TMX female mice treated with exercise increased the sarcomere length. Lack of Bmal1 was associated with a significant decrease in the A-band length in male animals. Both exercise and/or melatonin therapies showed no effect on groups. I-band length demonstrated nonsignificant changes among male groups. However, in females, the only significant increase was found in the TMX animals treated with melatonin. Length of H-zone length was significantly increased in the TMX male animals, and this increase was significantly resorted by interventions with aMT, and with exercise plus melatonin (Figure 6B).
Interestingly, the lack of Bmal1 induced a significant increase in the CSA of IFM in both sexes, and this increase was reduced by melatonin and exercise therapies. Furthermore, TMX caused a significant reduction in the IFM number, and this reduction was reversed in males and females with aMT intervention but not with exercise. The loss of Bmal1 induced a significant increase in the percentage of mitochondrial damage. Intervention with exercise maintained and even increased damage in males but reduced it in females. Melatonin therapy revealed a significant decrease in mitochondrial damage in both sexes. Exercise and aMT therapy together significantly reduced the TMX-induced IFM damage in both sexes (Figure 7B; see Supporting Information: Table S2).
4 DISCUSSION
The results here provide the first evidence demonstrating the need for the intrinsic muscle Bmal1 clock gene in the maintenance of skeletal muscle structure and function and in preventing frailty. Moreover, our findings in mice deficient in skeletal muscle Bmal1 share many similarities with other data obtained in the chronic, age-dependent mouse model of sarcopenia,19, 20 proving the former to be a useful tool for these studies.
Increasing evidence supports the relationship between chronodisruption and disease.32 Clock genes control most cycling functions including clock-controlled genes, and Bmal1 gene is of special relevance because its presence in the skeletal muscle is essential for the maintenance of skeletal muscle health.7-9 In view of these observations, a causal relationship between reduced Bmal1 expression in aged skeletal muscle and sarcopenia should be considered.
The association between functional decline and low muscle mass underlies muscle weakness, which is important in estimating mortality risk.33 In this work, we show that tamoxifen-induced Bmal1 knockout results in an increase in the FI, a relevant clinical sign of the frailty condition of mice.29 Similarly, in normal-aged mice, which also show a drop in Bmal1 expression,6 we identified the early onset of sarcopenia and the increase in the FI.19, 20 These data suggest that Bmal1 deficiency in skeletal muscle compromises its physical condition and agree with previous reports showing that inactivation of Bmal1 in skeletal muscle impairs gait, mobility, and muscle strength.7, 23 Other authors using a whole body Bmal1 knockout found a more dramatic phenotype, with reduced locomotor activity, body and muscle weight, muscle force, and short life spam. The global inactivation of Bmal1 also produced an immediate and complete loss of circadian rhythmicity in constant darkness.34, 35
Here, we analyzed whether the inactivation of Bmal1 locally in the skeletal muscle may also affect locomotor rhythm. Male and female iMS-Bmal1−/− mice have decreased activity in both light/dark and total darkness conditions compared to controls. As expected, the damage of the GM of Bmal1-deficient mice reduced its physical activity when it is higher, that is, at nighttime and subjective night. The minimal activity of mice during the daytime and the subjective day made it difficult to detect an effect, if any, of Bmal1 absence. We also observe that the loss of Bmal1 causes a phase advance in constant darkness compared to controls, with a drop in the endogenous period, affecting mainly females. Phase advance is linked to disruption of the muscle clock and disease,36 and, more specifically, the circadian rhythm of Bmal1 is associated with the onset and offset control of phases of activity.37 These sex differences had been also reported in humans, having women an earlier sleep phase and slightly shorter circadian periods than men of the same age,38 and these findings could explain some behavioral differences between sexes here found. In this line, we also detect that female mice have higher activity than males in all the experimental groups, including controls, as has already been reported in the literature.39, 40 A recent study in ovariectomized females and estrogen-supplemented males proved that estrogen, increased nitric oxide synthase activity, a downstream target of estrogen, and increased type 1 and decreased type 2 fibers expression are important mechanisms mediating the enhanced exercise performance in females,41 being a possible explanation for this.
The changes observed iMS-Bmal1−/− mice extend to muscle structure and composition. The absence of Bmal1 in skeletal muscle correlates with a reduction in the muscle fiber size and increased collagenous tissue, indicating atrophy and fibrosis, respectively, two hallmarks of sarcopenia,42 which we also reported in 24-month-old mice.19, 20 Age-related muscle weakness and wasting are associated with a fast-to-slow fiber type shift43 and, here, we show that the lack of Bmal1 in skeletal muscle leads to an increase in type 2A fibers, more oxidative, and a reduction in type 2B, more glycolytic fibers in deep GM of males, without affecting females, which agree with lactate reduction of GM in 24-month-old mice by in vivo spectroscopy, reflecting anaerobic respiration reduction with age.19 Our data also agree with other Bmal1 knockout models in soleus, extensor digitorum longus, and tibialis anterior muscles,7, 23, 30 and suggest a shift toward a more oxidative fiber type with age. Despite the higher percentage of oxidative fibers in iMS-Bmal1−/− animals, we detected a reduction in SDH activity in the deep, but not in the superficial region of GM in males and females, indicating a reduced mitochondrial oxidative capacity. This suggests that the shift toward a more oxidative fiber type could be a compensatory mechanism to counteract the drop of normal mitochondrial function. Because Bmal1 participates in mitochondrial muscle bioenergetics and dynamics5, 44 and mitochondrial bioenergetics is impaired in skeletal muscle with age and sarcopenia,45, 46 mitochondrial damage may underlie the development of sarcopenia associated with skeletal muscle Bmal1 loss. This suggestion is supported by the structure impairment here analyzed with an increase in mitochondrial damage and the reduction in their number, and fully supports the idea that intrinsic skeletal muscle Bmal1 is necessary for musculoskeletal health.7 The changes in the GM structure and function, including collagen deposits, are related to the absence of Bmal1 that inactivates prolyl 4-hydroxylase subunit alpha 1 (P4ha1),10 and the impairment of mitochondrial function which probably promotes an inflammatory phenotype that affects its myogenic function.5, 7-9 In fact, Bmal1 is related to the control of NF-kB immune activity and mitochondrial function and dynamics through the activation of two NAD+-dependent sirtuins, SIRT1 and SIRT3, respectively, and homeostatic processes.5, 47, 48 The impairment of these physiological mechanisms by the loss of Bmal1 may lead to the muscle phenotype developed in our model. To date, however, there is not enough molecular knowledge to explain all these changes, and future studies are needed to clarify the mechanism(s) through which loss of Bmal1 only in muscle fibers contributes to its impairment. Overall, the results above described depend on the lack of Bmal1 in the GM because after tamoxifen administration Bmal1 expression was blunted during the total duration of the experiments.
There is increasing evidence in mice and humans that physical exercise and melatonin have a protective role in age-related sarcopenia,22, 49-53 including studies from our laboratory.19, 26, 27 Exercise helps people with sarcopenia, although it is not sufficient to prevent or reverse this disease. Pineal melatonin, on the other hand, exerts chronobiotic functions, but also significant antioxidant and anti-inflammatory functions complemented with mitochondrial improvements, depending on its extrapineal production that also occurs in the skeletal muscle of mammals including humans.6, 14-19, 54 Interventions with exercise and/or melatonin conserve muscular mass and physical activity altered in iMS-Bmal1−/− male and female animals, with some synergistic effects between both. These treatments restored a normal FI, supporting their benefit for preventing frailty. Furthermore, exercise, but mainly melatonin, restores the normal activity/rest rhythm in both sexes. This may be probably because, as we show here, the lack of Bmal1 impairs mitochondria, the organelle where melatonin is synthesized,55 reducing its endogenous levels that are recovered after its administration to these mice. Moreover, besides the melatonin-clock genes connection,2, 6, 14 the indoleamine is able to control directly the rhythm of mice even in the absence of Bmal1 in the GM. The exercise, although it is known that it controls some aging genes,56 has, however, less chronobiotic strength than melatonin.57 Of note, melatonin was not able to recover the total activity of mice lacking Bmal1, which may depend in part on the melatonin, at much higher concentration than pineal melatonin, that is, when administered exogenously, is known to possess a time-dependent hypnotic effect promoting sleepy state in mice.58, 59 Moreover, the beneficial effects of melatonin together with exercise extend to maintain the normal architecture of the GM, increasing the fiber size and reducing the collagenous tissue. Both treatments re-established the normal fiber type profile and enhanced mitochondrial oxidative capacity in GM mice, preventing the shift towards a more oxidative fiber type caused by the loss of Bmal1. Although exercise is useful to modify mitochondrial plasticity to adapt them to the metabolic requirements,60 we found here that exercise further increases mitochondrial damage in TMX mice. This is consistent with findings showing that excessive exercise causes mitochondrial impairment in skeletal muscle.61, 62 This paradoxical effect probably depends on the excess of ROS produced by these damaged mitochondria. Melatonin, however, totally counteracted the damage to mitochondria induced by tamoxifen and restored the number and CSA of them, resembling the mitochondrial biogenesis and antioxidative effects of the former.19, 63, 64
From experimental studies,65, 66 we suggested that the human equivalent dose of melatonin is 50–500 mg/day. Studies conducted in resistance-trained athletes showed an alteration of their motor activity rhythm that was restored after daily administration of 100 mg melatonin for 4 weeks.67 On the other hand, exercise is known to improve sarcopenia in the older and it may prevent the loss of skeletal muscle fibers.49-51 So, a primary intervention in humans could in exercise plus 50 mg melatonin in a daily schedule, although it would be very interesting to do a clinical trial to assess these approaches.
Our findings may be summarized in: (1) GM of iMS-Bmal1−/− mice displays significant behavioral, morphological, and functional changes that clarify the participation of the intrinsic muscle clock (Bmal1) in the skeletal muscle homeostasis; (2) because iMS-Bmal1−/− mouse is prone to sarcopenia in the GM and shares many similarities with other models of sarcopenia including aged mice, it can be considered as an adequate acute model for the study of the disease; (3) the results support exercise and mainly melatonin as therapeutic tools to counteract sarcopenia by a mechanism that does not require the presence of Bmal1 in skeletal muscle, and (4) clock genes constitute emerging targets against age-related diseases.68
AUTHOR CONTRIBUTIONS
Darío Acuña-Castroviejo took responsibility for the study design; José Fernández-Martínez and Yolanda Ramírez-Casas performed the experiments; Paula Aranda-Martínez, Alba López-Rodríguez, and Ramy KA Sayed analyzed the data; Germaine Escames, José Fernández-Martínez, and Yolanda Ramírez-Casas prepared the manuscript, and Darío Acuña-Castroviejo critically revised the manuscript. All the authors participated in drafting the manuscript and approved the final version for publication.
ACKNOWLEDGMENTS
This study was partially supported by grants from the Instituto de Salud Carlos III through the grants PI19-01372 and CB/10/00238 (co-funded by European Regional Development Fund/European Social Fund “Investing in your future”); the Consejería de Economía, Innovación, Ciencia y Empleo, Junta de Andalucía (CTS-101), Spain. José Fernández-Martínez is supported by an FPU fellowship from the Ministerio de Educación, Spain; Yolanda Ramírez-Casas has a PFIS fellowship from the Instituto de Salud Carlos III; Paula Aranda-Martínez has a fellowship from grant no. P18-RT-698, and Alba López-Rodríguez has a fellowship from grant no. P18-RT-3222, from the Consejería de Economía, Innovación, Ciencia y Empleo, Junta de Andalucía. Funding for open access charge: Universidad de Granada/CBUA
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




