Melatonin protects against ischemic heart failure in rats
Abstract
Ischemic injury, which occurs as a result of sympathetic hyperactivity, plays an important role in heart failure. Melatonin is thought to have antiatherogenic, antioxidant, and vasodilatory effects. In this study, we investigated whether melatonin protects against ischemic heart failure (HF). In Wistar albino rats, HF was induced by left anterior descending (LAD) coronary artery ligation and rats were treated with either vehicle or melatonin (10 mg/kg) for 4 weeks. At the end of this period, echocardiographic measurements were recorded and the rats were decapitated to obtain plasma and cardiac tissue samples. Lactate dehydrogenase, creatine kinase, aspartate aminotransferase, alanine aminotransferase, and lysosomal enzymes (β-D-glucuronidase, β-galactosidase, β-D-N-acetyl-glucosaminidase, acid phosphatase, and cathepsin-D) were studied in plasma samples, while malondialdehyde and glutathione levels and Na+, K+-ATPase, caspase-3 and myeloperoxidase activities were determined in the cardiac samples. Sarco/endoplasmic reticulum calcium ATPase (SERCA) and caveolin-3 levels in cardiac tissues were evaluated using Western blot analyses. Furthermore, caveolin-3 levels were also determined by histological analyses. In the vehicle-treated HF group, cardiotoxicity resulted in decreased cardiac Na+, K+-ATPase and SERCA activities, GSH contents and caveolin-3 levels, while plasma LDH, CK, and lysosomal enzyme activities and cardiac MDA and Myeloperoxidase (MPO) activities were found to be increased. On the other hand, melatonin treatment reversed all the functional and biochemical changes. The present results demonstrate that Mel ameliorates ischemic heart failure in rats. These observations highlight that melatonin is a promising supplement for improving defense mechanisms in the heart against oxidative stress caused by heart failure.
Introduction
Among chronic diseases, heart failure (HF) is the most common single cause of hospitalization in industrialized countries with frequent re-admissions 1-3. It is associated with significant mortality and morbidity and is a major public health concern. At age 40, the lifetime risk for developing HF is one in five for both men and women 4.
Left anterior descending artery (LAD) ligation of rats has been commonly used for the induction of myocardial infarction and has been extensively used in cardiovascular research 5. Interruption of oxygen and substrate supply during cardiac ischemia is known to block the mitochondrial synthesis of ATP. Thus, glycolytic ATP production tries to compensate the lack of energy leading to the production of lactate, acidification of the cytosol, and activation of the sarcolemmal Na+/H+ exchanger. Furthermore, due to inhibition of the sarcolemmal Na/K-ATPase as a consequence of a decreased [ATP]/[ADP] ratio, cytosolic sodium ([Na+]i) concentrations increase 6, followed by a rise in cytosolic calcium ([Ca2+]i) via the Na+/Ca2+ exchanger 7-9. These alterations in calcium homeostasis were through a downregulation of sarcoplasmic reticulum Ca2+-ATPase (SERCA) 10, and increased frequency of oxidative stress and apoptosis 8 may also play a crucial role in the transition of cardiac hypertrophy to heart failure.
Melatonin (N-acetyl-5-methoxytryptamine) a molecule produced by a diversity of organisms, from algae to humans, has an evolution parallel to that of aerobic metabolism 11-13. In all organisms, melatonin is primarily produced during the night; this includes man 14. Melatonin and its metabolic derivatives are uncommonly effective direct free radical scavengers, while also stimulate the activities of several antioxidative enzymes 11. Lipid peroxidatin in the cell membranes devastate to the functional integrity of these structures and melatonin scavenging highly toxic hydroxyl radical that initiates LPO, protects cell membranes 15. Besides its ability to scavenge the highly toxic hydroxyl radical (OH.), melatonin is also effective in neutralizing the peroxynitrite anion (ONOO-), hydrogen peroxide, the superoxide anion radical (O2.-), singlet oxygen as well as other reactants 15. It has also been reported to increase the synthesis of glutathione and several antioxidant enzymes 16. Upon metabolism, melatonin converts to a number of antioxidant compounds such as N1-acetyl-N2- formyl-5-methoxy-kynuramine and N1-acetyl-5-methoxykynuramine 17. Therefore, melatonin is considered to be a broad-spectrum antioxidant 18. The pineal indoleamine reduces the organ damage in ischemia/reperfusion injury 19 and ionizing radiation damage 20. In these two processes, free radicals are considered to be responsible for the cell injury. More recently, Dominguez-Rodriguez et al. 21 investigating the relationships between circulating levels of melatonin and left ventricular (LV) remodeling in patients after acute MI demonstrated the cardioprotective effects of melatonin.
In light of above findings, we tested the hypothesis that melatonin could alleviate ischemic heart failure through its antioxidant effects, as well as by its modulatory role on cardiovascular functions. Therefore, in the LAD-induced ischemic heart failure model, therapeutic effects of melatonin on cardiovascular functions, as well as its protective effects against oxidative damage in target organs, were evaluated using various physiological and biochemical parameters.
Materials and methods
Animals
Male Wistar albino rats (200–250 g) were housed in an air-conditioned room with 12-h light and dark cycles, where the temperature (22 ± 2°C) and relative humidity (65–70%) were kept constant. All experimental protocols were approved by the Marmara University School of Medicine Animal Care and Use Committee. Rats were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg, ip). The chest was opened by a left thoractomy and the heart was exposed. The left anterior descending (LAD) was ligated and then the chest was closed 5. Rats surviving 24 hr after the surgery were randomly assigned to receive either melatonin (i.p.; once daily, at the dose of 10 mg/kg) or vehicle (%0.5 alcohol) for 4 weeks. The sham-ligated rats also received vehicle or melatonin. Body weight was recorded at the beginning of the study to obtain the basal levels. At the end of the 4th week of the initial treatments, body weight was repeated, while trans-thoracic echocardiography was performed to assess the cardiac function of the rats. After decapitation, trunk blood was collected and immediately centrifuged at 3000 g for 10 min to assay lactate dehydrogenase, creatine kinase, aspartate aminotransferas, alanine aminotransferase, and lysosomal enzymes (β-D-glucuronidase, β-galactosidase, β-D-N-acetyl-glucosaminidase, acid phosphatase, and cathepsin-D). To evaluate the presence of oxidative tissue injury, cardiac samples were taken and stored at −80°C for the determination of malondialdehyde and glutathione levels, myelopreoxidase, caspase-3 and Na+, K+-ATPase activities. To assess the role of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and caveolin-3 (Cav-3) in LAD-induced heart failure, SERCA and cav-3 were determined by western blot analysis and histological analyses.
Echocardiography
Echocardiographic imaging and calculations were performed according to the guidelines published by the American Society of Echocardiography 22 using a 12 MHz linear transducer and 5–8 MHz sector transducer (Vivid 3,General Electric Medical Systems Ultrasound, Tirat Carmel, Israel). Under ketamine (100 mg/kg, ip) anesthesia, measurements were taken from M-mode and two-dimensional images obtained in the parasternal long and short axes at the level of the papillary muscles after observation of at least 6 cardiac cycles. Interventricular septal thickness (IVS), left ventricular diameter (LVD), and left ventricular posterior wall thickness (LVPW) were measured during systole (s) and diastole (d). Ejection fraction, fractional shortening, and left ventricular mass were calculated from the M-mode images using the formulas [% EF = (LVDd)3 – (LVDs)3/(LVDd)3 × 100] for ejection fraction and [% FS = LVDd-LVDs/LVDd × 100] for fractional shortening, Relative wall thickness = 2 x (LVPWd/LVDd).
Blood assays
Plasma levels of lactate dehydrogenase (LDH) 23, creatine kinase (CK) 24, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) 25, β-D-glucuronidase 26, β-galactosidase 27, β-D-N-acetyl-glucosaminidase 28, acid phosphatase 29 and cathepsin-D 30 were determined spectrophotometrically using an automated analyzer (Bayer Opera biochemical analyzer, Leverkusen, Germany).
Measurement of malondialdehyde and glutathione in the cardiac tissue
Cardiac tissue samples were homogenized with ice-cold 150 mM KCl for the determination of malondialdehyde (MDA) and glutathione (GSH) levels. GSH measurements were taken using a modification of the Ellman procedure 31. Briefly, after centrifugation at 3000 rev./min for 10 min, 0.5 mL of supernatant was added to 2 mL of 0.3 m Na2HPO4.2H2O solution. A 0.2 mL solution of dithiobisnitrobenzoate (0.4 mg/mL 1% sodium citrate) was added and the absorbance at 412 nm was measured immediately after mixing. GSH levels were calculated using an extinction coefficient of 1.36 × 105 /m/cm. Results were expressed in μmol GSH/g tissue. The MDA levels were assayed for products of lipid peroxidation by monitoring thiobarbituric acid reactive substance formation as described previously 32. Lipid peroxidation was expressed in terms of MDA equivalents using an extinction coefficient of 1.56 × 105/m/cm, and results are expressed as nmol MDA/g tissue.
Measurement of myeloperoxidase, Na+,K+-ATPase, and caspase-3 activities in the cardiac tissue
Myeloperoxidase activity was measured in tissues in a procedure similar to that documented by Hillegass et al. 33. Tissue samples were homogenized in 50 mM potassium phosphate buffer (PB, pH 6.0), and centrifuged at 41,400 g (10 min); pellets were suspended in 50 mm PB containing 0.5% hexadecyltrimethylammonium bromide (HETAB). After three freeze-and-thaw cycles, with sonication between cycles, the samples were centrifuged at 41,400 g for 10 min. Aliquots (0.3 mL) were added to 2.3 mL of reaction mixture containing 50 mm PB, o-dianisidine, and 20 mm H2O2 solution. One unit of enzyme activity was defined as the amount of MPO present that caused a change in absorbance measured at 460 nm for 3 min. MPO activity was expressed as U/g tissue.
Measurement of Na+,K+-ATPase activity is based on the measurement of inorganic phosphate released by ATP hydolysis during incubation of homogenates with an appropriate medium. Since the activity of Na+,K+-ATPase, a membrane-bound enzyme required for cellular transport, is very sensitive to free radical reactions and lipid peroxidation, reductions in this activity can indicate membrane damage indirectly. The total ATPase activity was determined in the presence of 100 mm NaCl, 5 mm KCl, 6 mm MgCl2, 0.1 mm EDTA, 30 mm Tris HCl (pH 7.4), while the Mg2+-ATPase activity was determined in the presence of 1 mm ouabain. The difference between the total and the Mg2+-ATPase activities was taken as a measure of the Na+,K+-ATPase activity 34, 35. The reaction was initiated with the addition of the homogenate (0.1 mL), and a 5-min preincubation period at 37°C was allowed. Following the addition of 3 mm Na2ATP and a 10-min re-incubation period, the reaction was terminated with the addition of ice-cold 6% perchloric acid. The mixture was then centrifuged at 3500 g, and Pi in the supernatant fraction was determined by the method of Fiske and Subarrow 36. The specific activity of the enzyme was expressed as μmol Pi/mg protein/hr. The protein concentration of the supernatant was measured by the Lowry method 37.
The activity of caspase-3 was measured using ApoTargetCaspase-3/CPP32 Colorimetric Protease Assay (Invitrogen, Taastrup, Denmark) kit according to the manufacturer's instructions. Briefly tissue samples were homogenized and treated for 10 min with ice-cold lysis buffer supplied by the manufacturer. The cell lysates were subjected to three freeze-and-thaw cycles and 2 × 10 s sonication to fully disrupt the cells and disperse cell debris. The cell lysate was then centrifuged at 20,000 g for 5 min, and the supernatant was transferred to new sterile santrifuge tubes. Following protein determination, cytosol extracts were diluted in 50 μL lysis buffer and 50 μL reaction buffer (10 mM Dithiothreitol). Samples were placed into wells, and the microplate was equilibrated at 37°C for 10 min. The reaction was initiated by adding 5 μL of DEVD-pNA (Asp-Glu-Val-Asp p-nitroanilide) substrate (200 μm final concentration), and the reaction was carried out at 37°C for 2 hr in dark. The colorimetric release of p-nitroaniline (pNA) from the Ac-DEVD-pNA substrate was recorded at 405 nm using a microplate reader. Experiments were performed in triplicates. Results are presented as mean ± SD of six seperate experiments and expressed as fold-increase over the pretreatment level.
Western blotting of caveolin-3 and SERCA
After dissection from rat, tissues were treated to enrich cardiac muscles as follows. Tissues were then homogenized in phosphate-buffered saline supplemented with 1 μm phenylmethane sulfonylfuoride and treated with SDS sample buffer. Aliquots containing 10–30 μg proteins per lane were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane. The blotted membranes were probed with anti-caveolin-3 (clone26:BD Transduction Laboratories; 1:5000dilution) and SERCA antibody (Santa Cruz, CA, USA). The membranes were further treated with a 1:40,000 dilution of the horseradish peroxidase-conjugated secondary antibody (Pierce), and the reaction was detected with the Super Signal Chemiluminescent System (Pierce, Rockford, IL, USA) 38, 39.
Protein isolation and quantification
Proteins were extracted from 10 to 20 mg tissue in 200–400 μL tissue protein extraction reagent (T-PER; Pierce) containing protease inhibitors (Thermo Scientific Halt Protease Inhibitor Cocktail, Pierce, USA) on ice. Tissues were disintegrated with a scalpel on glass slides and fragmented tissues were transferred to tubes. The specimens were then homogenized and centrifuged at 10,000 g for 6 min at 14°C. The supernatants were stored at −20°C to be used for protein quantification and western blotting. Protein content was measured by a Bradford colorimetric assay (Bio-Rad, Hercules, CA, USA) 40.
Western blotting
Fifty microgram of protein was loaded on a 8% SDS-PAGE gel and transferred to nitrocellulose membrane (Pierce). The membranes were blocked with 1× blocking buffer (500 mL PBS, 5% non-fat dry milk, 250 μL Tween 20) 1 hr at room temperature (RT). The membranes were incubated in a primary antibody anti-serca2 (Santa Cruz, C-20) in 1× blocking solution for 1 hr at RT. After incubations, the membranes were rinsed five times in TBS-T (20 mm Tris, 150 mm NaCl, 0.05% Tween 20) and incubated with a secondary antibody of rabbit-anti-goat IgG (Santa Cruz) in 1× blocking solution for 1 hr at RT. The membranes were rinsed five times in TBS-T. Proteins were detected by chemiluminescence (Super Signal West Pico Chemiluminescent Substrate, Pierce). β-actin antibody (sc-47778, Santa Cruz) was used as an internal control to confirm equivalent protein loading.
Histopathological study
For light microscopic investigations, cardiac tissue specimens were fixed in 10% formaldehyde, dehydrated in alcohol series, cleared in toluene and embedded in paraffin. Paraffin sections (5 μm) were stained with hematoxylin and eosin (H&E) and examined under a photomicroscope (Olympus BH 2, Tokyo, Japan). All the tissue sections were examined microscopically for the characterization of histopathological changes by an experienced histologist in a blind fashion.
Immunohistochemistry
Cardiac tissues were fixed over night at room temperature in neutral-buffered 4% paraformaldehyde or zinc fixative (0.5% zinc chloride, 0.5% zinc acetate in TRIS-calcium acetate buffer, pH 7.4; prepared according to the protocol provided by Transduction Laboratories). Results were similar with the either fixative, although the zinc fixation gave better immunolabeling than paraformaldehyde fixation. Tissues were dehydrated through a graded series of ethanol, replaced with 1-butanol, and embedded in paraffin. Sections (6 μm thick) were placed on silane-coated slides, deparaffinized in xylene, and rehydrated through an ethanol gradient. Antigen retrieval was performed by incubating the sections at 90°C for 20 min in Antigen Unmasking Solution (Vector Laboratories, Burlington, ON, Canada). This treatment significantly enhanced the immunostaining signals of all the antibodies used in this study and was especially necessary for reproducible detection of the weak caveolin-3 signal in some tissues. Sections were treated for 15 min with a mixture of normal donkey and goat sera (5% each) and then incubated with the following primary antibodies at 4°C overnight: mouse monoclonal anti-caveolin-3 antibody (clone26, Transduction Laboratories; 1:500 dilution). Cells cultured on glass cover slips were fixed with neutral-buffered 3% paraformaldehyde for 10 min, permeabilized with 0.1% Triton X-100 for 5 min, and treated with 3% bovine serum albumin for 10 min before being immunolabeled. The cells were incubated with the following primary antibodies at room temperature for 20 min: mouse monoclonal anti-caveolin-3 antibody (clone 26, Transduction Laboratories; 1:250 dilution). Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody (Molecular probes; USA; 1:1000 dilution) and Cy3-conjugated donkey anti-mouse IgG antibody (Jackson Immuno Research Laboratories; 1:1000 dilution) were used as secondary antibodies. Negative controls were included for each experiment by incubating sections with non-immune mouse or rabbit IgGs at 0.5 μg/mL instead of the primary antibodies. The specimens were observed by a Zeiss LSM 5 PASCAL confocal laser-scanning microscope or by a Zeiss Axio Imager microscope 38.
Statistics
Statistical analysis was carried out using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA). Each group consisted of 6 animals. All data were expressed as means ± S.E.M. Data groups were compared with an analysis of variance (ANOVA) followed by Tukey's multiple comparison tests. Values of P < 0.05 were regarded as significant.
Results
The basal blood pressure and heart rate measurements (t1) recorded at the beginning of the experiment were not different among the four experimental groups (P > 0.05; Table 1). On the other hand, HR and BP were found to be decreased in the vehicle-treated HF group with cardiac injury. On the other hand, in the melatonin-treated HF group, both HR and BP values were found to be closer to the control. As expected, by the end of the 4 weeks, body weight measurements exhibited marked elevations in all groups, except the HF group (P < 0.001). But, heart weight was increased in the rats with HF (P < 0.001), and this effect was attenuated by melatonin treatment (P < 0.001) (Table 1).
| Sham group | HF group | |||
|---|---|---|---|---|
| Vehicle-treated | Melatonin-treated | Vehicle-treated | Melatonin-treated | |
| BP (mmHg) | ||||
| t1 | 117 ± 1.9 | 120 ± 1.8 | 124 ± 1.8 | 122 ± 1.7 |
| t2 | 119 ± 1.8 | 122 ± 2.6 | 104 ± 1.8***, ‡‡‡ | 118 ± 1.9††† |
| HR (beats/min) | ||||
| t1 | 398 ± 5.3 | 397 ± 6.1 | 406 ± 3.2 | 399 ± 2.9 |
| t2 | 400 ± 4.6 | 401 ± 5.4 | 402 ± 3.5 | 405 ± 2.9 |
| Body Weight (g) | ||||
| t1 | 266 ± 8.1 | 258 ± 4.3 | 271 ± 3.1 | 274 ± 3.9 |
| t2 | 310 ± 5.8*** | 307 ± 4.4*** | 279 ± 4.2‡ | 306 ± 6.3* |
| Heart Weight (mg) | 643 ± 22 | 617 ± 15 | 968 ± 79 ‡‡‡ | 688 ± 44†† |
| Heart/body weight ratio (mg/g) | 2.10 ± 0.06 | 2.06 ± 0.03 | 3.58 ± 0.19 ‡‡‡ | 2.40 ± 0.22††† |
- * P < 0.05, *** P < 0.001: vs t1 value.
- †† P < 0.01, ††† P < 0.001: vs Vehicle-treated HF group.
- ‡ P < 0.05, ‡‡‡ P < 0.001: vs Vehicle-treated Sham group.
Table 2 summarizes the transthoracic echocardiograpy measurements of both vehicle- and melatonin-treated rats with heart failure as compared to the control group. In the vehicle-treated rats with HF, LV end-diastolic and end-systolic dimensions were found to be significantly increased while percent fractional shortening and ejection fraction were significantly decreased (P < 0.05–0.001). However, melatonin treatment to the rats with LAD reduced the LV end-systolic and end-diastolic diameters and increased ejection fraction and fractional shortening (P < 0.05-0.001).
| Sham group | HF group | |||
|---|---|---|---|---|
| Vehicle-treated | Melatonin-treated | Vehicle-treated | Melatonin-treated | |
| IVS (mm) | 1.81 ± 0.12 | 1.83 ± 0.15 | 2.28 ± 0.15 | 1.83 ± 0.11 |
| PW (mm) | 2.28 ± 0.11 | 2.17 ± 0.09 | 1.64 ± 0.15** | 2.11 ± 0.10† |
| AW (mm) | 1.45 ± 0.09 | 1.32 ± 0.14 | 2.32 ± 0.17** | 1.43 ± 0.12†† |
| RWT | 0.52 ± 0.05 | 0.55 ± 0.05 | 0.75 ± 0.04* | 0.52 ± 0.06† |
| LVDs (mm) | 2.39 ± 0.18 | 2.55 ± 0.21 | 3.61 ± 0.20*** | 2.86 ± 0.13† |
| LVDd (mm) | 4.06 ± 0.13 | 3.89 ± 0.15 | 5.01 ± 0.28** | 4.21 ± 0.11† |
| EF (%) | 83.2 ± 3.8 | 77.3 ± 2.1 | 60.4 ± 3.9** | 78.1 ± 4.1† |
| %FS (%) | 38.7 ± 2.4 | 37.8 ± 2.3 | 20.5 ± 1.1*** | 32.9 ± 2.3†† |
- *P < 0.01, **P < 0.01, ***P < 0.001; compared to vehicle-treated Sham group.
- †P < 0.05, ††P < 0.01; compared to vehicle-treated HF group.
Plasma LDH activity showed a 2.5-fold increase in the heart failure group, indicating generalized tissue damage (P < 0.001), while treatment with the melatonin suppressed the LDH activity (P < 0.001, Table 3). In accordance with the plasma LDH activity, HF caused significant increases in the plasma levels of CK and lysosomal enzymes (β-D-glucuronidase, β-galactosidase, β-D-N-acetyl-glucosaminidase, acid phosphatase, and cathepsin-D) (P < 0.001). When melatonin was administered, all of these parameters were significantly reversed (P < 0.001) (Table 3).
| Sham group | HF group | |||
|---|---|---|---|---|
| Vehicle-treated | Melatonin-treated | Vehicle-treated | Melatonin-treated | |
| LDH (U/L) | 1366 ± 91 | 1291 ± 77 | 3344 ± 219*** | 1965 ± 192 ††† |
| CPK (U/L) | 1715 ± 146 | 1380 ± 146 | 2520 ± 169* | 1720 ± 193† |
| AST (U/L) | 62.6 ± 8.1 | 64.1 ± 5.8 | 109.5 ± 9.1** | 76.5 ± 7.7† |
| ALT (U/L) | 57.8 ± 5.4 | 65 ± 6.4 | 117.7 ± 13.9 ** | 70.3 ± 10.2† |
| β-D glucorinidase (U/mL) | 0.35 ± 0.02 | 0.30 ± 0.03 | 0.66 ± 0.05*** | 0.37 ± 0.05††† |
| β- galactosidase (U/mL) | 0.47 ± 0.02 | 0.37 ± 0.05 | 0.79 ± 0.05*** | 0.50 ± 0.05†† |
| β-D-N-acetyl-glucosaminidase (U/mL) | 0.69 ± 0.05 | 0.58 ± 0.06 | 1.36 ± 0.09*** | 0.73 ± 0.10††† |
| Acid phosphatase (U/mL) | 1.77 ± 0.08 | 1.62 ± 0.12 | 2.57 ± 0.21** | 1.94 ± 0.12† |
| Cathepsin-D (U/mL) | 0.49 ± 0.03 | 0.41 ± 0.03 | 0.92 ± 0.07*** | 0.43 ± 0.06††† |
- * P < 0.05, *** P < 0.001; compared to vehicle-treated Sham group.
- †P < 0.05, ††P < 0.01, †††P < 0.001; compared to vehicle-treated HF group.
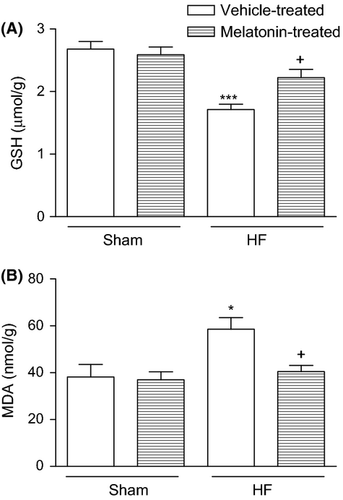
Heart failure caused a significant decrease in cardiac GSH level (1.71 ± 0.09 μmol/g; P < 0.001) when compared to the vehicle-treated sham group (2.68 ± 0.12 μmol/g); while in the melatonin-treated HF group, GSH content of the cardiac tissue was found to be preserved (2.23 ± 0.13 μmol/g; P < 0.05), being not significantly different from that of the control group (Fig. 1a). When compared to the vehicle-treated sham group (38.2 ± 5.3 nmol/g), MDA content of cardiac tissue was found to be increased in the vehicle-treated HF gorup, while melatonin treatment significantly decreased HF-induced MDA elevations (40.5 ± 2.6 nmol/g, P < 0.05; Fig. 1b).
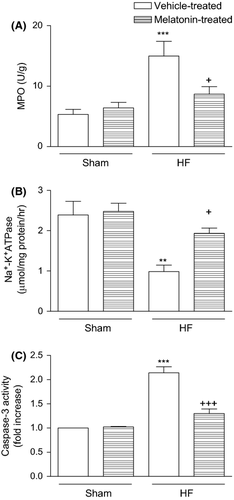
Myeloperoxidase activity, which is accepted as an indicator of neutrophil infiltration, was significantly higher in the cardiac tissues of HF group (14.9 ± 2.4 U/g, P < 0.001) compared to that of the vehicle-treated sham group (5.3 ± 0.9 U/g; Fig. 2a). However, in the melatonin-treated HF group, MPO activity of the cardiac tissue was significantly depressed (8.7 ± 1.2, P < 0.05), reaching to a level that was not different than that of the control group. When compared with the vehicle-treated sham group (2.39 ± 0.34 μmol/mg protein/hr), Na+,K+-ATPase activity was reduced in the HF group (0.98 ± 0.16 μmol/mg protein/hr, P < 0.01), indicating an impaired transport function and membrane damage in the cardiac tissues (Fig. 2b). On the other hand, in the group treated with melatonin, the measured Na+,K+-ATPase activity (1.93 ± 0.13 μmol/mg protein/hr, P < 0.05) was not different from the sham groups. The cardiac tissue caspase-3 activity in the sham groups was elevated by HF (P < 0.001), while melatonin treatment significantly decreased HF-induced caspase-3 elevation (P < 0.001; Fig. 2c).
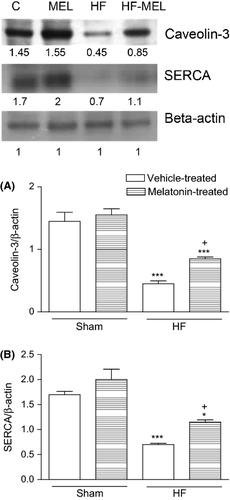
Caveolin-3 levels were found significantly lower in the vehicle-treated HF group compared to the vehicle-treated sham groups (P < 0.05). It was observed that these increments in the caveolin-3 levels were significantly prevented in the melatonin-treated HF group compared to the vehicle-treated HF group (P < 0.05, Fig. 3a). Sarco/endoplasmic reticulum calcium ATPase (SERCA) activity were significantly reduced in the saline-treated HF group compared to the saline-treated control group (P < 0.01–0.001). On the other hand, SERCA activities were significantly higher in the melatonin-treated HF group than those in vehicle-treated HF group (P < 0.05, Fig. 3b).
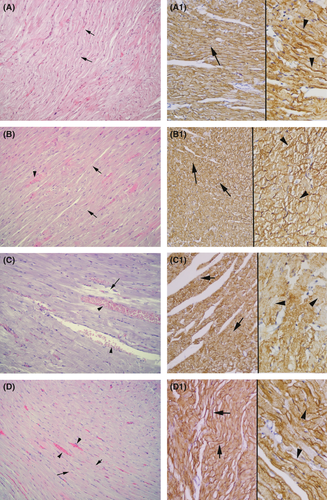
Vehicle (Fig. 4a)- and melatonin (Fig. 4b)-treated sham groups demonstrated regular alignment of both cardiac cells and interstitium. In the vehicle-treated HF group (Fig. 4c), deterioration of cardiac cells and intense capillary congestion were the prominent features. Melatonin treatment of the HF group (Fig. 4d) showed the regression of both capillary congestion and deterioration of cardiac cells. In the Cav-3 immunohistochemistry applied vehicle (Fig. 4a1) and melatonin (Fig. 4b1) sham groups, a strong staining was observed along the cardiac muscle cell membranes. Vehicle-treated HF group (Fig. 4c1) demonstrated pale staining throughout the membranes. The melatonin treatment (Fig. 4d1) prominently stained the cardiac cell membranes.
Discussion
Acute myocardial infarction (MI) remains a leading cause of morbidity and mortality worldwide. Myocardial infarction occurs when myocardial ischemia, a diminished blood supply to the heart, exceeds a critical threshold and overwhelms myocardial cellular repair mechanisms designed to maintain normal operating function and homeostasis. Ischemia at this critical threshold level for an extended period results in irreversible myocardial cell damage or death 41. Despite intensive research on the myocardial response to ischemia, molecular changes that lead to apoptosis and necrosis of the myocardium still remain unknown.
In the present study, increases in lipid peroxidation, and myeloperoxidase activity due to ischemia were accompanied by significant reductions in glutathione level, SERCA and Na+,K+-ATPase activity in the cardiac tissue, implicating the presence of oxidative tissue damage. In addition, elevated serum levels of AST, ALT, LDH, CK, and lysosomal enzymes (β-D-glucuronidase, β-galactosidase, β-D-N-acetyl-glucosaminidase, acid phosphatase, and cathepsin-D) were observed. Caveolin-3 was determined by Western blot analysis and histological analyses. Moreover, systolic blood pressure was significantly reduced and myocardial functions were improved. The results also demonstrate that melatonin treatment through its anti-oxidant effects, as observed by reversal of the changes in all the measured parameters, prevented the severity of ischemic heart failure by alleviating the extent of oxidative stress.
The hemodynamic examinations such as systolic blood pressure and echocardiographic measurements were improved in the melatonin-treated HF group compared with the vehicle-treated HF group. On the other hand, coronary artery ligation did not cause any effect on heart rate. This finding is in agreement with other studies showing that the heart rate remained unchanged in the rats with HF 42, 43. In view of the unchanged heart rate after the coronary artery ligation, it appears that the maintenance of high output status at early stages relied on the Frank–Starling reserve and the intact contractile function 44. Dominguez-Rodriguez et al. demonstrate that circulating melatonin levels were reduced in patients with coronary artery disease correlating with the severity of the disease. However, it is not clear whether this reduction is as a result of either melatonin ‘consumption’ caused by scavenging of the elevated free radical production, or lower melatonin synthesis in these patients. In a phase II clinical trial, melatonin has been shown to inhibition of I/R damage when administered intravenously immediately before primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction 45.
Lysosomes, acidic vacuolar organelles possessing several dozen hydrolytic enzymes, play the most important role in the degradation of damaged cellular components 46, 47. The stability and integrity of lysosomal membrane is vital to maintain normal levels of lysosomal enzymes (β-D-glucuronidase, β-galactosidase, β-D-N-acetyl-glucosaminidase, acid phosphatase, and cathepsin-D) in body fluids 48. Brunk et al. 49 established that the extent of lysosomal damage determines the cells' fate. We have noted a significant increase in the activities of lysosomal hydrolases in serum in ischemic heart failure rats. Similar results have been reported by others 50. Ravens and Gudbjarnason observed that the release of hydrolytic enzymes from lysosomes after coronary occlusion may be a causative factor for the development of myocardial cellular destruction 51. In our study, treatment with melatonin decreased the activities of lysosomal hydrolases in serum of ischemic heart failure rats. This effect might be due to the inhibitory effect of melatonin on lipid peroxidation of lysosomal membrane, thereby reducing the extent of lysosomal damage induced by ischemia in the myocardium.
In the current study, the results obtained regarding the increased concentrations of MDA (an indicator of lipid peroxidation) and decreased GSH concentration in the heart clearly indicate that ischemia is able to induce oxidative stress. Our results are in agreement with the study of Mukherjee et al., where ISO increased the level of lipid peroxidation products and decreased the reduced glutathione levels in cardiac tissue indicating that this synthetic catecholamine induces oxidative damage following oxidative stress. Furthermore, they showed that pretreatment with melatonin at a dose of 10 mg/kg body weight, restored both hemodynamic parameters and the activities and the levels of antioxidant enzymes which were found to be altered by ISO treatment. 52
Enhanced peroxidation of lipids in intra- and extracellular membranes results in the damage to the cells, tissues, and organs. Several studies have demonstrated that ischemia in the heart is associated with lipid peroxidation, which is an autocatalytic mechanism leading to oxidative destruction of cellular membranes, and their destruction can lead to the production of toxic, reactive metabolites, and cell death 53, 54. On the other hand, reduced thiol agents, such as GSH, which are capable of interacting with free radicals to yield more stable elements, are known for their ability to repair membrane lipid peroxides 55. In this sense, GSH and other antioxidants play a critical role in limiting the propagation of free radical reactions, which would otherwise result in extensive lipid peroxidation. In the present study, ischemic heart failure significantly depleted tissue GSH stores, indicating that GSH was used as an antioxidant for the detoxification of toxic oxygen metabolites. On the other hand, melatonin treatment restored the GSH in the rats with heart failure, suggesting that melatonin possessed antioxidant effects and preserved the cellular antioxidant stores.
As confirmed histologically, in rats with heart failure changes of cardiac morphology include various degrees of cell damage, hemorrhage, and the recruitment of leukocytes into the damaged tissue. In the present study, we used MPO as an index of tissue neutrophil infiltration. Because MPO is an essential enzyme for normal neutrophil function, and when neutrophils are stimulated by various stimulants, MPO, as well as other tissue damaging substances are released from the cells 56. In the present study, ischemic heart failure-induced increase in MPO activity was significantly decreased by melatonin, depending on blockade of neutrophil infiltration. As activation of neutrophils might lead to the generation of reactive oxygen metabolites, the reduction in tissue neutrophil accumulation may also result in reduced lipid peroxidation and attenuated tissue injury. Melatonin treatment markedly decreased the MPO activity. Singh et al. 57 showed that melatonin profoundly repressed the overexpression of COX-2 level that coincides with the decreased PGE2 level, MPO activity, and tissue injury. It was likely that the repression of COX-2 induction via melatonin reduces the inflammatory PGE2 synthesis and thus dramatically reduces the MPO activity.
Na+/K+-ATPase is the energy-transducing enzyme that maintains the normal physiological gradients of Na+ and K+ across the plasma membranes 58. Since Na+K+-ATPase regulates intracellular calcium homeostasis, reduced density of myocardial Na+K+-ATPase is prerequested for heart muscle contraction 59, 60. In our study, we observed decreased activity of Na+K+-ATPase in rats with heart failure while melatonin treatment significantly increased the pump activity. This increase in pump activity leads to reduction in intracellular Ca2+, thereby protecting tissue against excessive Ca2+.
The intracellular Ca2 + overload as a consequence of oxidative stress may also play a crucial role in the transition of cardiac hypertrophy to heart failure. It has been demonstrated that reactive oxygen species decrease the activity of the sarcoplasmic reticulum Ca2+ ATPase (SERCA), a membrane calcium pump that plays an important role in cardiac calcium handling and is a determinant of myocardial contractility 61. In agreement with this hypothesis, our results showed that hypoxia-induced increase in free radical levels and decreased SERCA activities in cardiac tissue might cause intracellular Ca2+ overload which known to be involved in ischemic myocardial injury. On the other hand, melatonin reversed the increased ROS production and intracellular Ca2+ overload in ischemic injury-damaged myocardium. This is in consistent with findings in previous studies suggesting that impaired myocardial and calcium handing functions are improved by melatonin treatment 62, 63. Furthermore, Chen et al. demonstrated that there is a diurnal variation in cardiac [Ca(2+)+Mg2+]-dependent ATPase (Ca2+ pump) activity, which is influenced by pinealectomy and melatonin. Their in vitro studies in which cardiac tissue was incubated in the presence of melatonin showed that the Ca2+ pump had been stimulated. These findings support the importance of melatonin in normal cardiac physiology 64.
Caveolin-3 is a component of caveolae in cardiac and skeletal muscles. It is localized to the sarcolemma and functions in formation of caveolae membranes, serving as a scaffolding protein to interact with and organize lipid and protein constituents in caveolae 65, 66. Oka et al. 67 showed that decrease in the content of caveolin-3 may contribute to pathological changes in the membrane function of hypertrophied cardiac myocytes. Accordingly, since we found reduced caveolin-3 in heart tissues, we speculate that decrease in caveola might cause decrease in both Na+K+-ATPase and SERCA activities, and these protein disorganizations might contribute to hemodynamic impairment. Melatonin treatment, on the other hand, significantly increased the levels of caveolin-3 in heart failure rats demonstrates that melatonin regulates the caveolin-3 status which might play an essential role in ischemic heart failure.
The present findings demonstrated that the cardioprotective effects of melatonin in ischemia-induced oxidative damage may be due to an augmentation of the endo-genous antioxidants and inhibition of lipid peroxidation by maintaining a balance in oxidant–antioxidant status, inhibiting neutrophil infiltration and modulating lipid metabolism. Furthermore, our results also show for first time that melatonin regulating membrane proteins, caveolin-3, Na+K+-ATPase, and SERCA provides beneficial effects against ischemic myocardium.
Acknowledgement
This research is supported by The Marmara University Scientific Research Committee with the project number SAG-C-YLP-040310-0032.




