Poly-L-Lactic acid increases collagen gene expression and synthesis in cultured dermal fibroblast (Hs68) through the TGF-β/Smad pathway
Abstract
Objective
Poly-L-Lactic Acid (PLLA) is a synthetic polymer which possesses biocompatible and biodegradable properties, and is widely used in the clinical filler material. This study focuses on the potential role of PLLA on the collagen production of dermal fibroblasts and its mechanism.
Methods
The dermal fibroblast Hs60 was treated with different concentration of PLLA. RT-qPCR was conducted for the determination of mRNA levels of collagen type I (COL1) alpha 1 (COL1A1), COL1 alpha 2 (COL1A2), elastin, matrix metalloproteinase 1 (MMP-1), tissue inhibitor of metalloproteinase 1 (TIMP-1), and TIMP-2. Procollagen Type I C-peptide (PIP) enzyme immunoassay (EIA) Kit assay was carried out to analyze procollagen production. Western Blot was employed to examine the effect of PLLA and transforming frown factor (TGF-β) receptor-specific inhibitor (SB431542) on protein levels of COL1A1 and TGF-β/Smad signaling pathway related proteins.
Results
With the increase of PLLA concentration, the production of procollagen gradually increased, and both protein and mRNA levels of COL1A1 and COL1A2 gradually increased (p < 0.001). Elevated PLLA concentrations increased elastin, TIMP-1, and TIMP-2 levels and attenuated MMP-1 expression. PLLA increased TGF-β levels in a dose-dependently manner. p-Smad2 and p-Smad3 protein levels were also increased by PLLA, but the influences were reversed by SB431542 (p < 0.001). Similarly, increased levels of COL1A1, COL1A2, TIMP-1, and TIMP-2 caused by PLLA were significantly inhibited by SB431542, whereas MMP-1 was typically elevated (p < 0.001).
Conclusion
Poly-L-Lactic Acid promotes the collagen production of dermal fibroblasts by activating the TGF-β/Smad signaling pathway. The findings may lay a foundation for clinical material applications of PLLA.
1 INTRODUCTION
Economic growth and increased longevity have given rise to the pursuit of beauty and the active search for new bioactive substances that can fight age and promote health.1 Skin aging is mainly characterized by structural and functional changes put into the epidermis and dermis.2 Collagen, a major component of the extracellular matrix (ECM), is synthesized by dermal fibroblasts to maintain the skin's strength and elasticity.3 However, UV rays, skin inflammation, intracellular metabolites, and aging can inhibit collagen synthesis, leading to skin aging occurrences such as wrinkle formation, sagging, and laxity.4 While the current anti-aging process cannot be permanently improved, injectable fillers are a simple, fast-acting, less-invasive, and fast-recovery cosmetic procedure to combat skin aging.5
As demand increases, safe and effective facial fillers are being updated, such as biodegradable calcium hydroxyapatite, polycaprolactone, hyaluronic acid, and non-biodegradable poly-meth methacrylate silicone, polyacrylamide, and autologous fat grafting.6, 7 Poly-L-Lactic Acid (PLLA) is a biocompatible, biodegradable, absorbable, immunologically inert, malleable, and easy-to-process polymeric synthetic polymer derived from plants that have been used in previous studies of synthetic suture materials. It was approved by the food and Drug Administration (FDA) in 2004 for the treatment of facial fat atrophy following immunodeficiency virus infection and in 2009 for nasolabial folds and other dermal wrinkles.8 The volume-expanding of PLLA post-injection is caused by the thinner. As the thinner is absorbed, the PLLA particles slowly degrade, promoting the formation of fibrous tissue and new collagen. Therefore, besides facial fillers, PLLA is also a good material for acne scarring and trunk plasticity in clinical practice.9 However, the potential mechanisms by which PLLA regulates dermal collagen production and expression are worth exploring.
Various cytokines have been shown to regulate collagen gene expression in fibroblasts. Among them, transforming frown factor (TGF)-β is a major regulator of ECM synthesis. TGF-β regulates fibroblast growth, apoptosis, and collagen synthesis by inducing the phosphorylation of smad2 and smad310 and inhibits matrix-degrading enzymes.11 Therefore, the present study attempted to explore whether the mechanism of PLLA-induced collagen synthesis is related to the TGF-β/Smad signaling pathway.
2 MATERIALS AND METHODS
2.1 Cell culture
The human dermal fibroblast cell line (Hs60) was obtained from the American Typical Culture Collection (ATCC). Cells were maintained in DMEM culture containing 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin in an incubator containing 5% CO2 at 37°C. For this study, the generation of cells was between 5 and 10th generations. PLLA, purchased from Sculptra®, is injected into sterile water in configurations and final concentrations of 0.1, 0.5, and 1 mg/ml, respectively. SB431542, a specific inhibitor of the TGF-β receptor, was configured according to the manufacturer's instructions. According to the results of previous studies12, 13 and preliminary experiments, 10 μM SB431542 was applied to cells and 2 h incubation was complished.
2.2 Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Hs60 cells were inoculated into 6-well plates, and when the cell density reached about 60–80%, 0.1, 0.5, and 1 mg/ml of PLLA were added. Hs60 cells were labeled with TRIzol reagent and cleaved in a shaker at 4°C to extract and isolate total RNA. NannoDrop ND-1000 spectrophotometer was used to quantify RNA purity and concentration. When OD260/280 was 1.8–2.0, high purity was confirmed. RQ1 RNAse-Free DNAse Kit removes genomic DNA and PrimeScript RT reagent Kit performs the reverse transcription reaction to synthesize complementary cDNA. Finally, the primers and cDNA (template) were mixed in TB Green Fast qPCR Mix Kit for amplification reaction analysis on Bio-Rad C1000/CFX PCR instrument. Ploidy changes in gene expression were calculated by the 2−ΔΔCT formula relative to the internal reference GAPDH. The relevant primer sequences used for RT-qPCR were shown in Table 1. RT-qPCR was performed with the following thermos cycling conditions: an initial 1 cycle at 95°C for 10 min, 40 cycles at 95°C for 15 s, 60°C for the 20 s, and 72°C for elongation for 60 s.
| Amplified gene | Type of primer | Primer sequence (5′-3′) |
|---|---|---|
| COL1A1 | F | TTCTGCAACATGGAGACTGG |
| R | CGCCATACTCGAACTGGAATC | |
| COL1A2 | F | GTGCTAAAGGAGAGAAAGGAAC |
| R | ACCAGGGAAACCAGTCATAC | |
| Elastin | F | AAAGTTCCTGGTGTCGGTCTTCCA |
| R | AGCAGCTCCATACTTAGCAGCCTT | |
| MMP-1 | F | TGCTGCTGCTGCTGTTCTGGG |
| R | GGCCGATGGGCTGGACAGGA | |
| TIMP-1 | F | CTCGTCATCAGGGCCAAGTT |
| R | GTAGGTCTTGGTGAAGCCCC | |
| TIMP-2 | F | TAGTGATCAGGGCCAAAGCG |
| R | CAGGCTCTTCTTCTGGGTGG | |
| GAPDH | F | GAGAAGGCTGGGGCTCATTT |
| R | CACAATGCCGAAGTGGTCGT |
- Abbreviations: COL1A1, collagen type I alpha1; COL1A2, collagen type I alpha 2; F, forward primer; MMP-1, matrix metalloproteinase-1; R, reverse primer; TIMP-1, tissue inhibitor of metalloproteinase 1; TIMP-2, tissue inhibitor of metalloproteinase 2.
2.3 Western blot
Hs68 cells were spiked with protein lysate containing 1% protease inhibitor and lysed on a shaker at 4°C for 5 min. The precipitation was removed by centrifugation, and the concentration was determined by a BCA assay kit. Configure sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and add 20 μg of protein for 120 mA 80 min electrophoresis. Then, the separated proteins in the gel were then transferred to PVDF membranes at 90 V for 110 min. Membranes were blocked in TBS 5% bovine serum albumin (BSA) buffer for 2 h at room temperature. Anti-collagen type I alpha 1 (COL1A, Col-1, sc-59 775, Santacruz Biotechnology), anti-TGF-β1 (sc-146, Santacruz Biotechnology), anti-p-Smad2 (18338T, Cell Signaling Technology), anti-p-Smad3 (9520T, Cell Signaling Technology), and anti-GAPDH (60004-4-Ig, Proteintech) were diluted according to the manufacturer's instructions and incubated overnight at 4°C and washed 3 times with TBST. Goat antibody secondary antibodies were incubated at room temperature for 1 h. Results were subsequently visualized using chemiluminescence detection reagents. Optical density measurements were performed by NIH Image J to analyze the scanned membranes.
2.4 Procollagen production
A Procollagen Type I C-peptide (PIP) enzyme immunoassay (EIA) assay kit was applied to analyze the levels of procollagen. Hs60 was inoculated into 6-well plates and incubated for 24 h in an incubator with 0.1, 0.5, and 1 mg/ml PLLA after 24 h. The supernatant of each well was collected and assayed according to the PIP EIA commercial assay kit (Takara Bio Inc.).
2.5 Statistical analysis
The results are illustrated as mean ± standard error (SD). Each experiment contained 3 biological replicates per condition and was repeated more than three times. Statistical differences were measured using ANOVA analysis followed Tukey test in GraphPad prism 6.0. A p value of <0.05 was considered statistically significant.
3 RESULTS
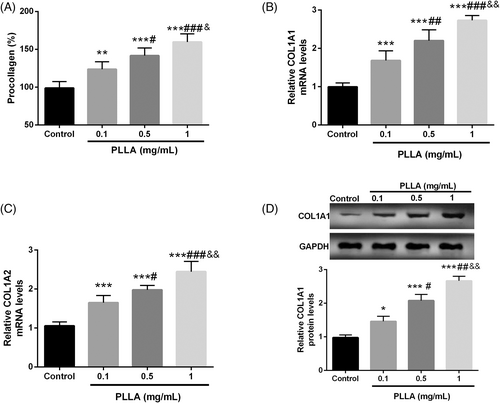
3.1 PLLA enhances the synthesis of type 1 collagen
To elucidate the effect of PLLA on collagen synthesis, the PIP EIA assay kit was first employed to determine the level of procollagen products in PLLA-treated dermal fibroblasts Hs60. As demonstrated in Figure 1A, PLLA typically increased procollagen production in a concentration-dependent manner (p < 0.05), and the mRNA levels of COL1A1 and COL1A2, which encode both strands of collagen, also showed a PLLA dose-dependent increase (p < 0.001, Figure 1B,C). Finally, COL1A1 protein levels were upregulated significantly in a dose-dependent manner with the increase of PLLA concentration, which was confirmed by Western blot (p < 0.001, Figure 1D).
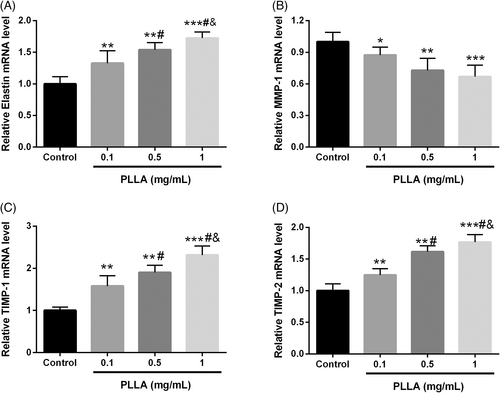
3.2 PLLA regulates the levels of elastin, MMP-1, TIMP-1, and TIMP-2
Together with collagen, elastin forms the ECM produced by dermal fibroblasts, providing elasticity and flexibility to the skin.14 As illustrated in Figure 2A, Elastin mRNA levels were significantly increased with higher PLLA concentrations (p < 0.001). Matrix metalloproteinases (MMPs) produced by dermal fibroblasts degrade collagen and elastin in the ECM. With the increase of PLLA level, the mRNA level of MMP-1 was gradually suppressed, while the inhibitors of MMPs, tissue inhibitor of metalloproteinase 1 (TIMP-1), and TIMP-2 were significantly enhanced (p < 0.001, Figure 2B–D).
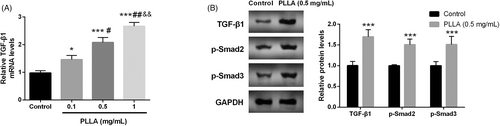
3.3 PLLA enhanced the activity of the TGF-β/Smad signaling pathway
The mechanism of PLLA in regulating type I collagen in dermal fibroblasts was subsequently explored. TGF-β/Smad signaling pathway was identified to be critical in dermal collagen synthesis. RT-qPCR confirmed that TGF-β mRNA levels were significantly elevated with the increase of PLLA dose (p < 0.001, Figure 3A). What's more, Western blot revealed that TGF-β protein was also gradually enhanced, and p-Smad2 and p-Smad3 proteins were also increased in a dose manner (p < 0.001, Figure 3B).
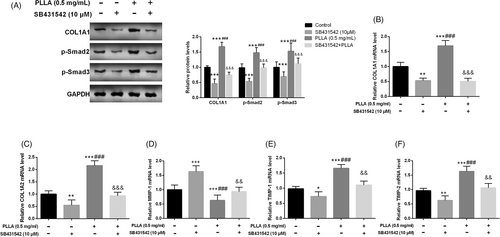
3.4 TGF-β inhibitor reverses the promotion of collagen synthesis by PLLA in dermal fibroblasts
SB431542 was added to PLLA-treated Hs60 as an inhibitor of TGF-β. SB431542 significantly reversed PLLA-induced increase in COL1A1, p-Smad2, and p-Smad3 proteins (p < 0.01, Figure 4A). Compared with PLLA treatment, SB431542 attenuated the promotion of mRNA levels of COL1A1 and COL1A2 induced by PLLA (p < 0.01, Figure 4B,C). Meanwhile, SB431542 also typically promoted MMP-1 expression and inhibited the mRNA levels of TIMP-1 and TIMP-2 compared to PLLA-treated cells (p < 0.01, Figure 4D–F).
4 DISCUSSION
The appearance of skin and face is considered an essential factor in happiness and health, and as a result, aesthetic procedures have proliferated worldwide.15, 16 They are less invasive, have the fastest recovery, and have less scarring to achieve the best results.15 PLLA is a synthetic polymer obtained from 100% natural sources such as corn starch and sugar cane17 and is biocompatible, biodegradable, and immunologically inert.18 As PLLA particles degrade, they gradually regain volume by stimulating collagen synthesis, and thus, PLLA is widely used as a dermal filler to improve appearance and repair imperfections, as well as in various areas of medicine.19, 20 The Hs68 cell line has the typical characteristics of primary dermal fibroblasts and is the most widely used dermal fibroblast cell line in the study of dermal physiology, which can regulate various components of the cell matrix including collagen.4, 21 Therefore, we explored the regulation of collagen synthesis in dermal cells by PLLA in Hs68 cells and focused on its potential molecular mechanisms.
In our study, we first examined the effect of PLLA on collagen synthesis, and the PIP EIA assay confirmed that procollagen synthesis in dermal fibroblasts gradually increased with increasing PLLA concentration. It is known that type I collagen is the most common type of collagen in the human body, consisting of a type I collagen α 1 chain and a type I collagen α 2 chains, encoded by the COL1A1 and COL1A2 genes.22 Here, we confirmed that the mRNA levels of COL1A1 and COL1A2 gradually increased with increasing PLLA concentration. The protein level of COL1A1 also increased gradually. Our findings are consistent with previous studies23 that found PLLA promotes collagen synthesis.
In our study, elastin levels were also found to increase gradually with increasing PLLA concentration. Previous studies have confirmed that elastin, a type of dermal ECM cross-linked fiber, together with collagen, forms the thicker connective tissue of the dermis, proving the mechanical framework and elasticity needed for the skin.24, 25 Therefore, cosmetic skin materials should mainly be biomaterials based on collagen and elastin. In our study, it was also confirmed that the PLLA gradient inhibited MMP-1, but significantly increased the levels of TIMP-1 and TIMP-2. Previous studies have shown that overexpression of MMPs blocks the synthesis of skin collagen or elastin by disrupting ECM and promoting its cracking, leading to rough wrinkles and sagging skin and accelerating skin aging.26 MMP-1 can form a tight complex with TIMP-1, and its activity can be specifically inhibited by TIMP-1 and TIMP-2.27
A TGF-β/Smad signaling pathway is a powerful activator of connective tissue synthesis and fibroblast proliferation, which physiologically and pathologically regulates fibroblast and collagen production and extracellular matrix renewal.28 Firstly TGF-β could regulate fibroblast differentiation and proliferation through paracrine and autocrine regulation to promote wound healing.29 Second, TGF-β is involved in the regulation of MMP-1 and TIMP-1 in ECM. High levels of TGF-β will damage the expression of MMP-1 and lead to the enhancement of the TIMP-1 reaction, which will increase collagen expression of collagen and reduce the degradation of ECM.30 Galangin resisters H2O2/UVB-induced collagen degradation of dermal fibroblasts in vivo and in vitro via the TGF-β/Smad signaling pathway.31 Oyster (Crassostrea Gigas) hydrolysate (OH) was shown to stimulate collagen synthesis by regulating the TGF-β/Smad signaling pathway.32 Compound extracts of Astragalus membranaceus and Salvia militarize extract regulate collagen synthesis in keloid fibroblasts by mediating the TGF-β/Smad signaling pathway.33 Pyropia yezoensis as a marine alga promotes collagen synthesis by activating the TGF-β/Smad signaling pathway in dermal fibroblasts.34 Based on the above study background, we attempted to investigate whether the TGF-β/Smad signaling pathway is involved in the mechanism by which PLLA promotes collagen gene expression and synthesis in dermal fibroblasts. It was found that with the increase of PLLA, the protein and mRNA levels of TGF-β were significantly decreased, and the protein levels of p-Smad2 and p-Smad3 were also significantly reduced, but this reduction was significantly reversed by TGF-β receptor-specific inhibitors SB431542. Finally, PLLA-induced elevation of COL1A1, COL1A2, TIMP-1, TIMP-2, and reduction of MMP-1 were significantly reversed by SB431542. This study does have several limitations that needed to be considered when interpreting the findings. For example, the lack of animal models and clinical data are potential limitations of this study, which is also the direction of our further research. Additionally, we confirmed the mRNA levels of MMP but did not use a more visual gel enzyme profiling assay to confirm its activity is another limitation of this study.
Collectively, our study provides evidence that PLLA stimulates collagen expression and synthesis in dermal fibroblasts through activation of the TGF-β/Smad signaling pathway. In conclusion, our study lays the foundation for the clinical application of PLLA.
CONFLICT OF INTEREST
None.
ETHICAL APPROVAL
All procedures were approved by the Ethics Committee of Tongde Hospital of Zhejiang Province.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




