Controlled inhalation improves central and peripheral deposition in cystic fibrosis patients with moderate lung disease
Conflict of interest: None declared.
Abstract
Aim
With progressive impairment of lung function, deposition of inhaled drug in the lungs becomes progressively more central, limiting its effectiveness. This pilot study explored the possibility that long slow inhalations might improve delivery of aerosol to the lung periphery in cystic fibrosis patients with moderate lung disease.
Methods
Five subjects aged 12–18 years (mean FEV1 72%; range 63–80%) inhaled a radiolabelled aerosol from a jet nebuliser on two occasions. Two inhalation techniques were compared: breathing tidally from a standard continuous output nebuliser and using long slow inhalations from the AKITA® JET system.
Results
Long slow breaths resulted in much lower oropharyngeal deposition with higher lung doses. Importantly, the peripheral lung increased proportionately. The increased lung dose is attributable to more of the larger inhaled droplets passing into the lower airways. This would be expected to increase the central deposition unless significantly more of the smaller droplets were able to penetrate deeper into the lungs. The data support improved delivery of drug to the distal lung when compared with tidal breathing.
Conclusion
These pilot data suggest that this approach may prove to be clinically relevant in improving the efficacy of inhaled medication in those with moderate-severe lung disease.
What is already known on this topic
- In those with airways disease the deposition of inhaled aerosols becomes more central and less homogenic as the lung function deteriorates.
- This is likely to significantly impair the effectiveness of inhaled drugs such as antibiotics as the disease progresses.
- Improvements might be achieved by using smaller droplets though this significantly increases treatment times or by using a slow inspiratory breath with a dosimetric device that only delivers aerosol during inhalation.
What this paper adds
- In this pilot study, patients reported the controlled inhalation was easy to use and would be happy to use the device in routine care.
- When compared with a standard jet nebuliser, drug delivery to the peripheries of the lungs was improved in patients with moderately severe cystic fibrosis lung disease using a dosimetric jet nebuliser that delivered aerosol during long slow inhalations.
Nebulisers are widely used in the treatment of cystic fibrosis (CF) lung disease. They are used to deliver drugs to the airways which cannot be delivered orally, such as DNAse and hypertonic saline. They also can deposit high doses of drugs, such as antibiotics on the luminal surface.1 The paradox of inhaled medication is that drug delivery is most ‘uniform’, and hence most effective, in those with healthy lungs.1-4 Diseases affecting the conducting airways cause drug deposition to become progressively more central and inhomogeneous as the disease progresses, significantly reducing delivery of aerosol to more peripheral airways in moderate to severe disease.1-4
Two potential strategies may improve the penetration of antibiotic-containing droplets into the peripheral regions of the conducting airways. One option would be to reduce the particle size of inhaled droplets,5, 6 but with current technology, this would inevitably result in a reduced rate of drug output and significantly increased treatment times.7 At present, there are no commercial devices that permit tailoring of droplet size to disease severity.
An alternative and, currently, more practical approach is to reduce central deposition by controlling inhalation flows. A number of electronically controlled breath-actuated (EBA) nebulisers are now available that limit the delivery of aerosol to the inspiratory phase of the respiratory cycle and which can modify the period of drug delivery to match the duration of the patient's inspiratory effort. There is a substantial body of literature demonstrating the benefits of long slow inhalation (LSI) when attempting to deliver drugs deeper into the lungs.6, 8, 9 Low inspiratory flows substantially reduce the impaction of aerosolised particles in the upper and central pulmonary airways resulting in more deposition in peripheral regions of the lungs. The same principles are likely to be beneficial in those with significant airways disease.
Previous studies in adults have shown that LSI using an EBA nebuliser increases the rate of delivery of aerosol to the lung by reducing the upper airways deposition, resulting in shorter inhalation times to deliver the same dose.10, 11 One of these studies, using a pharmacodynamic approach, suggested that peripheral delivery may have also improved10 but, to date, there have been no deposition studies that have explored the impact of this approach on the delivery of aerosol to distal airways in patients with moderate airways disease. In this pilot open-labelled, radiolabelled deposition study, the distribution of aerosol deposited in the airways with LSIs from an EBA nebuliser was compared with tidal breathing from a standard jet nebuliser in subjects with moderate CF lung disease.
Methods
The Princess Margaret Hospital Research Ethics Committee approved the study and the study conformed to standards set out in the Declaration of Helsinki. Informed consent was obtained from participants and parents. Subjects had a confirmed diagnosis of CF, were 12–18 years of age with moderate lung disease. They were clinically stable for at least 2 weeks prior to each study visits which were separated by 7–10 days. Their FEV1 at visit 2 was ±5% that at visit 1.
At each visit, subjects performed airway clearance with a physiotherapist and, after training, used one of two neubliser systems. (i) A Pari LC sprint jet nebuliser with continuous flow Pari Boy SX compressor was used with tidal breathing. (ii) A Pari LC sprint with AKITA® JET delivery system which provides a driving flow only during inhalation and which adjusts delivery to a patient's specific pattern of breathing12 was used with LSIs. The inspiratory time adopted for each patient was based on an assessment of the individual participant's ability to maintain a regular cycle of LSI of 6–8 s. The order was randomised.
The nebulisers were charged with 4 mL 99mTechnetium diethylene-triamine-pentaacetate (99mTc-DTPA) in isotonic saline (maximum of 1.75 MBq/mL). This does not alter droplet size. The subjects completed ten 6–8 s slow inhalations with the AKITA® JET device and breathed tidally for approximately 160 s with the standard nebuliser so that the total inspiratory times were approximately equal for the two arms. This provided sufficient 99mTc deposition to provide robust data while minimising the radiation dose and loss of isotope from the lung prior to imaging.13 The half-life residence time in the lungs of 99mTc-DTPA is only 80 min. Nose clips were worn during the study.
Immediately afterwards, simultaneous anterior and posterior planar images of the chest and abdomen with lateral images of the head and neck were obtained using a double-headed gamma camera (Ecam, Siemens, Erlangen, Germany). Separate count rates were determined for the right and left lungs (including central and peripheral lung regions), stomach, oesophagus, mouth and oropharynx and subsequently corrected for background counts, tissue attenuation and decay time. The geometric means of corresponding anterior and posterior count rates were calculated.14, 15 Participants provided subjective ratings (using a scale of 0–10) regarding the ease of use of the two techniques.
Results
Five subjects participated with a median age of 14.6 (12–18) years. Four were male. Their median FEV1 was 72% (63–80) predicted. Of 13 potential subjects, 2 subjects were not clinically stable and 6 subjects declined to participate.
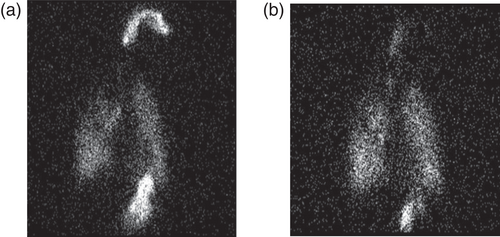
The AKITA® JET system was associated with significantly reduced oropharyngeal deposition as a percentage of total deposition (Table 1). Figure 1 is an example of the images obtained from a single patient. There was no significant difference in the mean peripheral to central (P:C) deposition ratios (Table 1).
| Standard delivery | Dosimetric delivery | P value | |
|---|---|---|---|
| Lung dose (%) | 31.97 (8.62) | 74.24 (8.39) | <0.001 |
| Orogastric dose (%) | 25.75 (8.72) | 18.01 (7.58) | <0.01 |
| Peripheral:central ratio | 2.13 | 2.16 | 0.90 |
Participants reported both techniques were ‘very easy’ (9/10 tidal breathing, 9.4/10 LSI) and all participants said they would be happy to use the AKITA® JET system again.
Discussion
The results from this pilot study support the suggestion that using LSI can also significantly increase the peripheral deposition of inhaled medication in CF patients with moderately severe lung disease. As anticipated, LSI greatly reduced deposition in the upper airway with the majority of the deposited dose being in the lungs. Importantly, the increased delivery to the lung did not alter the P:C ratio indicating that both the central and, crucially, the peripheral dose would have increased proportionately.
The slow breath permits the larger droplets contained in the nebuliser's polydispersed aerosol to avoid impaction in the upper airways and so deposit in the larger conducting airways, while the smaller droplets that would normally reach the lungs with tidal breathing are able to penetrate deeper into the lungs. In this case, the sum of these two changes is to increase the total lung dose and peripheral lung dose thus maintaining the P:C ratio unaltered.
Recruitment was limited by the low number of potential subjects, with less than 3.5% of patients at this centre having a FEV1 less than 80%. The median FEV1 was 72% and therefore represented a relatively mild population of patients with moderately severe disease. It is likely that the benefits of LSI might be greater in a cohort with lower lung function though. However, at the more severe end of the lung disease spectrum, its role is likely to be limited as in these subjects even inhaled gases fail to reach significant areas of the lungs.17, 18
Importantly, the subjects found the LSI technique at least as acceptable as using tidal breathing consistent with previous studies using this approach to decrease the time required for nebulisation.18
Conclusion
These results suggest further work assessing the potential benefits of LSI with EBA nebulisers for the treatment of patients with moderately severe CF and related airways diseases is warranted.
Acknowledgements
This study was funded by the Perth Children's Hospital Foundation and the AKITA® JET nebulisers were kindly provided by Vectura. Open access publishing facilitated by The University of Western Australia, as part of the Wiley - The University of Western Australia agreement via the Council of Australian University Librarians.




