Transcriptional suppression of tryptamine 5-hydroxylase, a terminal serotonin biosynthetic gene, induces melatonin biosynthesis in rice (Oryza sativa L.)
Abstract
Rice tryptamine 5-hydroxylase (T5H) is the second enzyme in melatonin biosynthesis, catalyzing tryptamine into serotonin. Transgenic rice plants, in which the expression of endogenous T5H was either overexpressed or repressed, were examined for alteration in melatonin biosynthesis. Unexpectedly, the overexpression genotypes showed reduced levels of melatonin, while the repression genotypes had elevated levels with an average increase of fourfold. With regard to melatonin intermediates, tryptamine and serotonin levels decreased, but tryptophan and N-acetylserotonin were unaltered in the overexpression genotypes compared with the wild type. In contrast, the repression genotypes had sevenfold higher tryptamine levels than the wild type. In addition, tryptophan and 5-hydroxytryptophan were present at higher levels in the repression genotypes than in both the wild-type and the overexpression genotypes. The enhanced melatonin synthesis in the repression genotypes was closely associated with a transcriptional increase in TDC1. When these rice plants were challenged by oxidative stressors such as herbicides, much higher melatonin synthesis was also observed in the repression genotypes than in either the wild-type or overexpression genotypes. These results suggest that the tryptamine increase through the suppression of T5H plays an important signaling role in triggering melatonin biosynthesis in rice, although the exact role of tryptamine remains to be uncovered.
Introduction
Melatonin is found in both animals and plants and plays common roles in both groups, such as having antioxidant and antiradical properties. It acts as a primary metabolite in animals 1, but seems to behave as a secondary metabolite in plants as there is currently no clear evidence that melatonin is an essential molecule in plant growth and development 2. However, no direct evidence exists for melatonin as a secondary metabolite influencing ecological interactions between plants and their environment. Thus, we cannot rule out the possibility of melatonin acting as a primary metabolite participating in nutrition and essential metabolic processes within plant cells. For example, melatonin is implicated in root and cotyledon promotion 3-5, and its promotional activity on root growth seems to be independent of auxin, suggesting a novel plant growth molecule 6. In addition, melatonin is associated with the delay in flowering 7, flower development 8, 9, and protection against oxidative damage 10-16.
In parallel with an increasing body of physiological studies on melatonin in plants, the biosynthetic genes encoding enzymes catalyzing melatonin were recently cloned. As in animals, melatonin synthesis in plants starts from tryptophan, which is first catalyzed into tryptamine by tryptophan decarboxylase (TDC) followed by tryptamine 5-hydroxylase (T5H), resulting in serotonin synthesis. TDC was first cloned from Catharanthus roseus to study the biosynthetic pathway of monoterpene indole alkaloids including vinblastine and ajmaline 17 and further cloned in rice using sequence homology 18. The T5H gene, which is a cytochrome P450 enzyme, was cloned in rice via a map-based cloning strategy using Sekiguchi lesion (sl)-mutant rice 19. The last two biosynthetic reactions in melatonin biosynthesis are referred to as the key regulatory steps as the metabolic flow from serotonin to melatonin is dramatically diminished 20, 21. The genes involved in these steps are serotonin N-acetyltransferase (SNAT) and N-acetylserotonin methyltransferase (ASMT), which were recently cloned in rice plants 22-24. Although all four genes for melatonin biosynthesis in rice plants were cloned, so far, neither gain-of-function nor loss-of-function analyses have been performed using these endogenous melatonin biosynthetic genes. A few studies have reported on gain of function using either the animal melatonin biosynthetic gene 25 or the plant melatonin-degrading gene 26.
As an initial step in searching for the rate-limiting gene or genes responsible for melatonin biosynthesis in rice, we chose the second gene, T5H, as a rice T5H knockout mutant called Sekiguchi lesion showed altered levels of serotonin and melatonin in detached senescent rice leaves 20. In the present study, we generated transgenic rice in which the expression of endogenous T5H was either stimulated or suppressed to determine whether these transgenic lines exhibit altered levels of melatonin production. We found that melatonin is elevated in T5H-repressed rice plants in contrast to the T5H-overexpressing rice plants. The melatonin increase in the T5H-repressed rice plants was attributed to an elevation in both 5-hydroxytryptophan (5-OH-Trp) and tryptamine. The main reason for melatonin rise in T5H-repressed rice plants was closely associated with the enhanced expression of TDC1 mRNA leading to the tryptamine increase. The induction of TDC1 mRNA may be due to tryptamine oxidation by monoamine oxidase 27, generating hydrogen peroxide (H2O2), a possible signal molecule for inducing TDC1 mRNA.
Materials and methods
Production of transgenic rice plants overexpressing and repressing T5H
To generate transgenic rice plants overexpressing T5H, we employed the pIPKb002 binary vector 28. The full-length T5H and N-terminal 37-amino-acids-deleted T5H were amplified by polymerase chain reaction (PCR) using appropriate primers harboring the attB recombination sequences. The resulting products were cloned into the pDONR221 Gateway® vector (Invitrogen, Carlsbad, CA, USA). The pDONR221-T5H and pDONR221-∆37T5H entry vectors were then recombined with the pIPKb002 Gateway destination vector via LR recombination to generate pIPKb002-T5H and pIPKb002-∆37T5H. To repress T5H, we used the pANDA RNAi binary vector 29. The N-terminal 530-bp T5H gene was amplified with the following primers: forward 5΄-AAA AAG CAG GCT CCA TGG AGC TCA CCA TGG-3΄; reverse 5΄-AGA AAG CTG GGT CGA CGG GCT TCG-3΄. The resulting 530-bp T5H cDNA fragment was further amplified by PCR using primers flanking the attB recombination sequences (forward 5΄-GGG GAC AAG TTT GTA CAA AAA AGC AGG CT-3΄; reverse 5΄-GGG GAC CAC TTT GTA CAA GAA AGC TGG GT-3΄). The PCR product was gel-purified by DE81 membrane (Whatman, Clifton, NJ, USA) and cloned into the pDONR221 Gateway® vector (Invitrogen) via a BP recombination reaction. The pDONR221 530-bp T5H gene entry vector was recombined with the pANDA RNAi binary vector via LR recombination reaction to create pANDA-T5H. The pIPKb002-T5H, pIPKb002-∆37T5H, and pANDA-T5H binary vectors were transformed into Agrobacterium tumefaciens LBA4404. Agrobacterium-mediated transformation was used to generate transgenic rice plants using scutellum-derived rice calli (Oryza sativa cv. Dongjin) as previously described 30.
Plant material and herbicide treatments
T2 homozygous transgenic rice (Oryza sativa cv. Dongjin) plants expressing rice T5H under sense overexpression or RNAi repression were used. Rice seeds were surface-sterilized and sown on half-strength Murashige and Skoog (MS) medium (MB Cell, Seoul, Korea) in vertically oriented square polystyrene dishes (SPL Life Sciences, Pocheon-si, Korea). The seeds were incubated for 7 days at 28°C under 12-hr light/12-hr dark conditions. For herbicide application, the 7-day-old seedlings were treated with 0.1 μm butafenacil and placed in a plant growth room at 28°C under a 12-hr light/12-hr dark cycle for 48 hr, as described previously 15. The rice seedlings were harvested, frozen in liquid nitrogen, and stored at −80°C until analysis.
RT-PCR analysis
Total RNA was isolated from the rice tissues using an RNeasy Plant Mini Kit (Qiagen Tokyo, Japan). Reverse-transcription (RT)-PCR analysis was performed as described previously 23. The following primers (5′→3′) were used: for tryptophan decarboxylase (TDC1), forward ATG ACC TGC CTC GAC TGC ACC and reverse CTT GTT CAG CCG CTC CAT CAG; for tryptamine 5-hydroxylase (T5H), forward CCT CGT CCT GGA CAT GTT CGT C and reverse ATG GCG AAC GTG TTG ATG AAC AC; for serotonin N-acetyltransferase (SNAT), forward GGG CTG CGG CAA CTT GGT CC and reverse AGA AAG CTG GGT CTA AAA TCT GGG GTA; for N-acetylserotonin methyltransferase (ASMT), forward TAC CGT CCA TGA CGG CG and reverse CGG CCG CCT TCT CGA CA; and for ubiquitin-5 (UBQ5), forward CCG ACT ACA ACA TCC AGA AGG AG and reverse AAC AGG AGC CTA CGC CTA AGC.
Analysis of tryptophan, 5-OH-Trp, tryptamine, and serotonin by high-performance liquid chromatography (HPLC)
Frozen rice leaves (400 mg) were homogenized to a fine powder using a TissueLyser II (Qiagen) and extracted with 3 mL of MeOH. The extracts were cleared by centrifugation, and water was added to a concentration of 80% methanol. Extracts were then passed through a C18 cartridge (Waters, Milford, MA, USA). The eluates were evaporated until dry and dissolved in 1 mL of 50 mm sodium phosphate buffer (pH 7.9). An aliquot of 10 μL was analyzed by HPLC as described previously 20. The analysis was performed in triplicate.
Analysis of melatonin by high-performance liquid chromatography (HPLC)
Frozen rice samples (400 mg) were ground to a powder in liquid nitrogen using a TissueLyser II (Qiagen) and extracted with 3 mL of MeOH followed by passage through a C18 cartridge (Waters) as described above. The eluates were evaporated until dry and dissolved in 1 mL of 50 mm sodium phosphate buffer (pH 7.9) followed by the addition of 20 μL 0.1 m KOH. The sample was further extracted with 800 μL of chloroform, and the solvent fraction was mixed with 500 μL 0.45 m Na-borate (pH 10). After vortexing and centrifugation, the chloroform fraction was evaporated until dry and dissolved in 100 μL of water. An aliquot with 10 μL was subjected to HPLC with a fluorescence detector system (Waters). The samples were separated on an Atlantis C18 column (3.9 × 150 mm; Waters) with an isocratic elution profile of 34% (v/v) MeOH in water at a flow rate of 0.22 mL/min. Melatonin was detected at an excitation of 280 nm and emission of 348 nm.
Statistical analysis
One-way ANOVA was used for statistical evaluations. A P-value < 0.05 was considered statistically significant.
Results
As an initial step in searching for a rate-limiting step for melatonin biosynthesis in rice plants, we first chose the T5H gene and generated transgenic rice plants either overexpressing or repressing T5H. For the T5H overexpression lines, two genotypes were achieved with one type overexpressing the full-length T5H gene and the other with the N-terminal 37-amino-acids-truncated T5H (∆37T5H) lacking the transmembrane domain (Fig. 1). The T5H transgenes were under the control of the maize ubiquitin promoter. Fourteen and 10 independent T1 transgenic lines were initially generated in the full-length T5H and ∆37T5H genotypes, respectively. For T5H RNAi, seven independent T1 transgenic lines were initially generated. From these T1 lines, we finally selected 4, 3, and 5 homozygous T2 lines with the full-length T5H, ∆37T5H, and T5H RNAi genotypes, respectively. We examined expression levels of the T5H transgene in these transgenic rice genotypes by RT-PCR analysis using a T5H-specific primer set. Total RNA isolated from 7-day-old seedlings were used for the full-length T5H and ∆37T5H genotypes, while total RNA isolated from 1-month-old detached leaves challenged by senescence treatment for 6 days was used for the T5H RNAi genotypes. The results are shown in Fig. 2. Both genotypes of the T5H overexpression lines showed that T5H mRNA transcripts were constitutively overexpressed in the transgenic genotypes, whereas the control nontransgenic lines (wild type) had no T5H transcripts, suggesting that both the full-length T5H and ∆37T5H genotypes expressed T5H constitutively under the control of the maize ubiquitin promoter (Fig. 1A, B). In addition, T5H transcripts were moderately suppressed in the T5H RNAi genotypes compared with those in the wild type (Fig. 1C), confirming that the T5H RNAi construct was downregulating T5H transcripts in the T5H RNAi genotypes.

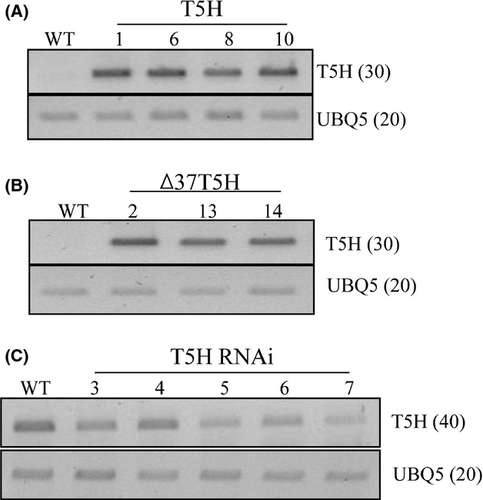
To examine whether the transcriptional overexpression or suppression of T5H in these transgenic genotypes can alter the levels of melatonin intermediates, we quantified the tryptophan, tryptamine, serotonin, and N-acetylserotonin (NAS) content. As shown in Fig. 3, T5H overexpression did not alter tryptophan levels, but levels were elevated in T5H repression genotypes due to the suppression of T5H expression (Fig. 3A). For tryptamine, T5H overexpression led to a decrease in tryptamine levels only in the full-length T5H overexpression genotype, but not in the ∆37T5H genotypes. In contrast, highly elevated levels of tryptamine were observed in the T5H repression genotypes as expected (Fig. 3B). In contrast to our expectation, serotonin levels decreased in all transgenic genotypes (Fig. 3C), and a more severe reduction in serotonin levels was present in the T5H overexpression genotypes than in the T5H repression genotypes. The reason for serotonin reduction in the T5H overexpression genotypes is not clear. NAS levels were similar in both the transgenic genotypes and nontransgenic wild type. These results suggest that the alteration in T5H expression levels caused significant changes in melatonin precursor levels, such as tryptophan, tryptamine, and serotonin, suggestive of a possible controlling role of T5H in melatonin biosynthesis in rice plants.
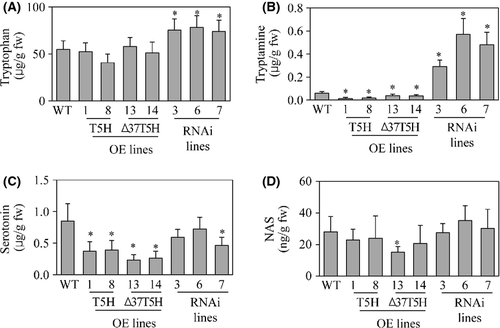
To address the reason for the high production of serotonin in the T5H repression genotypes relative to the T5H overexpression genotypes, we measured 5-OH-Trp levels, which are known to be an alternative pathway for serotonin synthesis in plants 20. The 5-OH-Trp levels were higher in the T5H repression genotypes than in the T5H-overexpression genotypes (Fig. 4A). This suggests that the suppression of T5H expression creates a bypath in which tryptophan is catalyzed into 5-OH-Trp by a putative 5-OH-Trp synthase followed by serotonin synthesis via the TDC enzyme 31. Next, we measured melatonin levels in these transgenic genotypes. Melatonin levels in the T5H overexpression genotypes were comparable with those in the wild type, but fourfold higher melatonin levels were observed in the T5H repression genotypes compared with the wild type (Fig. 4B). These results indicate that an inverse correlation exists between T5H expression and melatonin production, albeit the exact mechanisms remain to be elucidated. To examine whether T5H suppression affects the melatonin biosynthetic gene profiles, we performed RT-PCR analysis to monitor the expression levels of TDC, SNAT, and ASMT. As shown in Fig. 4C, the transcript levels of SNAT and ASMT were almost identical in the transgenic genotypes and wild type, whereas the TDC1 transcript was significantly overexpressed in the T5H repression genotypes compared with the wild-type and T5H overexpression genotypes. This suggests that T5H suppression induces the transcription of TDC1 leading to higher tryptamine synthesis than in the T5H overexpression genotypes.
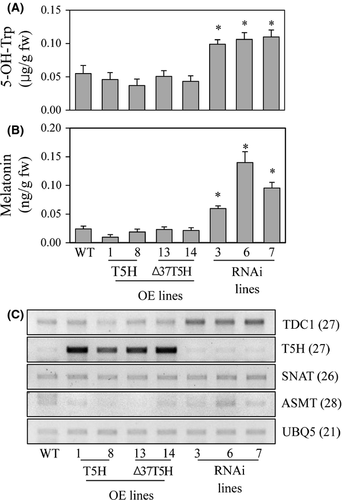
Melatonin synthesis in plants is known to be induced in response to oxidative stress treatments via the transcriptional activation of melatonin biosynthetic genes 15. To see whether the T5H repression genotypes also produce melatonin at greater levels than the other genotypes when challenged by oxidative stress (such as the herbicide butafenacil), we quantified melatonin levels as well as other metabolites (such as serotonin, tryptamine, and 5-OH-Trp) 48 hr after 0.1 μm butafenacil treatment. As shown in Fig. 5, melatonin and tryptamine levels were higher in the T5H repression genotypes than in the other genotypes. However, the levels of serotonin and 5-OH-Trp in the T5H repression genotypes were equal to those of the other genotypes. These results confirm that T5H suppression gives rise to melatonin biosynthesis in rice plants. RT-PCR data following butafenacil treatment also revealed that the enhanced TDC1 expression in the T5H repression genotypes is due to the elevated synthesis of melatonin and tryptamine (Fig. 6).
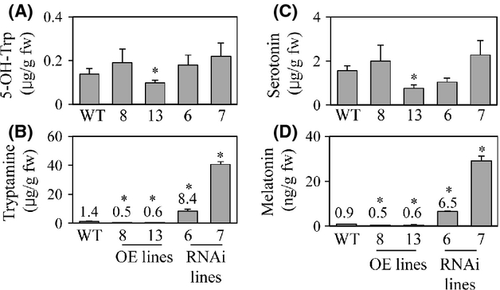
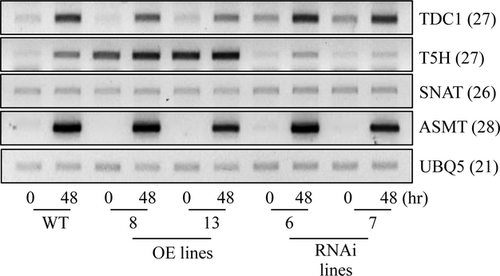
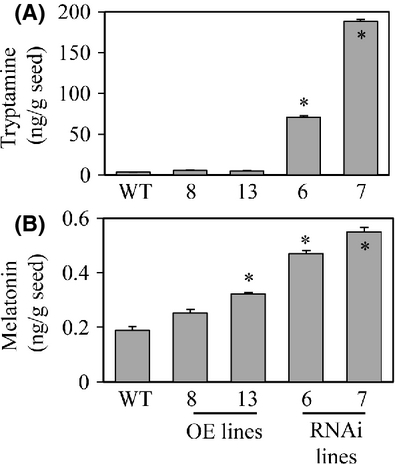
Finally, we quantified the levels of melatonin in dehusked rice seeds. As shown in Fig. 7, the average melatonin level in the T5H repression genotypes was at least twice that in the other genotypes. The tryptamine levels were also higher in the T5H repression genotypes than in the other genotypes. These results were consistent with the data observed in the seedlings. Taken together, T5H suppression leads to an increase in melatonin levels in rice seedlings and seeds, as well as in seedlings exposed to oxidative stress. The exact mechanism by which T5H suppression leads to this biochemical phenotype awaits further in-depth studies.
Discussion
Recently, all of the melatonin biosynthetic genes were cloned in rice plants and included TDC 18, T5H 19, SNAT 22, and ASMT 23. The main enzymatic products of these enzymes start with tryptamine, serotonin, N-acetylserotonin, and melatonin. Additionally, an alternative bypath seems to exist starting from 5-OH-Trp, serotonin, N-acetylserotonin, and melatonin 20, 32. This bypath is initiated by a putative 5-OH-Trp synthase catalyzing tryptophan into 5-OH-Trp. In animals, 5-OH-Trp is catalyzed by tetrahydropterin-dependent tryptophan hydroxylase 33, but these types of amino acid hydroxylase homologues are absent in the plant genome. Thus, the putative plant 5-OH-Trp synthase may be a plant 2-oxoglutarate-dependent dioxygenase 34, but no information on plant 5-OH-Trp synthase has been reported.
According to the kinetic features of plant melatonin biosynthetic enzymes, the T5H enzyme has the highest substrate affinity with a Km of 7.3 μm toward tryptamine 19, but other enzymes, including TDC, SNAT, and ASMT, had low substrate affinities of 690 μm (tryptophan), 864 μm (serotonin), and 385 μm (N-acetylserotonin), respectively. At first glance, T5H does not have a regulatory role in controlling melatonin biosynthesis in plants. However, a T5H-mutant rice (Sekiguchi lesion) exhibited a lesion phenotype related to pathogen resistance 19, suggesting that T5H may be involved in regulating serotonin or melatonin metabolism during lesion formation. Therefore, we initially chose the T5H gene and constructed transgenic rice plants either overexpressing or suppressing endogenous rice T5H expression (Figs 1, 2). In contrast to our expectation, T5H overexpression genotypes did not result in the overproduction of serotonin and melatonin. However, the T5H suppression genotypes produced more melatonin than the wild type via a concomitant increase in 5-OH-Trp synthesis (Fig. 4A, B). Melatonin increase in the T5H repression genotypes was positively correlated with an increase in tryptamine levels by suppression of T5H transcription via RNA interference (Fig. 3B, Figs 4B, 5B, 5D).
At a molecular level, we propose that the suppression of T5H expression induces TDC1 transcription, after which the enhanced TDC protein in turn catalyzes 5-OH-Trp into serotonin followed by melatonin increase in the T5H repression genotypes. In addition, the tryptamine increase in the T5H repression genotypes probably underwent oxidation by a monoamine oxidase, which in turn generated H2O2, a signaling molecule inducing an array of gene expressions including TDC1 27. An unknown molecule or transcription factor(s) triggered by H2O2 may also participate in the suppression of melatonin degradation as no biosynthetic genes such as SANT and ASMT were transcriptionally activated in the T5H repression genotypes. To verify these hypotheses, further gain-of-function and loss-of-function studies of melatonin biosynthesis using either TDC isoforms or cloning melatonin-degrading gene(s) 26 are needed. Another possible explanation for the high level of melatonin production in the T5H repression lines is the presence of a novel melatonin biosynthetic pathway in mitochondria. As mitochondria are generally considered to be the major site of melatonin biosynthesis in animals 35, we cannot rule out the possibility that plants synthesize melatonin in chloroplasts and mitochondria 36. Based on this hypothesis, our results suggest that the biosynthesis of melatonin in chloroplasts was inhibited by the suppression of T5H, but that mitochondrial melatonin biosynthesis was induced via 5-OH-Trp with high catalytic efficiency relative to that in chloroplasts.
In summary, the T5H gene plays a regulatory role in controlling melatonin biosynthesis in rice plants, and the T5H gene suppression enhances the levels of melatonin in plants, which can be utilized as health-promoting foods 1, 37-41.
Acknowledgements
This research was supported by grants from the Next-Generation BioGreen 21 Program (SSAC Project No. PJ00949105) from the Rural Development Administration as well as the National Research Foundation of Korea (2011-0013092) and the Priority Research Centers Program (2010-0020141) through the NRF of the Ministry of Education, Science and Technology (MEST), Republic of Korea. The T5H cDNA was kindly provided by the National Institute of Agrobiological Sciences 42.




