Cross-protection between a Naturally Occurring Mild Isolate of Potato virus M (PVM) and a More Virulent Isolate in Datura metel Plants
Abstract
Cross-protection is a phenomenon that occurs when infection with a mild virus prevents or suppresses the harmful effects of subsequent infection by a related severe virus. Isolates of Potato virus M (PVM, genus Carlavirus, family Betaflexiviridae), like isolates of other viruses, are likely to occur in plants in multiple infections, but the knowledge about intrahost interactions between PVM isolates and their impact upon the host response to infection is none. Here, we report that pre-infection with a mild isolate I-38 successfully protected Datura metel plants against destructive effects of infection by a severe isolate Uran. The latter was not detected by reverse transcription-PCR in plants pre-inoculated 7 days earlier by I-38, which indicates that it was excluded by host defence mechanisms triggered by the low virulent isolate. Our results provide the first evidence for the occurrence of cross-protection between PVM isolates within a common host plant.
Introduction
Potato virus M (PVM, genus Carlavirus, family Betaflexiviridae) has a single-stranded, positive-sense RNA genome of approximately 8.5 kb in length (Zavriev et al. 1991; Adams et al. 2012). In nature, the virus is transmitted non-persistently by several aphid species (Kerlan 2009; Palukaitis 2012). It can also be transmitted mechanically, which is used for experimental purposes.
PVM has a worldwide distribution (Xu et al. 2010; Palukaitis 2012; Ali-Ali et al. 2013), but a range of its natural hosts is narrow. The main host is potato (Solanum tuberosum), but PVM also infects other solanaceous plants, such as Capsicum annuum or Datura metel (Brunt 2001). PVM is still economically important in potato crops in some countries of Central and Eastern Europe and Asia, including Poland (Kerlan 2009) and China (Li et al. 2013). The virus induces mostly mild chlorosis and leaf distortion, but in susceptible cultivars, certain PVM isolates can cause mottle, mosaic, crinkling and rolling of leaves and plant stunting. Severity of symptoms and tuber yield losses can vary depending on the virulence of PVM strain and the tolerance of the cultivar (Cavileer et al. 1998; Loebenstain 2009).
Molecular and serological diversity of PVM is so far poorly recognized (Halterman et al. 2012). PVM isolate named PVM-ID has been reported to greatly differ from the ordinary virus strain in detectability by antisera raised against type PVM antigen (Cavileer et al. 1998), which may imply the existence of different PVM serotypes. Recently, the phylogenetic trees revealed the segregation of PVM isolates originating in different countries into two main divergent evolutionary lineages (Xu et al. 2010; Tabasinejad et al. 2014), the majority of the isolates being arranged within the two clades irrespective of their country of origin (Tabasinejad et al. 2014).
PVM isolates, like those of many other plant viruses, may be expected to occur in nature in multiple infections. However, nothing is known about a type and outcomes of probable interactions between PVM isolates invading the same host plant. Closely related viruses mostly interact with one another in an antagonistic manner (Syller 2012). A typical antagonistic interaction is cross-protection. It refers to a situation in which a previous infection with a mild (protecting) virus isolate prevents or suppresses the harmful effects of subsequent infection by a related severe (challenging) virus isolate (Syller 2012; Zhou and Zhou 2012).
In this study, the results indicating the existence of antagonism between PVM isolates in plants of Datura metel (called the Devil's trumpet or Downy thorn-apple) are demonstrated. The response of plants to sequential or simultaneous inoculation by low virulent and highly virulent isolates is characterized. Additionally, coat protein sequences of the two isolates are compared.
Materials and Methods
Virus isolates, plants, inoculation and diagnostic tests
PVM isolates I-38 and Uran (AY311394) were chosen from the laboratory-maintained collection of PVM isolates. The isolate Uran was collected in the 1970s in Polish potato cv. Uran (Chrzanowska 1976), and I-38 was found in 2012 in plants of cv. Irga, grown in small vegetable gardens in Młochów. The choice was made on the basis of a striking difference between these isolates in a level of virulence to D. metel plants, revealed in preliminary trials involving also Chenopodium quinoa, Gomphrena globosa, Lycopersicon chilense, Solanum lycopersicum (tomato) cv. Najwcześniejszy and Solanum rostratum.
In three subsequent independent experiments, D. metel seedlings were mechanically inoculated (two leaflets/seedling; 10, 15 or 30 plants/experimental combination, depending on the experiment) with the inocula containing I-38 or Uran alone, or a mixture of both isolates (1 : 1, v/v). In another combination, each seedling was inoculated with I-38, and after 7 days, the same leaflets were inoculated with the isolate Uran. To prepare the inocula, sap from L. chilense leaves systemically infected with the relevant isolates was diluted 1 : 3 (v/v) in phosphate buffer (0.057 m K2HPO4, pH 8.0). After inoculation, the plants were kept in a greenhouse or in a phytotron (23°C), depending on the experiment.
PVM infection in plants was evaluated by DAS-ELISA performed two or three times for each plant, using monoclonal antibodies against PVM (Neogen Europe Ltd – ADGEN Phytodiagnostics, Ayr, Scotland, UK), according to the manufacturer's recommendations. A sample from each plant consisted of the tissue taken from the top non-inoculated leaves. Leaf sap extracted from each sample was diluted 1 : 4 with extraction buffer. Absorbance readings at 405 nm were recorded using an ELISA reader (Dynex MRX II; Dynex Technologies, Inc., Chantilly, VA, USA) 1 and 2 h after adding the substrate.
Reverse transcription–polymerase chain reaction (RT-PCR), sequencing and sequence alignment
For total RNA isolation and cDNA synthesis, the procedure described by Syller and Grupa (2014) was employed. The RT-PCR was performed as described by Xu et al. (2010), using primers PVM 3 and PVM 4 reported by these authors. RT-PCR products of amplification were visualized in 1.4% agarose gels with TBE buffer and ethidium bromide (0.5 mg/ml). To discriminate between I-38 and Uran, the RT-PCR products were digested with a mixture of restriction enzymes HindIII and FokI (Fermentas, Hanover, MD, USA), used to generate different restriction fragments length polymorphism (RFLP) patterns. Detection of possible restriction sites in the coat protein sequences of the isolates was performed using nebcutter V2.0 (http://tools.neb.com/NEBcutter2/index.php). Ten-microlitre aliquots of the RT-PCR products were digested with the enzymes, 0.5 μl each (10 U/μl), and incubated for 4 h at 37°C. The digested products were separated on 2% agarose gels and visualized with ethidium bromide staining. Sizes of the RFLP bands were determined by comparison with a 1000-bp DNA ladder (Fermentas).
The PCR product for the isolate I-38 was purified using GenElute™ PCR Clean-Up Kit (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer's instructions, and sequenced using the set of primers described above.
Detection of possible restriction sites in the coat protein sequences of PVM isolates I-38 and Uran was performed using nebcutter V2.0. The sequence of the coat protein gene of the isolate I-38 was analysed using blastn and blastp programs (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequences of the isolate I-38 have been deposited in the NCBI GenBank and given an accession number KJ365309.
The predicted amino acid sequence of protein products of the I-38 coat protein gene was deduced from the nucleotide sequence using expasy translate tool (http://web.expasy.org/translate/). Multiple sequence alignment was performed using ebi clustalw (2.1) program (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Results and Discussion
In the preliminary trials, PVM isolate Uran induced in D. metel severe symptoms, leading to the premature plant death, whereas I-38 caused very mild or no symptoms. No such striking differences, if any, in the response of plants of the other five species to infection with these isolates were observed.
In the three main experiments, Uran repeatedly induced in D. metel distinct symptoms, which appeared as early as 2–3 days postinoculation (dpi). The symptoms consisted of chlorotic local lesions, progressively developing systemic stem and leaf necrosis, descending of the inoculated leaves, reduced plant growth and irreversible plant wilting and withering, resulting in the premature plant death (Fig. 1a,c). By contrast, I-38 again caused no or mild symptoms that included slight chlorotic local lesions followed by incidental wilting and descending of the inoculated leaves (Fig. 1a,b).
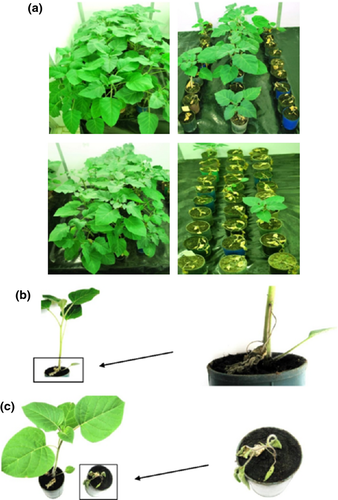
Plants of D. metel that were simultaneously inoculated with I-38 and Uran varied in the response to double infection. Approximately one-fourths of the plants successfully withstood the destructive effects of infection, whereas the remaining 75% of plants exhibited severe symptoms, leading to plant death (Fig. 1a). By contrast, the plants that were pre-inoculated with I-38, and after 7 days were challenge-inoculated with Uran, displayed symptoms resembling those exhibited by the plants infected with I-38 alone (Fig. 1a,c). The response of plants to double infection with I-38 and Uran indicated that within-host interactions between these isolates are antagonistic in type. In addition, the way in which the plants sequentially inoculated with the two isolates responded to infection strongly suggested the occurrence of within-host exclusion of the highly virulent isolate Uran by the low virulent isolate I-38. The presence of PVM in singly or doubly inoculated plants was confirmed by ELISA (some plants were not tested as they had died within several days after inoculation with the isolate Uran). However, it was not possible to discriminate between PVM isolates using this assay.
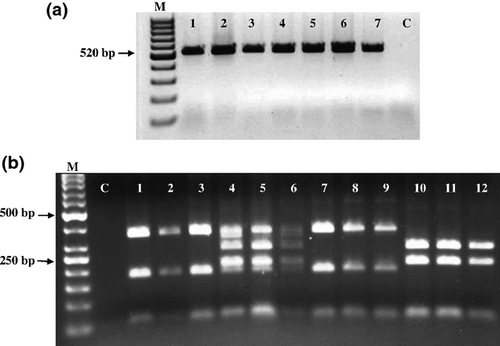
Molecular assays were consequently applied to identify the presence of I-38 and/or Uran in doubly inoculated D. metel plants. For the RT-PCR-RFLP assay, leaf samples were taken from the upper non-inoculated leaves 17 days postinoculation with Uran. Virus detection was carried out using PVM-specific primers designed for the coat protein-encoding region. A product of 520 bp, expected for PVM, was obtained with each of the examined samples collected from singly or doubly inoculated plants (Fig. 2a, lanes 1–7). To discriminate between I-38 and Uran, the RT-PCR products were further examined using restriction enzymes HindIII and FokI to generate different restriction sites for I-38 and Uran (Fig. 2b, lanes 1-3 and 10-12, respectively). As seen in Fig. 2b, the RT-PCR-RFLP products obtained from plants pre-infected with I-38 and challenge-inoculated with Uran indicated the presence of I-38, but not of Uran (lanes 7–9). In contrast, five PCR products were obtained from the plants simultaneously inoculated with these isolates (Fig. 2b, lanes 4-6), which indicates that both isolates were present in these plants.
The above results provide evidence for the occurrence of cross-protection between PVM isolates in plants of D. metel. This species proved to be a suitable host for studying the competitive interference between PVM isolates. The host defence mechanism(s) triggered by I-38 appeared to be efficient enough to protect D. metel plants against dramatic effects of subsequent infection by Uran. Interestingly, remarkable effects of the protection were still visible more than two months after plant inoculation (not shown). The phenomenon of cross-protection may rely on a prevention of the disassembly of the challenging virus by the expression of the coat protein of the protecting virus, or on the induction of RNA silencing by the protecting virus, most probably by sequence-specific degradation of the challenging virus RNA (Nishiguchi and Kobayashi 2011). However, none of the proposed mechanisms fully elucidates the molecular basis of cross-protection. It may be further speculated that multiple mechanisms rather than a simple one underlie this phenomenon, and different strategies can be used by different viruses (Nishiguchi and Kobayashi 2011; Zhou and Zhou 2012).
A comparison of the I-38 nucleotide sequence with those in the NCBI GenBank databases showed 97% identity with the coat protein genes of the isolate Uran or another Polish PVM isolate M57 (accession no. AY311395.1). In turn, the BLASTp analysis of the amino acid sequence of the I-38 coat protein resulted in 99% homology with that of the coat protein of PVM isolate from Germany (ACF05255.1). In the next step of analysis, the ClustalW alignment of the amino acid sequences of the coat proteins of I-38 and Uran revealed three substitutions: proline for leucine at position 31, isoleucine for valine at position 138 and alanine for valine at position 156 (Fig. 3). It would be premature to connect these substitutions with the different levels of virulence of I-38 and Uran. However, such a possibility cannot be excluded because it is known that mutations in the coding regions of plant viruses can modify certain biological properties of these viruses, including symptoms type and/or severity (e.g. Lindbeck et al. 1992; Xu et al. 2012; Hasiów-Jaroszewska et al. 2013). A presumption drawn from the preliminary trials that the competitive interference between I-38 and Uran can be host species dependent in form also remains to be verified.

To the best of our knowledge, this is the first report of cross-protection between isolates of PVM invading the same host plant.
Acknowledgements
The authors thank Zofia Wasilewska for excellent technical assistance.




