A Novel Technology to Boost Natural Production of Hyaluronic Acid in the Skin Tissue: Human Histology Study
Funding: This work was supported by BTL Industries.
ABSTRACT
Introduction
Combining monopolar radiofrequency (RF) with targeted ultrasound (TUS), this study investigated whether these modalities promote facial rejuvenation through the production of hyaluronic acid (HA) in human skin.
Methods
Seven subjects (51–64 years, BMI 21.1–29.8 kg/m2) were enrolled and divided into three treatment groups in this single-center study; Group A (n = 3, simultaneous RF + TUS), Group B (n = 3, stand-alone RF), and control (n = 1, no treatment). Both treated groups underwent four (4) 60-min treatments on the face delivered 7–14 days apart. Punch biopsies (3 mm in diameter) were collected from the infra-auricular area at baseline and both follow-up visits and stained for HA by using hyaluronic acid binding protein. Digital photographs were taken to document changes in visual appearance. Finally, the subjects' comfort and satisfaction were assessed.
Results
There was a statistically significant (p < 0.05) average increase at 1 month in the HA-stained area of +112 358.7 μm2 in group A (RF + TUS) representing an increase of 48.65%. The treatment effect peaked at 3 months with an increase of +156 345.2 μm2, corresponding to a 67.69% increase in the HA-stained area. In Group B, there was no significant difference in the average increase of the HA-stained area between 1 month (+14 830 μm2) and 3 months (+20 995 μm2) corresponding to a 6.76% and 9.56% increase, respectively. The control samples did not indicate any changes throughout the study. Digital photographs of the RF + TUS group showed both a decrease in rhytids and tighter skin. Therapies were comfortable with no adverse events.
Conclusion
Overall, this study has shown that the combination treatment of RF + TUS has a more pronounced and sustained effect on facial rejuvenation compared to RF alone. The measurable increase in the production of HA with simultaneous use of RF + TUS peaked at a 3-month follow-up, suggesting the gradual advancement of the treatment effect and overall improvement in facial appearance.
Trial Registration
ClinicalTrials.gov identifier: NCT05987917
1 Introduction
Hyaluronic acid (HA) is a naturally occurring polysaccharide (glycosaminoglycan) that is abundantly present in human tissues, particularly the skin [1]. It has garnered significant attention as an ideal tool for facial rejuvenation due to its unique properties and versatility [1, 2]. Hyaluronic acid plays a crucial role in tissue hydration, lubrication, and cellular function [3]. It possesses exceptional water-binding capabilities, allowing it to retain moisture and provide optimal hydration to the skin [3, 4]. This property makes it a key contributor to skin elasticity, texture, and overall skin quality.
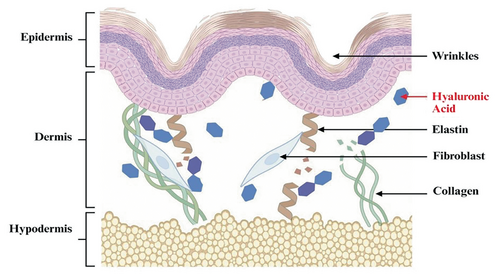
However, the natural aging process, in combination with environmental factors, can lead to a decline in HA levels. As HA levels decrease, skin may lose its plumpness, resulting in diminished facial volume and contour. This HA deficiency can lead to a range of skin-related consequences, such as the appearance of wrinkles, fine lines, and volume loss (see Figure 1) on the skin [5]. Facial rejuvenation, therefore, has become increasingly popular in recent years, as individuals strive to achieve a more youthful and vibrant appearance [6].
Hyaluronic acid is commonly associated with dermal fillers or injection treatments that are now a common choice for addressing age-related changes in areas such as the cheeks, nasolabial folds, and lips [7]. For example, HA fillers introduce synthetic HA into the skin, which binds to water and blends with the naturally present HA to enhance facial volume. These fillers use the ability of HA to hold volume and serve as facial volume implants which are usually delivered below the skin, sometimes even below muscles. However, such application is not focused on restoring and rejuvenating the depleted skin structure itself [8, 9]. Therefore, there is a compelling need for comprehensive and noninvasive technological solutions capable of actively promoting intrinsic alterations in the skin to address age-related facial changes.
One such approach is a novel technology (EXION, BTL Industries Inc., Boston, MA) that simultaneously emits monopolar radiofrequency (RF) and targeted ultrasound (TUS) to enhance the production of HA. In prior veterinary studies, it was suggested that the concurrent application of monopolar RF in conjunction with TUS resulted in a notable increase in endogenous HA production. Furthermore, these two studies conducted a comparison of this technology with standalone RF and RF combined with non-TUS [10]. Both of these alternative approaches did not appear to produce a significant impact on the synthesis of natural HA. It is hypothesized that this novel technology uses its ultrasound component to alter the impedance of the skin tissue, increase cell permeability, and, most importantly, deliver mechanical stimuli to the fibroblasts located in the reticular dermis [11-13]. The RF field creates an oscillating electrical current that induces collisions between charged ions and molecules in the skin, which in turn generates heat [14, 15]. The rise in temperature in the dermis initiates neocollagenesis and elastogenesis [16]. Additionally, the combination of RF and TUS results in greater HA production within the dermis, leading to significant improvements in facial rejuvenation.
Consequently, evaluating the histological changes associated with enhanced HA production is crucial for optimizing facial rejuvenation procedures [17]. By analyzing the dermal architecture and HA synthesis, researchers can objectively elucidate the mechanisms underlying the effectiveness of any facial therapy [18]. It can also allow for the identification of any potential structural alterations caused by any therapeutic intervention [19]. This study conducted a histological examination of the facial skin tissue before the treatment and posttreatment to assess the changes in HA levels resulting from simultaneous monopolar RF and TUS application. These results were then compared with facial skin tissue samples obtained from patients treated solely with RF and with those from one control subject.
This study aims to evaluate the clinical efficacy and safety of the therapy for facial rejuvenation through documentation of the changes in the skin tissue related to the levels of HA and to investigate whether the observed effects in previous veterinary studies can be replicated in human skin.
2 Methods
2.1 Study Population
This study was a single-center, three-arm, open-label design. It was approved by the Advarra Institutional Review Board (IRB ID: Pro00049067) and followed the 1975 Declaration of Helsinki guidelines (ClinicalTrials.gov Identifier: NCT05987917).
Seven healthy subjects (51–64 years, BMI 21.1–29.8 kg/m2) were recruited and participated in this study. Written informed consent was obtained from all participating subjects based on inclusion (above 21 years old, BMI ≤ 35 kg/m2 and presence of clearly visible wrinkles in the treated area) and exclusion criteria (bacterial or viral infection, acute inflammations, impaired immune system, skin related autoimmune diseases, poor healing, or unhealed wounds in the treatment area).
- Group A: n = 3, treated with a device simultaneously emitting RF and TUS, mean age 56.7 years, BMI 25.9 kg/m2, skin type II–IV.
- Group B: n = 3 treated with a device utilizing only RF energy, mean age 57 years, BMI 23.6 kg/m2, skin type II–III.
- Control: n = 1 did not receive any treatment, 58 years, BMI 25.3 kg/m2, skin type III.
2.2 Treatment Protocol
Group A was treated using the face applicator delivering the RF and TUS energies simultaneously (Group A). Group B was treated by standalone monopolar RF.
The subjects of study Groups A and B received four treatments of the entire face area, including the forehead, upper and lower cheeks (comprising infra-auricular area as well), and the area around the eyes. Individual treatments were spaced by 7–14 days. The entire area was assessed for wrinkles and skin laxity reduction.
No anesthetic agents were used prior to or during treatment. A conductive gel was applied to the treatment area to ensure good energy flow. In accordance with the protocol and clinical guidelines, the RF intensity was adjusted based on patient tolerability and was set to 70% for each treatment.
Before treatment, all jewelry was removed and the skin was cleared of any cosmetic products. Subjects were provided with a headband to pull hair off their face and a black neck drape to conceal their clothing. Each treatment session lasted up to 60 min. The control subject did not receive any treatment.
2.3 Punch Biopsy
Punch biopsies of the skin tissue from the infra-auricular area were performed at baseline, followed by 1- and 3-month follow-up visits. Subjects were put in a supine position, and a 1% lidocaine anesthesia was applied to the biopsied area prior to the sampling to minimize pain and discomfort.
Full-thickness biopsies, measuring 3 mm in diameter, were taken from the subjects with a depth of 1.5–2.0 mm. Following the procedure, the biopsy wounds were carefully closed, disinfected, and closely monitored throughout the study for the healing process. The subjects were provided with comprehensive instructions for home wound care to ensure adequate and optimal healing.
The control samples were acquired from the face of the control subject at the corresponding time points (baseline—at least 1 week before the treatments, 1 and 3 months) in order to mirror the sampling regimen in the treated groups. Each participant provided three biopsy samples of facial skin tissue for the evaluation.
2.4 Histological Examination
The taken samples were processed for histological examination. The samples were fixed, embedded, cut, and stained by conventional methods using ethanol-formalin-glacial acetic acid (ethyl alcohol 70%, formalin 10% of 37% solution, water 15%, glacial acetic acid 5%).
Each biopsy sample was sectioned for staining and subsequent analysis employing routine procedures. Samples were specifically embedded in paraffin wax, sliced into 5-μm-thick slices, and stained for the visualization of HA and surrounding tissue structures under a microscope. All samples were labeled using the subject ID and the description of the corresponding visit to prevent any mix-ups.
A clinical histologist analyzed the stained samples to determine the levels of HA, including any pathological changes, in order to rule out any acute or lasting skin damage resulting from the study treatment.
2.5 HA Staining and Imaging
The staining mechanism (see Figure 2) employed conventional HA-specific HABP and biotinylated-HABP staining for the visualization of HA under light and confocal microscopy, which enabled a detailed visual assessment of HA and the general tissue structure. The HA in the samples was visualized by increased intensity of brown color.
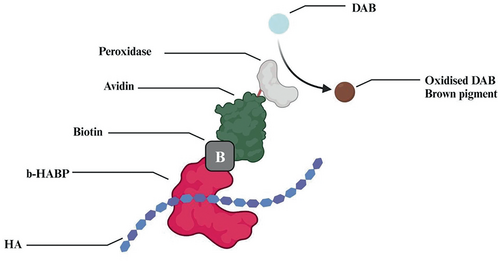
For HA staining, a biotinylated HA-binding protein (b-HABP; Seikagaku Corp., Tokyo, Japan) at 5 μg/mL was added and incubated at 4°C overnight. The slides were washed with peripheral blood smear (PBS) before incubation with fluorescence avidin-D (20 μg/mL; Vector Laboratories, Burlingame, CA) at room temperature for 1 h. After a final washing step, the stained samples were mounted with Cytoseal XYL Thermo Scientific microscopy slides. The slides were observed, analyzed, and photographed by confocal laser scanning microscope (TCS-40; Leica Microsystems, Cambridge, UK).
Hyaluronic acid quantification involved using ImageJ software and applying semiautomatic segmentation in the hue–saturation–brightness (HSB) color system. The amount of HA was finally expressed in terms of the occupied area (square micrometers) in analyzed images.
2.6 Photography and Questionnaires
At the baseline visit, after the last (4th) treatment, at the 1- and 3-month follow-up visits, 2D and 3D digital photographs of the treated areas were taken from subjects in all groups.
A photographic imaging system LifeViz Mini (QuantifiCare S.A. France) was used to take 3D photographs from the left, right, and front views of the face at multiple angles. These images were used for therapy effectiveness assessment by three independent evaluators using the Global Aesthetic Improvement Scale (GAIS) and Fitzpatrick Wrinkle and Elastosis Scale (FWES). Before and after each visit, the examination for possible adverse effects was performed in all subjects.
The 5-point Likert scale Subject Satisfaction Questionnaire (SSQ) was administered to the subjects from Groups A and B after the final treatment and at all follow-up visits to assess their satisfaction with the therapy results according to this criteria: (5) Strongly agree, (4) Agree, (3) Neither agree nor disagree, (2) Disagree, and (1) Strongly disagree. The SSQ consisted of the following questions: Q1: Wrinkles in the treatment area have been reduced after treatments; Q2: Skin laxity in the treated area has improved; Q3: Overall appearance of the skin has been improved after treatments; Q4: I am satisfied with the treatment results.
To further document patient comfort during treatments, the Therapy Comfort Questionnaire (TCQ) was administered after the final treatment session in Groups A and B with a 5-point Likert scale question. Subjects responded to the question; Q1: I found the treatment comfortable according to the same criteria as SSQ. The TCQ also included a Numerical Analog Scale (NAS), where pain scores ranged from 0—no pain, to 10—worst possible pain, experienced during the treatment.
2.7 Statistical Analysis
The descriptive statistic was calculated (mean and standard deviation). Student's t-test and Friedman test were performed with a significance level set at α = 0.05 to show statistically significant differences between the groups.
3 Results
In total, seven subjects were enrolled in the study. Both treated groups (A and B) completed four treatments and two follow-up visits. There was no withdrawal from the study.
Collected punch biopsies healed properly with no additional medical care needed. The histological examination showed no pathological abnormalities in the skin tissue. Digital photographs were taken to document changes in visual appearance while the subject's comfort and satisfaction were assessed.
There were no adverse events reported throughout the study, and no treatment-related downtime was experienced by any subject.
3.1 Histology Examination
Histological examination revealed an increased amount of HA in posttreatment samples, with increased intensity and extent of HABP staining shown as the visualization of the brown color under microscopy (Figures 3 and 4).
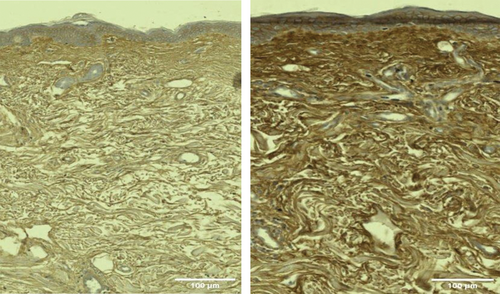
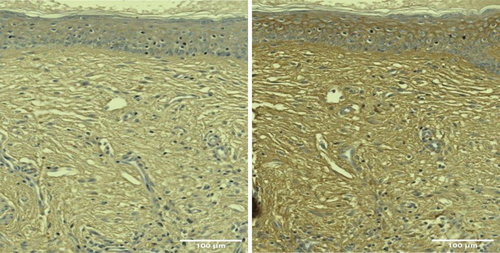
There was a statistically significant (p < 0.05) average increase at 1 month in the HA-stained area of +112 358.7 μm2 in group A (RF + TUS) in Figure 5, representing an increase of 48.65%. The treatment effect peaked at 3 months with an increase of +156 345.2 μm2, corresponding to a 67.69% increase in the HA-stained area (see Table 1).
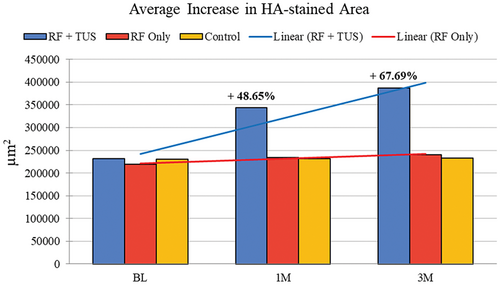
| Parameters | BL | 1 M | 3 M |
|---|---|---|---|
| HA μm2 | 230 965.5 | 343 324.2 | 387 310.7 |
| Difference μm2 | N/A | 112 358.7 | 156 345.2 |
| Difference (%) | N/A | 48.65% | 67.69% |
- Abbreviations: 1 M, 1-month follow-up; 3 M, 3-month follow-up; BL, baseline; N/A, not available.
In Group B, there was an insignificant (p-value = 0.07) difference in the average increase of HA-stained area between 1 month (+14 830 μm2) and 3 months (+20 995 μm2) corresponding to a 6.76% and 9.56% increase, respectively (see Table 2).
| Parameters | BL | 1 M | 3 M |
|---|---|---|---|
| HA μm2 | 219 233.7 | 234 054.0 | 240 219.4 |
| Difference μm2 | N/A | 14 830 | 20 995.8 |
| Difference (%) | N/A | 6.76% | 9.58% |
- Abbreviations: 1 M, 1-month follow-up; 3 M, 3-month follow-up; BL, baseline; N/A, not available.
There were no observable changes or increases in HA-stained area indicated in the control samples at 1 and 3 months, as shown in Figure 5.
3.2 Photography Evaluation
Digital photographs of the RF + TUS group showed both a decrease in rhytids and a tighter skin (Figure 6). The aesthetic improvements in the treated subjects were evaluated using GAIS and FWES scales.
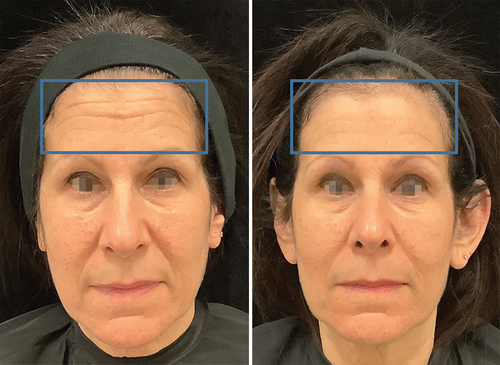
The subjects in Group A (RF + TUS) showed a significant GAIS improvement in facial appearance with smoothed lines. The most prominent improvement of 1.22 ± 0.38 points to 1.88 ± 0.19 was observed in the RF + TUS group at 3 months. In the RF only (Group B), there was a GAIS score improvement of 0.66 ± 0.33 to 0.77 ± 0.19 points at 3 months, with no changes observed by the evaluators.
Subjects treated with RF + TUS (group A) showed significant improvement from an average baseline FWES value of 5.33 ± 0.33 points to 3.11 ± 0.19 at 3-month follow-up visits. In group B (RF only), there was no significant decrease in wrinkle severity with an average baseline FWES value of 4.44 ± 0.19 and 4.11 ± 0.38 points at 3 months.
3.3 Subject Satisfaction and Therapy Comfort
The subjects from Group A found the treatment (RF + TUS) very comfortable (4.0/5) with mild discomfort (2.3/10) and reported a high satisfaction (4.0/5) with the treatment result, improvement in skin laxity, and overall skin appearance at 3 months. Subjects from Group B (RF only) were less comfortable with their treatment (3.3/5), found it to be moderately painful (5.3/10) and were less satisfied with their overall appearance (3.4/5).
3.4 Safety and Adverse Events
There were no adverse events reported throughout the study, and no downtime was experienced by any subject from both treated groups (A and B) due to the treatment.
4 Discussion
This clinical study evaluated the clinical efficacy and safety of the combined therapy of RF and TUS for facial rejuvenation through changes in facial skin tissue related to the levels of HA. The results obtained from this study support the use of monopolar RF combined with TUS as an effective technique for facial rejuvenation. This was evidenced by both quantitative histological assessments and qualitative visual evaluations carried out.
The histological analysis of the HA-stained area revealed statistically significant (p < 0.05) changes occurring within the facial skin following treatment. In Group A, which received the simultaneous RF + TUS treatment, an average increase of 48.65% was observed at 1 month compared to baseline. This substantial change signified a robust initial response to the combined therapy. Interestingly, the treatment effect exhibited a progressive improvement, peaking at 3 months posttreatment with an even larger increase of 67.69% in the HA-stained area. This pronounced enhancement at 3 months suggests a sustained and cumulative effect of the RF + TUS treatment on skin rejuvenation (see Figure 5). In contrast, Group B, which underwent the standalone RF treatment regimen, did not exhibit the same level of improvement. The average increases in HA-stained area observed at 1 and 3 months were statistically insignificant, corresponding to only 6.76% and 9.56% increases, respectively. There were no changes in the control samples.
This disparity in outcomes demonstrates that the combined treatment modality (RF + TUS) exerts a more pronounced effect on the skin's extracellular matrix. The absence of any HA-containing substances applied suggests that the increased HA levels can be attributed to the study treatment. HA production is proposed to result from the combined effects of RF-generated thermal energy and TUS's mechanical energy, which together promote fibroblast activity in the dermis [12, 14, 15, 20-23]. It was also observed from this present study that the treatment peaked at 3 months. This is consistent with previous research that has shown that structural remodeling in skin tissue after mechanical and thermal stimulation occurs approximately three months after treatment [21, 24-26]. Additionally, findings from previous animal studies using this combination therapy showed that the use of RF + TUS led to a significant increase in HA levels in porcine skin samples, while RF alone did not have the same effect. This suggests the crucial role of TUS in altering tissue composition for increased HA production. However, further research examining the standalone effect of TUS is recommended to gain a clearer understanding of this mechanism.
Furthermore, the digital photographs taken for the RF + TUS group provided additional supportive evidence of the combination treatment's efficacy. These photographs (Figure 6) were carefully assessed by three independent evaluators using the GAIS and FWES scales revealing a decrease in rhytids (wrinkles), improvement in skin laxity and overall facial appearance, which aligns with the quantitative data obtained from the HA-stained area measurements. These qualitative improvements validate the clinical relevance of the study's findings that the combination therapy not only induces structural changes at the cellular level but also translates into visible enhancements in facial appearance. The role of HA in maintaining skin hydration and volume has been investigated by Zhang et al. [3] Similarly, other researchers have also reported that HA acts as a natural moisturizer and provides support to the main fibers that form the extracellular matrix, contributing to skin elasticity and firmness [27-29]. These previous investigations correspond with the clinical observations of reduced wrinkles, increased skin laxity, firmness, and a refreshed overall appearance reported by the Group A (RF + TUS) subjects in this study.
Another important aspect of this study is the safety and tolerability profile of the combined RF and TUS therapy. The subjects from Group A found the treatment (RF + TUS) very comfortable and reported high satisfaction with the treatment results, further enhancing the clinical feasibility and attractiveness of this approach for facial rejuvenation. Subjects from Group B (RF only) were less comfortable with their treatment and found their therapy moderately painful. This suggests that comfort levels were enhanced by the ultrasound component of the combination therapy. Similar to the previous studies, during this investigation, there were no treatment-related adverse events. This further underscores the safety of the combined RF + TUS treatments used for skin rejuvenation, and the trust patients can place in the safety profile of the therapy.
Numerous studies on RF modalities used for facial rejuvenation emphasize their skin-tightening effects but generally do not indicate active promotion of the body's natural HA production within the dermal layers [15, 24]. The results of this study suggest that the conclusions drawn from previous veterinary studies regarding the effect of the simultaneous application of RF and TUS on natural HA enhancement are applicable to human skin as well. TUS waves are expected to activate cell receptors, causing changes in gene expression and protein synthesis within the fibroblasts. This modification enhances the permeability of cells and enables more effective transmission of RF energy to deeper skin layers, without causing any discomfort to the patient [12, 13]. By increasing the levels of HA in the dermis, skin hydration is improved, and the overall rejuvenation effect is completed.
Nonetheless, a few limitations of our study must be acknowledged. The sample size was small, and the study duration was limited to a 3-month follow-up period. Additionally, collagen and elastin content, which are the crucial extracellular matrix components involved in skin aging, were not analyzed following the combined RF + TUS application, as it was beyond the scope of this study. Future research is recommended to evaluate the effects of combined RF + TUS energies on dermal collagen and elastin, as well as to analyze TUS as a standalone treatment to compare its role in enhancing HA levels. Further investigations with larger sample sizes and extended follow-up periods may provide a more comprehensive understanding of the long-term efficacy and durability of the observed effects of this combination therapy.
5 Conclusion
Overall, this study has shown that the combination treatment of RF + TUS has a more pronounced and sustained effect on facial rejuvenation compared to RF alone. The combination of both monopolar RF and TUS enhanced the deposition of HA within the facial skin, leading to improved skin quality and texture. The treatment was well tolerated by all the subjects, and no adverse events were reported. By harnessing the body's natural ability to produce HA, this novel noninvasive technology offers an effective and safe alternative to invasive procedures such as dermal fillers or surgical interventions. In addition, the targeted nature of the treatments ensures that the production of HA is concentrated in specific areas, allowing for precise facial rejuvenation and improved skin quality. For these reasons, the findings from this study will have significant implications for the field of aesthetic medicine and antiaging treatments.
Ethics Statement
Author declares that human ethics approval was obtained for this study.
Conflicts of Interest
The author declares no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the Supporting Information of this article.