Effects of migration distance on life history strategies of Western and Semipalmated sandpipers in Perú
Abstract
enMigration distances of shorebird species correlate with life history strategies. To assess age-specific migratory preparation and adult wing-molt strategies, we studied Western Sandpipers (Calidris mauri) and Semipalmated Sandpipers (C. pusilla) with different migration routes at the Paracas National Reserve in Perú, one of the most austral non-breeding areas for these sandpipers, from 2012 to 2015. Western Sandpipers breed near the Bering Sea, ~11,000 km from Paracas. Semipalmated Sandpiper populations at Paracas are a mixture of short-billed birds from western Arctic breeding sites, plus long-billed birds from eastern sites, ~8000 km distant. Adults of both species arrive in October with primary feathers already partially renewed so wing molt starts at sites further north. Semipalmated Sandpipers with longer bills completed wing molt later than shorter billed birds. Adults of both species prepared for migration in February and March. No juvenile Western Sandpipers prepared for migration, confirming the “slow” over-summering life history strategy of more southerly non-breeding populations. Juvenile Semipalmated Sandpipers showed bimodality in strategies. Most showed no migratory preparation, but, during three non-breeding periods, from 27% to 31% fattened, molted, and partially replaced outer primaries during the pre-migratory period. Juveniles with longer culmens were heavier and tended to have more alternate plumage. Juveniles that were partially molting primaries had longer culmens and more alternate plumage. Juvenile Semipalmated Sandpipers from eastern-breeding populations thus have a higher propensity for a fast life history strategy, and western birds a slow one, at this non-breeding site in Peru. Western-breeding Semipalmated Sandpiper populations thus resemble Western Sandpipers, suggesting a common, possibly distance-related, effect on life history strategy.
Efectos de la distancia de migración sobre la estrategias de historia de vida en Calidris mauri y Calidris pusilla en Perú
esLa distancia de migración de aves playeras esta correlacionada con las estrategias de la historia de vida. Para determinar la preparación para la migración relacionada con la edad y la estrategias de muda en los adultos, estudiamos individuos de Calidris mauri y Calidris pusilla que tienen rutas de migración diferentes en la Reserva Nacional Paracas en Perú, una de las áreas de migración más australes para estas aves, entre 2012 y 2015. Calidris mauri se reproduce cerca del mar de Bering, ~11,000 km de Paracas. Las poblaciones de Calidris pusilla en Paracas son una mezcla de aves con pico corto de los sitios de reproducción del Oeste del Ártico y aves con pico largo de sitios reproductivos al este, distanciados por ~8000 km. Los adultos de ambas especies llegan en Octubre con las plumas primarias parcialmente renovadas, lo cual indica que la muda de invierno comienza en localidades más al norte. Los individuos de Calidris pusilla con el pico más largo, completaron la muda en las alas más tarde que los individuos de pico corto. Los adultos de ambas especies se prepararon para la migración en Febrero y Marzo. Ninguno de los juveniles de Calidris mauri se preparó para la migración, lo cual confirma una estrategia de verano más “lenta” de las poblaciones no reproductivas más sureñas. Los juveniles de Calidris pusilla mostraron bimodalidad en las estrategias. La mayoría de los individuos no mostro preparación alguna para la migración, pero, durante tres periodos no reproductivos, entre 27 y 31% de los individuos acumularon grasa, mudaron y reemplazaron parcialmente las primarias externas durante el periodo pre-migratorio. Juveniles con cúlmenes más largos fueron más pesados y tuvieron la tendencia a tener más plumaje alterno. Juveniles que mudaron las primarias parcialmente tuvieron cúlmenes más largos y mayor cantidad de plumaje alterno. Consecuentemente, los juveniles de Calidris pusilla de las poblaciones reproductivas del Este tienen una mayor propensidad a tener una estrategia más rápida en las historias de vida y las aves del Oeste una más lenta, en estos sitios no reproductivos de Perú. Poblaciones reproductivas del Oeste de Calidris pusilla son similares a Calidris mauri, sugiriendo un posible efecto común de la distancia sobre las estrategias de historia de vida.
Long-distance migration has been recognized as an evolutionary adaptation to maximize the survival and reproductive success of individuals by exploiting seasonal peaks of resource abundance and avoiding seasonal resource depression (Alerstam et al. 2003). Migration strategies thus balance costs against benefits, and studying the diversity of migration patterns will help identify the relative importance of different selective factors. Life history strategies of migratory shorebirds vary among short-, medium-, and long-distance migrants (Nebel et al. 2000, Fernández et al. 2004, Morrison et al. 2005, O'Hara et al. 2005, Buehler and Piersma 2008, Remisiewicz et al. 2014) and, for some species, among age classes (O'Hara 2002, Fernández and Lank 2007, Remisiewicz et al. 2010). As a dramatic example, not all individuals in populations migrate from non-breeding areas and attempt to breed every year; such over-summering birds spend a “gap year” in non-breeding areas (Loftin 1962, Johnson and Johnson 1983, McNeill et al. 1994, Hockey et al. 1998, Pyle 2008). Juveniles that over-summer are pursuing a slow life history strategy, postponing their first potential breeding opportunity. Proximate explanations to account for over-summering by juvenile shorebirds include sexual immaturity (Eisenmann 1951, Loftin 1962, Johnson and Johnson 1983), helminthic infestation (McNeill et al. 1994), sterility, injuries, or illness (Wetmore 1927), and less efficient foraging (Puttick 1979, Hockey et al. 1998). Ultimate explanations include low probabilities of having a successful breeding season (Summers et al. 1995), higher likelihood of surviving in non-breeding areas (Fernández et al. 2004), condition-dependence on primary feather wear (O'Hara 2002), and other distance-dependent costs (Myers et al. 1985, Lank et al. 2003, Ydenberg et al. 2004, 2007).
Feather molt is another factor integrated with migratory strategy. Birds generally avoid overlapping molting with other energy-expensive activities such as breeding or migration, adopting a variety of schedules to separate these activities (Ginn and Melville 1983, Zwarts et al. 1990). Molt strategy varies with breeding success, migration distance, food availability, non-breeding latitude, and type of habitat (Prater 1981, Barta et al. 2006, Howell 2010, Remisiewicz 2011, Rogers et al. 2014, Dietz et al. 2015). For example, adults of many species, such as Little Stints (Calidris minuta), Red Knots (Calidris canutus), and Wood Sandpipers (Tringa glareola), molt almost exclusively in non-breeding areas (Pearson 1984, Remisiewicz et al. 2009, Summers et al. 2010). Other species, like Wilson's Phalaropes (Phalaropus tricolor), start to molt at stopover sites, but then suspend it and resume in non-breeding areas (Jehl 1987). Molt suspension is common among shorebirds and is known to be a strategy for coping with temporary food scarcity (Prater 1981) and facilitating short-distance movements (Remisiewicz 2011). For example, adult Common Greenshanks (Tringa nebularia) arrive in wintering areas in Kenya with a suspended molt, but then resume molting from September to January (Pearson 1974). Molting at staging sites is also not uncommon. For example, Red Knots take advantage of seasonal peaks in food availability at specific stopover sites during fall migration to molt (Harrington et al. 1979). Finally, some taxa pursue alternative strategies, such as some subspecies of Dunlins (Calidris alpina) that start molting on or near breeding areas (Kania 1990, Holmgren et al. 2001, Warnock et al. 2013).
Molt strategies also vary with age-classes. Some juveniles molt all of their primaries before their first northward migration (e.g., Little Stints, Tree 1974), others retain their first set of primaries and complete the first migration with the same feathers (Prater et al. 1977), and some retain their first set of primaries for up to 17 mo (e.g., Western Sandpipers, O'Hara et al. 2005). A peculiar addition to this set of molt-chronology variability in juvenile shorebirds is the molt strategy known as the Partial Post-Juvenile Wing Molt (PPW), where juveniles lose and replace only 1–6 outer primaries on each wing (Gratto and Morrison 1981).
Western Sandpipers (Calidris mauri) and Semipalmated Sandpipers (C. pusilla) are among the smallest scolopacid sandpipers in the Americas. They overlap in morphological traits (Paulson 1993, Haig et al. 1997), look alike in basic plumage (Phillips 1975, Sibley 2000), and have similar breeding biology (Holmes 1972, Gratto-Trevor 1991, Ruthrauff et al. 2009). A proportion of the populations of each of these species spends the non-breeding season in South America, where they often flock together. However, they differ in the timing of southward migration, with Western Sandpipers migrating about one month before central and eastern populations of Semipalmated Sandpipers (Lank et al. 2003, Hicklin and Gratto-Trevor 2010, Franks et al. 2014).
Western Sandpipers migrate from their breeding range in western and northern Alaska and eastern Siberia to Perú along the Pacific coast, and to the east coast of the United States to Surinam on the Atlantic coast (Franks et al. 2014). They show a differential migration by sex, with a higher proportion of males at northern than at southern sites (Nebel et al. 2002). Previous studies report a slow, over-summering life history strategy for the juveniles spending their first non-breeding season in Panamá, in contrast to a fast life history for those in México, where most migrate north in their first year (Fernández et al. 2004, O'Hara et al. 2005).
Semipalmated Sandpipers breed on the Subarctic Alaskan coast, where they partially overlap the breeding range of Western Sandpipers, and east across the Canadian tundra to northern Quebec. They spend the non-breeding season from Florida to the central Brazilian coast on the Atlantic coast, and from southern México to Perú on the Pacific coast (Harrington and Morrison 2010, Hicklin and Gratto-Trevor 2010). Semipalmated Sandpipers exhibit a cline in bill length across their breeding range, with eastern birds having bills that are ~8–12% longer than those of western birds (Manning et al. 1956, Harrington and Morrison 2010, Gratto-Trevor et al. 2012), despite minimal population genetic differentiation (Miller et al. 2013). Most of the eastern population (84%) of Semipalmated Sandpipers spends the non-breeding season in northeastern South America (Morrison et al. 2012), whereas the western and central populations appear to favor western South America as a non-breeding site. Based on mean bill lengths, Gratto-Trevor et al. (2012) suggested that the central breeding population predominates in Perú. However, Tavera (2013) found a confluence of eastern and western populations on the Peruvian coasts, as assessed by bill lengths. The migration distance from western breeding sites of Semipalmated Sandpipers to Perú is ~11,000 km, whereas eastern populations migrate ~8000 km. Based on one year's data, Tavera (2013) showed differences in life history strategies between coexisting populations in Perú, including arrival timing and body molt-chronology prior to spring departure, which may be related to the differences in migration distance.
We examined non-breeding populations of Western and Semipalmated sandpipers in Perú to test predictions about the effects of migratory distance and age class on life history strategies. After inferring the geographical breeding origins of Semipalmated Sandpipers at Paracas, our study had three objectives. (1) We quantified the propensity for over-summering behavior by first-year birds. Nearly all young Western Sandpipers spending their first non-breeding season in Panamá over-summer, but few of those at México do so. We expected high rates in Perú. For Semipalmated Sandpipers, over-summering by juveniles has been reported (Spaans 1976, Pyle 2008), but has not been quantified at any location. We used changes in mass, plumage, and wing molt during the adult pre-migratory season as potential indicators of migration propensity. (2) We compared wing-molt chronologies of adult and juveniles of each species. For Western Sandpipers, southward-migrating adults have been reported to drop the first five primaries almost simultaneously upon arrival in Panamá (Watts 1998). Semipalmated Sandpipers are reported to begin primary molt after arrival in Brazil (Spaans 1981), but patterns of molt have not been documented further south for either species. For juveniles, we expected Western Sandpipers to follow the slow life history strategy previously shown in Panamá and, therefore, to not undergo wing molt during their first non-breeding season. For Semipalmated Sandpipers, a study at James Bay revealed variation in the extent of PPW, ranging from half to all migrants originally banded as juveniles and recaptured as first-year birds (Gratto and Morrison 1981). However, the probability of PPW varying among breeding or non-breeding populations and whether those birds undergoing PPW have a different probability of pursuing a fast versus slow life history strategies are unknown. (3) For juvenile Semipalmated Sandpipers, we tested for differences in migratory strategies between breeding populations coexisting in Perú. Specifically, we expected that migratory strategies differed as a function of migration distance, using bill length as an indicator of the location of breeding populations. If longer-distance migrants have a greater propensity to over-summer, a smaller proportion of juvenile Semipalmated Sandpipers with shorter bill lengths should prepare for migration.
Methods
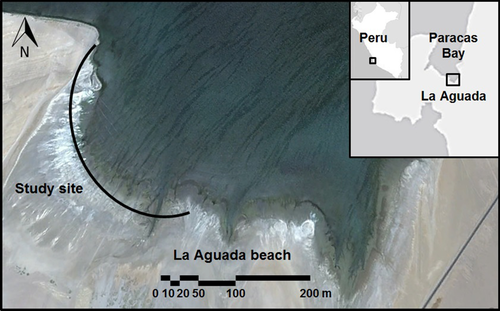
Study site
The Paracas National Reserve is a Natural Protected Area in Perú located in the Department of Ica. We conducted field work on La Aguada beach (13°51′35.47″S 76°16′16.16″W), an intertidal mudflat <2 km long and surrounded by coastal desert (Fig. 1). The near-shore section of the mudflat has no vegetation and most of the lower intertidal zone is covered with soft mud filled with polychaetes, fly larvae, microscopic sea shrimp, and beetles (Pellissier 2013), and covered by biofilm and decaying algae.
Data collection
Migrant Western and Semipalmated sandpipers arrive in Paracas in early October, and were captured during three consecutive non-breeding seasons (October 2012–March 2013, October 2013–April 2014, and December 2014–April 2015). Nine-day campaigns were conducted per month during the new moon phase. Shorebirds were captured primarily at night with mist-nets. Time of capture depended on the tide cycle; we always began three hours after the evening high tide and ended three hours before the next high tide, ranging between 21:00 and 06:00. Fewer than 10% of the birds were captured with bungie-powered whoosh nets on rising tides between 06:00 and 09:00. Captured birds were banded on the right tarsus with an incoloy metal band (CORBIDI Bird-Banding Program, the Peruvian bird-banding scheme). A three-character-coded yellow flag was placed on the left tibia, following the Panamerican Shorebird Program protocol (Myers et al. 1983, Myers 1984), to identify individuals, allow collection of local resighting data, and to document long-distance movements. Birds were assigned age classes as juveniles or adults based on plumage. Juveniles (individuals in their first non-breeding season from 0 to 12 mo old) were aged by the retained juvenile-type inner greater coverts, and adults (12+ mo old) were aged by wing and flight feather characteristics (Prater et al. 1977, but see Franks et al. 2014). Birds were weighed using a digital scale (± 0.5 g).
Alternate plumage scores
Most small sandpipers undergo a pre-alternate molt from basic (dull non-breeding) into alternate (bright breeding) plumage in spring (Prater et al. 1977, Wilson 1994, Hicklin and Gratto-Trevor 2010, Franks et al. 2014). Alternate plumage of captured birds of both species was identified using feather characteristics for each species, including the degree of black-centered and rufous-edged coloration (Prater et al. 1977). The crown, cheeks, mantle, upper scapulars, and tertials were scored following O'Hara et al. (2005) as: 1 = no rufous anywhere, 2 = trace of rufous on any tract such as the mantle, upper scapulars, tertials, or crown, 3 = traces of rufous on more than one tract, 4 = presence of rufous in three or four tracts, 5 = rufous on lower scapulars and other tracts, and 6 = full alternate plumage.
Wing molt scores
We classified captured birds as having completed wing molt (all new feathers), being in suspended molt (e.g., having old and fully grown feathers, but no missing or pin feathers), being in active molt, or not having started wing molt (all old feathers). Individual primaries were numbered in the order they normally molt, from the innermost primary (P1) to the outermost (P10) (Pyle 2008). The stage of primary molt was recorded as a molt formula using the British Trust for Ornithology method (Ginn and Melville 1983), a string of 10 digits, whereby individual feathers were assigned a score (0 = old feather, 1 = feather in pin, 2 = brush stage, 3 = two-thirds grown, 4 = four-fifths grown, and 5 = new feather). The sum of the scores for all 10 primaries was the primary molt score (PMS), which ranged from 0 (all old primaries, molt not started) to 50 (all new primaries, molt completed).
Data analyses
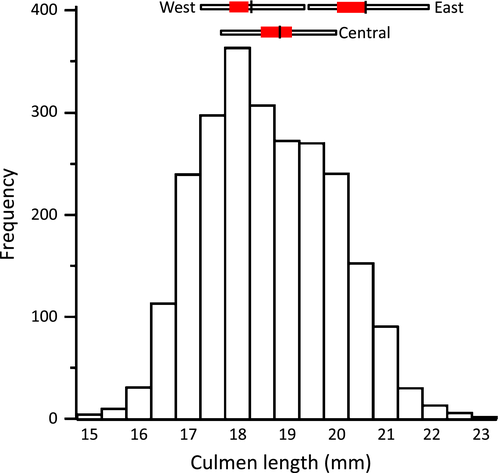
To infer the geographical origins of the Semipalmated Sandpipers captured at Paracas, we compared the frequency distribution of culmen lengths with (1) the range of population mean values for western, central, and eastern populations (Gratto-Trevor et al. 2012), and with (2) the mean and standard deviation of simulated regional populations with 50:50 sex ratios, derived from metrics of museum specimens (Table 2 in Harrington and Morrison 2010), with 1 mm added to account for shrinkage. For the Paracas population, we determined measures of centrality and skewness, and tested for deviation from normality with the Kolmogorov–Smirnov D statistic.
Patterns of mass change and the alternate plumage status were examined by dividing the non-breeding season into “residency” and “pre-migratory” periods. Because individuals of both species of sandpipers started to increase in mass above non-breeding levels starting in early February, we defined the residency period as extending from the beginning of field work (October) through the end of January, and the pre-migratory period as starting on 1 February. Mass and plumage variation were compared among age categories and analyzed separately for each period and for each year of the study. For the pre-migratory period only, we tested whether the potential indicators of migratory readiness were concordant using logistic regression of plumage scores as a function of mass, and by modeling the presence/absence of PPW as a function of date-specific mass and plumage. We included data from first captures of all birds (N = 2965) plus individuals recaptured more than 4 weeks later (N = 236) that were treated as independent observations. Only captures with both mass and plumage scores were used. Species were analyzed separately.
We quantified patterns of mass with general linear models in relation to the fixed effects of time, age, and their interaction. Alternate plumage scores were treated as an ordinal response variable, and analyzed with respect to age, mass, date, and year using logistic regression models. We initially tested full models including interactions for year and age differences, and reran reduced models eliminating non-significant interaction terms. All tests used a Type III SS, which controls for other model effects, to assess the significance of particular variables (α = 0.05 for main effects, and α = 0.10 for interactions). We expected our potential indices of migratory readiness to positively covary during the pre-migratory period, and tested for a relationship between mass and plumage class with logistic regression models. For juvenile Semipalmated Sandpipers, we tested for correlations among PPW, body mass gain, and plumage score during the pre-migratory period with logistic regression models.
To look for evidence that life history traits of Semipalmated Sandpipers varied with migration distance, we tested whether the timing of post-breeding wing molt of adults during the residency period, and the pre-migratory mass, plumage score, and PPW of juveniles, varied with respect to culmen length, which was used as a proxy for their breeding population of origin. We modeled mass using multiple regression and plumage score with logistic regression, with predictors of culmen length, date, and their potential interaction. We modeled the presence or absence of PPW for birds captured after 8 March, when PPW was first detected, using logistic regression, with mass at capture, body molt score, and culmen length as predictors. To provide a detailed picture of these relationships, we plotted the frequency distributions of culmen lengths for samples with or without PPW, and compared these with sex-specific distributions of culmen lengths from eastern and western breeding areas (Gratto-Trevor et al. 2012). Western Sandpipers can be reliably sexed by bill length differences, but Semipalmated Sandpipers cannot. Preliminary analyses of sex effects for Western Sandpipers showed no differences in molt or plumage variables, thus these were not considered further. Potential effects of sex differences on our interpretation of patterns in Semipalmated Sandpipers are considered in the discussion. All analyses were performed using SAS (SAS Institute 2012; v. 9.4). We used AIC scores to choose the most parsimonious of competing models. We report significance of individual factors in logistic regression with Wald χ2 and two-tailed tests.
Results
Non-breeding population of Semipalmated Sandpipers
Semipalmated Sandpipers captured at Paracas had a distribution of culmen lengths that spanned that of their entire breeding range (Fig. 2). The distribution was positively-skewed and non-normal (N = 2439, mean = 18.6 mm, median and mode = 18.5mm, skewness = 0.20, Kolmogorov-Smirnov normality test D = 0.05, P < 0.01), showing a species-wide distribution with an over-representation of western birds.
Residency period
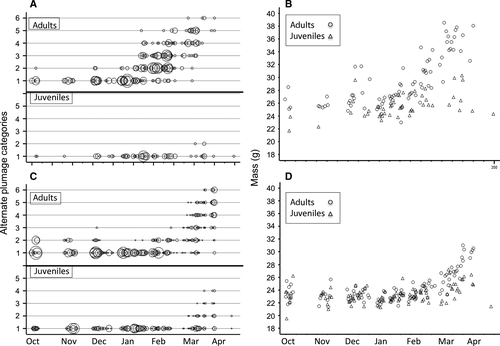
Both species had low masses and stable basic plumage scores during the non-breeding residency period, followed by fattening and the development of alternate plumages by most, but not all, individuals in the populations during the pre-migratory period (Fig. 3, Table 1). During the residency period, adult Western Sandpipers were heavier than juveniles in all years (Table 1, Fig. 3), with no significant interactions among years or age classes (all P > 0.18). Masses were stable during 2012–2013 and 2014–2015, but increased for both age classes during 2013–2014. Adult Western Sandpipers started to develop alternate plumage by the end of January, but no juveniles did so. Adult Semipalmated Sandpipers were heavier than juveniles during 2012–2013, but we found no other age differences in either plumage scores or mass (Table 1, Fig. 3).
| Residency period | Pre-migratory period | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | ||||||||||||
| Species | N | F | P | Mean adult | Mean juvenile | N | F | P | Mean adults | Mean juvenile | ||
| WESA | 2012–2013 | Age | 54 | 7.4 | 0.01 | 26.8 | 24.9 | 188 | 3.6 | 0.059 | 31.9 | 27.1 |
| Day | - | 0.04 | 0.84 | - | - | - | 9.5 | 0.002 | - | - | ||
| Age*Day | - | - | - | - | - | - | 5.4 | 0.021 | - | - | ||
| 2013–2014 | Age | 288 | 25.9 | <0.001 | 26.4 | 24.7 | 81 | 7.6 | 0.007 | 31.1 | 27.2 | |
| Day | - | 25.6 | <0.001 | - | - | - | 4.1 | 0.047 | - | - | ||
| Age*Day | - | - | - | - | - | - | 12.0 | <0.001 | - | - | ||
| 2014–2015 | Age | 104 | 32.5 | <0.001 | 28.5 | 25.7 | 42 | 33.1 | <0.001 | 33.5 | 26.9 | |
| Day | - | 0.2 | 0.66 | - | - | - | 4.1 | 0.05 | - | - | ||
| Age*Day | - | - | - | - | - | - | - | - | - | - | ||
| SESA | 2012–2013 | Age | 533 | 5.5 | 0.02 | 23.1 | 22.7 | 310 | 7.5 | 0.007 | 25.8 | 23.8 |
| Day | - | 1.4 | 0.25 | - | - | - | 28.9 | <0.001 | - | - | ||
| Age*Day | - | - | - | - | - | - | 11.5 | <0.001 | - | - | ||
| 2013–2014 | Age | 1060 | 0.3 | 0.56 | 22.8 | 22.7 | 299 | 17.5 | <0.001 | 27.4 | 24.2 | |
| Day | - | 3.0 | 0.08 | - | - | - | 117.3 | <0.001 | - | - | ||
| Age*Day | - | - | - | - | - | - | 32.1 | <0.001 | - | - | ||
| 2014–2015 | Age | 138 | 0.5 | 0.50 | 23.0 | 23.2 | 81 | 6.7 | 0.011 | 27.9 | 25.1 | |
| Day | - | 0.2 | 0.66 | - | - | - | 5.1 | 0.026 | - | - | ||
| Age*Day | - | - | - | - | - | - | 9.0 | 0.003 | - | - | ||
Pre-migratory period
From early February onwards, adult Western Sandpipers gained mass, but juveniles did not (Fig. 3, Table 1). Although mean masses of juvenile Western Sandpipers were consistently lower than those of adults during the pre-migratory period, we only detected a significant difference in the third year of our study (Table 1). Adults acquired alternate plumage, whereas only three juveniles did so (age × date terms, 1 df: 2012–2013: Wald χ2 = 34.4, P < 0.0001; 2014–2015: χ2 = 20.9, P < 0.0001; insufficient data for 2013–2014).
Adult Semipalmated Sandpipers were heavier than juveniles during the pre-migratory period (Table 1). Adults started to molt into alternate plumage by early February, and 27% of juveniles did so starting in early March (55/206 with plumage classes > 1, pooled over years). We found no significant differences in plumage scores between age classes during the first 2 years (age × date, 1 df: 2012–2013: Wald χ2 = 0.4, P = 0.50; 2013–2014: Wald χ2 = 1.7, P = 0.19). In year 3, significantly more adults than juveniles molted into alternate plumage (Wald χ2 = 9.3, P = 0.002).
Mass and plumage scores provided similar signals of migratory propensities (Table 2). For both age classes of Semipalmated Sandpipers and for adult Western Sandpipers, mass had a substantial positive effect in logistic models of plumage category that included date in season and year. Juvenile Western Sandpipers that were not expected to prepare for migration exhibited little variation in either plumage or mass (Fig. 3).
| Estimate ± SD | Wald 95% (CL) | Wald χ2 | Pr > χ2 | |
|---|---|---|---|---|
| WESA Adult | 0.217 ± 0.039 | 0.139–0.294 | 30.0 | <0.001 |
| WESA Juvenile | 0.068 ± 0.192 | −0.307–0.444 | 0.1 | 0.72 |
| SESA Adult | 0.262 ± 0.036 | 0.191–0.333 | 52.5 | <0.001 |
| SESA Juvenile | 0.409 ± 0.078 | 0.256–0.561 | 27.6 | <0.001 |
Primary molt
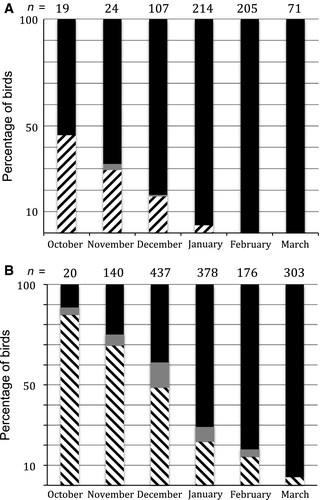
Adult Western Sandpipers captured in October had an average Primary Molt Score of 33 (replacement of 1–6 inner primaries); 84% were in active primary molt, and the rest had already completed it (Fig. 4A). By January, only 4% were still molting primaries, with an average PMS of 47 (molt almost completed except for half of the last outer primary). Suspended molt was found in only two birds, one in mid-November and one in mid-December, both with a PMS of 35 (1–7 new inner primaries were new), and all adults finished primary molt by February (Table 3).
| Month | Western Sandpipers | Semipalmated Sandpipers | ||
|---|---|---|---|---|
| N | Mean PMS | N | Mean PMS | |
| October | 19 | 33.5 ± 7.4 | 398 | 28.3 ± 10.1 |
| November | 24 | 45.3 ± 5.7 | 232 | 35.0 ± 9.6 |
| December | 107 | 44.7 ± 5.5 | 842 | 40.8 ± 7.9 |
| January | 214 | 47.1 ± 2.4 | 445 | 43.6 ± 4.8 |
| February | 205 | 50 | 242 | 43.3 ± 9.3 |
| March | 71 | 50 | 428 | 46.5 ± 2.5 |
Adult Semipalmated Sandpipers were also in active molt by early October, with 88% having an average PMS of 28 or greater, indicating that they had completed more than half of the wing molt (Fig. 4B). The remaining 12% had either completed primary molt or had suspended molt (4%), with an average PMS of 30 (1–6 inner primaries were new). The proportion of birds with suspended molt appeared to increase in December. By February, 85% of the individuals had completed primary molt and the rest were almost finished, with an average PMS of 43 (1–9 inner primaries were new). No adults were found with suspended molt by March, when 96% had a full set of new primaries. Adults captured in different molt status categories (active, suspended, or completed; only two were scored as not started) differed in culmen length (F4,1634 = 15.8, P < 0.0001 controlling for day in season and day in season squared). Adults with completed molt had significantly shorter bills than those in active or suspended molt (Tukey's post hoc tests, P < 0.05), and those with suspended molt had the longest mean bill lengths (Table 4).
| Molt status | N | Mean culmen length (mm) | 95% confident limits |
|---|---|---|---|
| Active | 556 | 18.73 | 18.63–18.83 |
| Suspended | 55 | 19.04 | 18.72–19.37 |
| Completed | 543 | 18.55 | 18.44–18.66 |
No juvenile Western Sandpipers molted primaries. By contrast, 31% of juvenile Semipalmated Sandpipers captured during March were in active outer primary molt. Annual average PMS varied from 8 to 14, meaning that these birds were replacing between two to four outer primaries, and even six in a few cases (Table 5).
| Year | N | PPW (%) | Mean PMS ± SD | Primaries molted |
|---|---|---|---|---|
| 2013 | 77 | 22 | 10.8 ± 6.24 | P8–P10 |
| 2014 | 39 | 38.4 | 8.8 ± 4.58 | P9–P10 |
| 2015 | 27 | 44.4 | 13.8 ± 5.74 | P7–P10 |
Life history differences among juvenile Semipalmated Sandpipers
Did culmen length, acting as a surrogate for breeding population of origin, predict the likelihood of migration as assessed by mass, plumage development, and/or wing-molt strategy? For mass, a full model including culmen length, date, and their interaction was significant (Table 6). The interaction occurs because there was no relationship early in the season, but culmen length predicted mass positively and strongly only during the last part of the pre-migratory period when birds with longer bills tended to be heavier. For plumage category, logistic regression as a function of culmen length and date showed a strong date effect and a non-significant, but positive, trend with culmen length (estimate for culmen length = 0.205 ± 0.14 [SD], 95% Wald CI = −0.07–0.48, Wald χ2 = 2.2, P = 0.14, N = 208). For PPW, juvenile Semipalmated Sandpipers with more alternate plumage, lower masses at capture, and longer culmen lengths were more likely to show PPW (Table 7). We had expected mass to increase with PPW probability, as two potential indicators of migratory propensity, but the opposite occurred. PPW was more likely to occur in years 2 and 3 of our study than in year 1. We found no significant effect of date on its correlation with other variables (Table 7).
| Parameters | df | Estimate | SD | F or t | P |
|---|---|---|---|---|---|
| Full model | 3, 203 | - | - | 22.3 | <0.0001 |
| Culmen | 1 | −0.913 | 0.89 | −1.0 | 0.31 |
| Date | 1 | −0.160 | 0.11 | −1.5 | 0.14 |
| Culmen*Date | 1 | 0.010 | 0.01 | 1.8 | 0.07 |
| Parameters | df | Estimate | SD | Wald 95% (CL) | Wald χ2 | Pr > χ2 |
|---|---|---|---|---|---|---|
| Date | 1 | −0.058 | 0.148 | −0.348–0.233 | 0.2 | 0.67 |
| Year 1 | 1 | −2.178 | 1.234 | −4.597–0.241 | 3.1 | 0.08 |
| Year 2 | 1 | −0.010 | 1.528 | −3.005–2.985 | 0 | 0.99 |
| Year 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Plumage score | 1 | 0.728 | 0.332 | 0.0781–1.378 | 4.8 | 0.03 |
| Mass | 1 | −0.565 | 0.137 | −0.833–−0.297 | 17.0 | <0.0001 |
| Culmen | 1 | 0.555 | 0.187 | 0.189–0.921 | 8.8 | 0.003 |
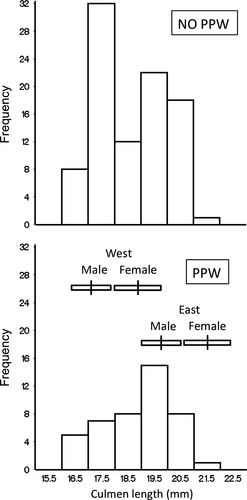
The culmen length frequency distribution of juveniles showing PPW was unimodal and biased toward longer-billed birds, whereas that of birds not showing PPW was bimodal (Fig. 5, Table 7). The smaller mode (17.5 mm) was missing from the PPW distribution (Fig. 5). To provide a breeding population context, we provide the mean and standard deviations of male and female culmen lengths from western and eastern-breeding regions (Fig. 5).
Discussion
Slow vs. fast life history strategies
We present evidence for inter- and intraspecific differences in life history strategies of first-year Western and Semipalmated sandpipers preparing to migrate north from southern Perú. Juvenile Western Sandpipers did not increase in mass or undergo a pre-alternate molt, behavior strongly indicative of over-summering birds and similar to patterns reported in Panama, but contrasting with those in México where most juveniles prepared for northward migration (Fernández et al. 2004, O'Hara et al. 2005). In contrast, a quarter to a third of juvenile Semipalmated Sandpipers in our study prepared for migration during their first April, based on the proportion either molting into alternate plumage or replacing outer primaries. Patterns of mass in juvenile Semipalmated Sandpipers have a complex interaction with molt strategy, our third predictor of migratory propensity (see below). Censuses and resighting of individually marked birds at Paracas conducted during the breeding season (June and July 2015) confirm that small groups (150–300) of juvenile Western and Semipalmated sandpipers feed in the area (P. Pellissier, pers. comm.), corroborating our inference of over-summering based on indicators of migratory preparation.
For Semipalmated Sandpipers, bimodality in first-year life history strategy was previously suggested based on observations of both over-summering juveniles in northern South America (Spaans 1979) and recaptures of first-year breeders on southward migration (Gratto-Trevor 1988). No information was previously available with respect to either the breeding or non-breeding geographical distribution of the two life history strategies. Our results provide direct validation of a difference in life history strategies based on data from a single non-breeding area; both strategies occur with reasonable frequency, at least in Perú. Although this is not likely to be the case elsewhere, we found that from 27% to 31% of juvenile Semipalmated Sandpipers were preparing for migration in different years, which is consistent with Gratto and Morrison's (1981) suggestion that about a third of Semipalmated Sandpipers return to breeding areas in their first year of life.
The migration strategies of Western Sandpipers are geographically segregated, being largely a function of migration distance from their compact breeding range around the Bering Sea. Despite overlap in strategies at a single site, we suggest that a migration-distance explanation may similarly apply to the difference in life-histories of juvenile Semipalmated Sandpipers. Shorter-billed juvenile Semipalmated Sandpipers, which are partially sympatric with breeding Western Sandpipers in Alaska (~11,000 km from Paracas), were less likely to prepare for migration than longer-billed birds (~8000 km from breeding areas in eastern Canada), as assessed by patterns of mass, molt into alternate plumage, and likelihood of molting their outer primaries. Thus, western populations of Semipalmated Sandpipers follow a strategy similar to that of Western Sandpipers, spending the non-breeding season at Paracas. These results provide intraspecific support for the hypothesis that longer migration distances are associated with slower life history strategies (e. g., Myers et al. 1985, O'Hara et al. 2005).
An alternative interpretation of the effects of culmen length in Semipalmated Sandpipers would invoke sex-specific life history strategies. Biases consistent with our results could occur if males, with short bills, were less likely to migrate than females, with longer bills, combined with particular non-breeding sex ratios. The location of the PPW mode relative to the breeding distributions (Fig. 5) indicates that the larger culmen length bias detected was not simply due to females being more likely to engage in PPW than males, but, rather, there is a bias toward PPW in eastern- rather than western-breeding birds. The extent of sex ratio clines in non-breeding Semipalmated Sandpipers and their sex ratio at Paracas are unknown.
The presence of two populations at Paracas is also indicated because Semipalmated Sandpipers with longer mean culmen lengths arrive at the site later in the season (Tavera 2013). The increased prevalence of birds with suspended molts in December probably reflects a wave of eastern migrants.
Variation in primary molt strategies.
Adult Western and Semipalmated sandpipers at Paracas conform to a Southern Hemisphere molt strategy (Pyle 2008, Howell 2010), with the timing of adult primary molt extending from October through February. Western Sandpipers are 3–4 weeks ahead of Semipalmated Sandpipers, consistent with their earlier breeding and migration (Lank et al. 2003).
At least some adults of both species appeared to initiate wing molt prior to arrival in Paracas. About 4% of both species clearly did so because they were captured with suspended molt, providing a minimum estimate of occurrence. Significant numbers of sandpipers begin arriving during September and, by October, most birds (84% of Westerns and 88% of Semipalmated Sandpipers) had already molted more than half of their primaries and most birds were in active molt. Many of these may have arrived with suspended molt in September or early October, but resumed molt by the time of capture. Western Sandpipers tend to initiate molt rapidly at Panamá Bay (Watts 1998), where they typically dropped 4–6 inner primaries upon arrival. Watts (1998) documented a peak population present in October, with numbers decreasing thereafter and suggesting continued southward migration. Wing molt of Western Sandpipers can begin as far north as Kansas (Senner and Martinez 1982), but Semipalmated Sandpipers do not appear to start wing molt until they are further south. In Kansas, there was no evidence of molt of Semipalmated Sandpipers, but a small proportion of Western Sandpipers were undergoing wing molt (S. Franks, pers. comm.), and all primary feathers were old when birds arrived in Suriname (Spaans 1979, 1981). Geolocator data from an individual Semipalmated Sandpiper captured at Nome, Alaska, in May 2014, and recaptured in May 2015, showed a migration route and timing that fits with molting farther north (E. B. Kwon, pers. comm.). The bird arrived on the coast of Ecuador in mid-August, where it stayed for three months before flying to northern Perú. Thus, northern South America seems a probable site for migrant Semipalmated Sandpipers to undergo partial molt prior to continuing on to Paracas.
Adult Semipalmated Sandpipers captured with different molt statuses during the residency period differed in average culmen lengths, controlling for date of capture. Those with longer bills, indicative of eastern population origins, were biased toward having either suspended or active molt, whereas those with shorter bills were biased toward having completed molt. This pattern is consistent with later seasonal breeding, migration, and molt timing by eastern birds, but also with a higher probability of engaging in a partial molt stopover by eastern birds, leading to suspended molt.
About a third of juvenile Semipalmated Sandpipers captured during March in Paracas were undergoing PPW (Table 5). Individuals with more alternate plumage, lighter in mass and with longer culmens (eastern population origins) were more likely to express PPW. More alternate plumage is expected if PPW correlates with migratory propensity, but, contrary to what one might expect, these birds were lighter rather than heavier. However, this makes sense in the context of molting, when birds typically reduce their body mass for energetic and safety reasons (Swaddle and Witter 1997, Hedenström and Sunada 1999, Lind and Jakobsson 2001).
Partial post-juvenile wing molt is assumed to be an economizing compromise supporting long migratory flights by juveniles attempting to breed in their first year, by renewing some, but not all, of the primaries they grew rapidly in the breeding areas (Spaans 1976). The PPW strategy usually involves between two to six outer primaries (Videler 2005, Remisiewicz et al. 2010), those experiencing the most wear. Some species with long-distance flights perform a complete wing molt prior their first northward migration (e.g., Ringed Plovers, Charadrius hiaticula; Tree 1977). At the other extreme, shorter-distance migrants may not molt at all, such as Western Sandpipers spending the non-breeding season in México where juveniles perform three migrations with the same set of feathers (Fernández et al. 2004). For Semipalmated Sandpipers, the difference in migration distance between 8000 and 11,000 km likely alters the selective balance between undergoing PPW, migrating, and attempting to breed in the first year versus retaining juvenile flight feathers and oversummering or, in Pyle's (2008) terms, following a northern versus southern molt strategies.
Conclusion
Among the hypotheses accounting for life history variation outlined in the introduction, the broad patterns of our results are consistent with being driven by differences in migration distance. The mechanisms driving a relationship between wing-molt strategy and migration distance remain unclear. Direct degradation of flight efficiency is an obvious possibility (O'Hara 2002), but other incremental costs associated with predation risk during migration (Lank et al. 2003, Ydenberg et al. 2004, 2007), weather (Xu et al. 2015), and energetic risks (Baker et al. 2004) might influence molt and migration chronology decisions. The decision to migrate or not is likely related to the cost of migration. If this cost increases sufficiently with distance (Pienkowski and Evans 1985) and the reproductive payoff of juvenile breeders is low (Gratto et al. 1983), over-summering behavior would be favored.
Acknowledgments
A number of people played key roles in obtaining all the data for our study, but we would like to give especial thanks to CORBIDI shorebird banding crew: E. Ortiz, P. Pellissier, O. Custodio, A. Mendez, Y. Tenorio, R. Huayanca, L. Burga, P. Colchao, P. Alcázar, and all the volunteers for their constant effort and help during capture and sampling processes. We deeply appreciate T. Valqui for his patience, support and first-hand cooperation during all the years of fieldwork. We are grateful to the staff of Paracas National Reserve, especially to P. Saravia for assistance in obtaining the permits with no further complications and to the head of the Reserve in those years S. Marthans for all his support and cooperation. We thank R. Ydenberg, C. Smith, and M. Drever for their support and scientific advice during the last year of fieldwork. This study was held under the permit of the Peruvian National Service of Protected Natural Areas (SERNANP). Funding for this project was provided by two grants from the Neotropical Migratory Bird Conservation Act Program administered by the U.S. Fish and Wildlife Service, and also by Environment Canada and the Centre for Wildlife Ecology at Simon Fraser University. Capture and sampling methods performed in this study followed guidelines recommended by the Canadian Council on Animal Care as approved by CCAC committee of the Simon Fraser University (Animal Care's permit number: 1043B-03).




