Change in Chemical Composition of Sweet Basil (Ocimum basilicum L.) Essential Oil Caused by Alfalfa mosaic virus
Abstract
The effects of Alfalfa mosaic virus (AMV) infection on essential oil (EO) content and composition of a Sweet Basil cv. Gigante di Napoli were evaluated. A 10-fold lower extraction yield from infected plants was observed, suggesting that morphological alterations induced by the disease may affect abundance and efficacy of secretive tissues. Organoleptic properties and thus quality of EO were severely affected and EO composition resulted severely altered, with a great increase in sesquiterpenes (from 72.8 to 19.8%) and a decrease in both monoterpenes (from 35 to 11%) and phenylpropanoids (from 44.5 to 15.8%, despite a slight increase in eugenol). Such report is indicative of possible direct or indirect metabolic consequences of AMV in a commercially important species like Ocimum basilicum is. The possible consequences of linalool and trans-β-farnesene content changes on the dispersion of viruliferous aphids are also examined and discussed.
Introduction
Sweet basil (Ocimum basilicum L.) is an economically important herb crop in several Mediterranean countries. Approximately 80 ha are grown annually in Italy, 30 ha in France, and 20 ha in Israel. Basil is also an important fresh and processed (frozen and pesto sauce) crop in U.S. markets. This herb is used fresh, dried and processed for flavouring and fragrances and in traditional medicines (Garibaldi et al. 1997). In particular, O. basilicum essential oil (EO) is characterized by a great degree of chemodiversity and different chemotypes are known, being the linalool/estragole (sweet basil), the methylchavicol, the eugenol and the methylcinnamate rich the most common ones. As with most agricultural commodities, diseases impose significant production constraints, affecting both yield and overall quality of basil. Many diseases afflict basil and it is noteworthy that quali-quantitative modifications of secondary metabolites, hereby included essential oils, can be related to the phytopathological status of plants (Bruni et al. 2005). The effect of phytopathogenic bacteria, fungi or phytoplasmas on various aromatic plants has been found to be responsible for significant variations in the composition of marketed essential oils (Zechini D'Aulerio et al. 1995; Hudaib et al. 2001, 2002). However, the knowledge on the influence of viral diseases on terpenic biosynthesis is quite limited, since epidemiological studies on virus presence in aromatic and medicinal crops became systematic only during the last decade (Bellardi et al. 1999; Bellardi and Rubies-Autonell 2001, 2003). One of the most common virus affecting sweet basil in Europe is Alfalfa mosaic virus (AMV), transmitted by aphids. Generally AMV elicit bright yellow mosaic in sweet basil (Fig. 1). A recrudescence of this disease has been observed since 2003 in several cultivations sites in Campania region (Southern Italy), with symptomatic plants ranging from 5 to 20%, depending from the inspected field (Parrella et al. 2011b). In the present preliminary study, the effects of AMV infection on EO content and composition of a sweet basil local ecotype were evaluated.

Materials and Methods
Plant material, isolation of essential oil, CG and CG-MS analysis
Leaves of Gigante di Napoli variety, grown in open field in Southern Italy (Campania), naturally infected by AMV isolate belonging to subgroup I (Parrella et al. 2000, 2011a; Bruni et al. 2006) and healthy material of the same variety, were steam-distilled for 2 h with a commercial Clevenger apparatus. The essential oil samples were dried over anhydrous sodium sulphate and stored in glass vials with Teflon-sealed caps at −18 ± 0.5°C in the absence of light.
GC analysis was performed on a Fisons (Rodano, Milano, Italy) 9130–9000 series gas chromatograph equipped with a Fisons EL980 processor, a FID detector and a MEGA SE52 (Mega, Legnano, Italy) 5% polydiphenyl 95% dimethylsiloxane bonded phase column (i.d. = 0.32 mm, length 30 m, film thickness = 0.15 μm). Operating conditions were as follows: injector temperature 280°C; FID temperature 280°C, Carrier (Helium) flow rate 2 ml/min and split injection with split ratio 1:40. Oven temperature was initially 45°C and then raised to 100°C at a rate of 1°C/min, then raised to 250°C at a rate of 5°C/min and finally held at that temperature for 10 min. One microlitre of each sample dissolved in CH2Cl2 was injected. The percentage composition of the oils was computed by the normalization method from the GC peak areas, without using correction factors. Essential oil constituents were analysed by a Hewlett Packard HP5890 series II plus gas chromatograph equipped with a HPMS 5989b mass spectrometer operating on EI mode. The GC conditions were the same reported for GC analysis, and the same column was used. The MS conditions were as follows: ionization voltage, 70 eV; emission current, 40 μA; scan rate, 1 scan/s; mass range, 35–300 Da; ion source temperature, 200°C.
Identification of the compounds
The Mass Spectrometry (MS) fragmentation patterns were checked with those of other essential oils of known composition, with pure compounds and by matching the MS fragmentation patterns with NBS75K mass spectra libraries and with those in the literature (Adams 2002). The relative amounts of the individual components were obtained from GC analysis based on peak areas without FID factor correction. The constituents of the volatile oils were also identified by comparing their GC retention indices. A mixture of aliphatic hydrocarbons (C8–C24) in hexane (Sigma, Saint Louis, MO, USA) was injected under the above-mentioned temperature program to calculate the retention indices using the generalized equation by Van del Dool and Kartz (1963).
Results and Discussion
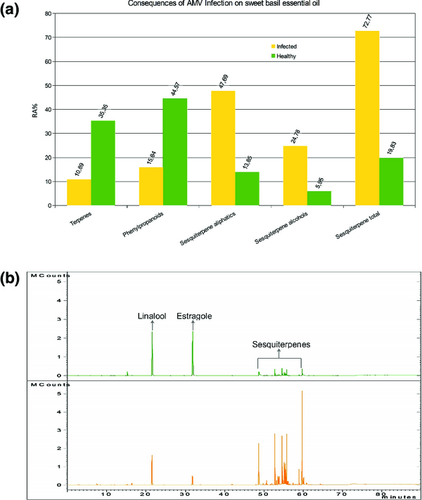
A 10-fold lower extraction yield from AMV-infected plants was observed compared to healthy control, suggesting that the disease may affect abundance and efficacy of secretive tissues (Table 1). A total of 54 compounds were identified (Table 1), and qualitative composition of healthy basil was comparable to estragole (methyl chavicol)-rich chemotype of sweet basil (Hiltunen and Holm 2003). Organoleptic properties and thus quality of EO were greatly affected and EO composition resulted severely altered, with a large increase in sesquiterpenes (from 19.8 to 72.8%) and a decrease in both monoterpenes (from 35 to 11%) and phenylpropanoids (from 44.5 to 15.8%, despite a more than two time increase in eugenol) (Fig. 2a,b). AMV infection drastically changed the chemotype of the Gigante di Napoli variety. In particular, linalool and estragole, the two main constituents and aroma contributors of sweet basil were reduced from 31.5 to 9.5% and from 39.3 to 3.3%, respectively, in AMV-infected plants (Fig. 2a), whereas eugenol increased from 5.1 to 12.35% in the same plants. Interesting, eugenol, which increased in AMV-infected basil, possess several important biological activities such as antiseptic and anaesthetic properties (Jadhav et al. 2004): it is able to reduce the presence of some species of bacteria dangerous to humans that contaminate food (Gill and Holley 2004) and kills certain human colon cancer (Jaganathan and Supriyanto 2012). Eugenol presents also a strong repellent activity against mosquito (Nerio et al. 2010). Between sesquiterpenes, a remarkable increase was observed in sesquiterpene alcohols, with a huge increase of τ-Cadinol (Table 1), which is known to posses, as eugenol, important bactericidal activity (Claeson et al. 1992).
| Compound | R.T. | Infected | Healthy |
|---|---|---|---|
| 2-hexanal | 5.739 | nd | 0.06 |
| α-Pinene | 8.718 | tr | 0.08 |
| Sabinene | 10.940 | tr | 0.09 |
| β-Pinene | 11.211 | 0.05 | 0.15 |
| 1-Octen-3-ol | 11.622 | tr | 0.09 |
| Myrcene | 11.992 | 0.11 | 0.19 |
| Limonene | 14.894 | tr | 0.06 |
| 1.8 Cineole | 15.248 | 0.22 | 2.13 |
| E-Ocimene | 16.368 | 0.51 | 0.4 |
| trans-Ocimene | 17.340 | tr | 0.07 |
| γ-Terpinene | 18.673 | nd | 0.05 |
| Terpinolene | 19.751 | nd | 0.05 |
| Linalool | 21.491 | 9.51 | 31.52 |
| acetylmethylcyclohexene | 24.590 | 0.08 | nd |
| Camphor | 26.141 | tr | 0.18 |
| 4-Terpineol | 29.788 | tr | 0.23 |
| Estragole | 31.808 | 3.31 | 39.36 |
| Octanol Acetate | 33.855 | 0.13 | nd |
| Bornyl Acetate | 41.894 | 0.41 | 0.15 |
| α-Cubebene | 47.978 | 0.15 | 0.07 |
| δ-Elemene | 48.312 | 0.06 | nd |
| Eugenol | 48.716 | 12.35 | 5.21 |
| α-Copaene | 49.777 | 0.39 | 0.17 |
| β-Bourbonene | 50.191 | 0.13 | nd |
| β-Cubebene | 50.550 | 0.4 | 0.15 |
| β-Elemene | 50.671 | 0.89 | 0.34 |
| Methyl Eugenol | 51.681 | 0.18 | tr |
| α-cis-Bergamotene | 51.901 | 0.17 | tr |
| β-Caryophyllene | 52.017 | 0.34 | 0.08 |
| β-Cedrene | 52.163 | 0.13 | tr |
| β-Copaene | 52.533 | 0.19 | tr |
| α-trans-Bergamotene | 52.804 | 9.75 | 3.1 |
| Aromadendrene | 53.130 | 0.17 | 0.05 |
| Unk. Sesquiterpene | 53.248 | 0.75 | 0.28 |
| α-Humulene | 53.605 | 1.5 | 0.42 |
| trans-β-Farnesene | 53.734 | 0.67 | 0.16 |
| Muurola.4(14),5 diene cis | 53.891 | 1.49 | 0.41 |
| β-Acoradiene | 54.073 | 0.3 | nd |
| Germacrene D | 54.637 | 7.69 | 2.84 |
| g-Amorphene | 54.804 | 1.1 | 0.14 |
| Bicyclogermacrene | 55.183 | 4.38 | 1.28 |
| α-Bulnesene | 55.421 | 3.61 | 1.05 |
| Germacrene A | 55.586 | 2.51 | 0.71 |
| γ-Cadinene | 55.816 | 7.24 | 2.24 |
| δ-Cadinene + Calamenene trans | 56.006 | 0.93 | 0.16 |
| Muurol-5-en-4-β-ol cis | 56.446 | 0.21 | nd |
| α-Muurolene | 56.586 | 0.2 | 0.05 |
| Germacrene B | 57.255 | 0.05 | nd |
| ε-nerolidol | 57.413 | 0.39 | tr |
| Spathulenol | 57.878 | 0.25 | tr |
| Viridiflorol | 58.144 | 0.26 | tr |
| Gleenol | 58.402 | 0.36 | tr |
| Cubenol | 58.970 | 2.45 | 0.57 |
| τ-Cadinol | 59.716 | 17.39 | 4.66 |
| τ-Muurolol | 60.077 | 1.53 | 0.75 |
| Total | 94.89 | 99.75 |
- R.T., retention time; tr, lower than 0.05%; nd, not determined. The values of compounds showing higher concentration differences between infected and healthy plants are reported in bold.
Concerning compounds that can have a role in the vector (aphids)–virus–plant interactions, particular attention should be given to changes observed in healthy and infected plants of linalool and trans-β-Farnesene. Linalool, the second compound in term of quantity after estragole in healthy basil, decreased from 31.52% in healthy basil to 9.51% in AMV-infected basil (Table 1). High level of emission of this monoterpenes has been demonstrated to repel significantly Myzus persicae aphid, one of the major AMV vector (Aharoni et al. 2003). The reduction of this compound in the AMV-infected basil, combined with the bright yellow mosaic symptoms elicited by the same virus in this plant, could results in more attractive plants for aphids vector: once aphids arrive on infected plants, trans-β-Farnesene, which increases in infected basil, would act as alarm pheromone also a low concentration (Kunert et al. 2005), inducing the aphids to stop feeding and disperse all around, giving birth to winged forms which leave their host (infected basil). Overall, these changes of the basil compounds can cause the dispersion of viruliferous aphids to new host plants with the consequent AMV spreading.
Although preliminary, such report is indicative of possible direct or indirect metabolic consequences of AMV in commercially important species like O. basilicum is. Moreover, since AMV is a dangerous infectious pathogen of basil (Parrella et al. 2011b), further investigations on the virus spreading between plants, due to the dispersion of viruliferous aphids as consequence of changes in the content of some components in AMV-infected plants (and also to biogenetic and phytochemical effects of AMV infection), would be helpful.
Acknowledgements
Authors disclose any commercial affiliations as well as consultancies, stock or equity interests, and patent licensing arrangements that could be considered to pose a conflict of interest regarding the submitted manuscript.




