Criss-crossing autism spectrum disorder and adult neurogenesis
Frank Bicker and Leonardo Nardi contributed equally to this work.
Abstract
Autism spectrum disorder (ASD) comprises a group of multifactorial neurodevelopmental disorders primarily characterized by deficits in social interaction and repetitive behavior. Although the onset is typically in early childhood, ASD poses a lifelong challenge for both patients and caretakers. Adult neurogenesis (AN) is the process by which new functional neurons are created from neural stem cells existing in the post-natal brain. The entire event is based on a sequence of cellular processes, such as proliferation, specification of cell fate, maturation, and ultimately, synaptic integration into the existing neural circuits. Hence, AN is implicated in structural and functional brain plasticity throughout life. Accumulating evidence shows that impaired AN may underlie some of the abnormal behavioral phenotypes seen in ASD. In this review, we approach the interconnections between the molecular pathways related to AN and ASD. We also discuss existing therapeutic approaches targeting such pathways both in preclinical and clinical studies. A deeper understanding of how ASD and AN reciprocally affect one another could reveal important converging pathways leading to the emergence of psychiatric disorders.
Abbreviations
-
- AN
-
- adult neurogenesis
-
- ASD
-
- autism spectrum disorder
-
- BDNF
-
- brain-derived neurotrophic factor
-
- BrdU
-
- bromodeoxyuridine
-
- BTBR
-
- black and tan brachyury
-
- CBD
-
- Cannabidiol
-
- CBDV
-
- Cannabidivarin
-
- CHD8
-
- chromodomain helicase DNA binding protein 8
-
- CNS
-
- central nervous system
-
- DCX
-
- doublecortin
-
- DG
-
- dentate gyrus
-
- FMR1
-
- fragile X mental retardation 1
-
- FMRP
-
- fragile X mental retardation protein
-
- FXS
-
- fragile x syndrome
-
- GSK3
-
- glycogen synthase kinase 3
-
- HC
-
- hippocampus
-
- HDAC
-
- histone deacetylase
-
- hESCs
-
- human embryonic stem cells
-
- IGF
-
- insulin-like growth factor
-
- Mdm2
-
- murine double minute 2
-
- MECP2
-
- methyl-CpG-binding protein 2
-
- MEF2
-
- myocyte enhancer factor 2
-
- MWM
-
- Morris Water Maze
-
- NeuN
-
- neuronal nuclei
-
- NPCs
-
- neural progenitor cells
-
- NSCs
-
- neural stem cells
-
- NS-HSPG
-
- N-sulfated heparan sulfate proteoglycan
-
- OB
-
- olfactory bulb
-
- PFC
-
- prefrontal cortex
-
- PSD
-
- post-synaptic density
-
- PTEN
-
- phosphate and tensin homolog
-
- RBPJ
-
- recombining binding protein suppressor of hairless or recombination signal-binding protein for immunoglobulin kappa j region
-
- RTT
-
- Rett syndrome
-
- SGZ
-
- subgranular zone
-
- SHANK3
-
- SH3 and multiple ankyrin repeat domains protein 3
-
- SHH
-
- sonic hedgehog
-
- SMO
-
- smoothened
-
- SVZ
-
- subventricular zone
-
- TrkB
-
- tropomyosin-related kinase B
-
- UBE3a
-
- ubiquitin-protein ligase E3A
-
- VPA
-
- valproic acid
-
- WNT
-
- wingless-related integration site
1 INTRODUCTION
1.1 Autism spectrum disorder
Autism spectrum disorder (ASD) comprises a group of heterogeneous, complex, pervasive, and multifactorial neurodevelopmental disorders primarily characterized by the following two core features: 1) deficits in social interaction and 2) repetitive behavior (APA, 2013). ASD is further associated with various comorbidities, including seizures, intellectual disability, and increased anxiety among others (Goh & Peterson, 2012; Russell et al., 2019; Vasa & Mazurek, 2015; Viscidi et al., 2013). The number of ASD cases has dramatically increased over the past decade. According to the most recent study conducted by the Centers for Disease Control and Prevention (2020), ASD is diagnosed in approximately one in every 54 children in the U.S., and reported to occur in all racial, ethnic, and socioeconomic groups (Maenner et al., 2020).
The onset of the disorder occurs during development, typically in early childhood, but symptoms may not become fully manifest until later, when social demands exceed limited capacities. Thus, early assessment and intervention can support individuals’ long-term success. Typical so-called “early warning signs” can be observed earliest at the age of 6–12 months in manifestation of unusual interaction and communication skills like no smiling at people, no babbling, and pointing or performing any other age-specific gestures, respectively. These features can be followed by poor eye contact and not responding to sounds or voices, indicating that in general, sensory dysfunction is one of the most abundant links related to the clinical phenotype in ASD (Leekam et al., 2007; Robertson & Baron-Cohen, 2017; Tomchek & Dunn, 2007). Interestingly, sensory dysfunction itself may impair social functioning and could thereby also contribute to increased repetitive behavior (Suarez, 2012). In the context of sensory perception, chemo-signals are used in non-verbal interaction showing that both odor and olfaction play an important and meaningful role in social communication (Gelstein et al., 2011; Zhou & Chen, 2009). Interestingly, olfaction is strongly interconnected with the same socio-emotional pathways implicated in ASD (Sanchez-Andrade & Kendrick, 2009). Specifically, children with ASD show intact odor detection with reduced odor identification ability, while poor odor identification significantly correlates with autism symptom severity (Rozenkrantz et al., 2015). This indicates that olfactory dysfunction is directly related to the clinical phenotype in ASD (Crow et al., 2020; Sweigert et al., 2020) and could thereby serve as a diagnostic tool. However, compared to other sensory abnormalities, olfactory deficits in individuals with ASD are still poorly understood.
Individuals within the autism spectrum vary enormously but most will find it hard to cope with other people, to work or to function independently. Depending on the extent of manifestation, deficits are sufficiently severe to cause impairment in personal and social functioning observable in all life situations, although they may vary tremendously. Realistically, the majority of children with ASD can be expected to need some degree of assistance also as adults. In severe form, the condition may require intensive, specialized, lifelong care, and support.
There is currently no cure for ASD. The high heterogeneity of the ASD phenotype results from hundreds of different genetic and environmental factors, unfortunately reducing the potential effect size of an intervention. Research shows that early intervention can improve some ASD symptoms and, therefore, increase the quality of life of affected individuals, suggesting that the most effective interventions are likely to be those following the key principles of personalized medicine. However, there is still an urgent need for new therapeutic strategies, especially those targeting adult populations already living with ASD.
1.2 Adult neurogenesis
Current research links autism to a biologically altered brain, and advanced brain-imaging technologies like magnetic resonance imaging and positron emission tomography scans are used to examine this in detail. Magnetic resonance imaging studies, for example, substantiate neuroanatomical abnormalities in individuals with ASD in a variety of brain regions that are involved in the regulation of cognition, social and stereotypical behavior, including the hippocampus (HC), prefrontal cortex (PFC), and striatum (Ecker et al., 2017). In addition, ASD patients exhibit atypical numbers and morphologies of dendritic spines (Phillips & Pozzo-Miller, 2015). In sum, these studies suggest that neuroplasticity appears to be divergent in ASD, but only few studies to date have addressed the impact of post-natal and, in particular, adult neurogenesis on the ASD phenotype.
Adult neurogenesis (AN) is the process by which new functional neurons are created from neural stem cells (NSCs) existing in the post-natal brain. The entire event is based on a sequence of cellular processes, such as proliferation, specification of cell fate, maturation, and ultimately, synaptic integration into the existing neural circuits. Hence, AN is supposed to be implicated in structural and functional brain plasticity throughout life.
In humans and other mammals, the subgranular zone (SGZ) of the dentate gyrus (DG) of the HC is one of the two brain regions that can generate new neurons within the entire lifespan of the organism (Kempermann et al., 2015). Newborn neuroblasts in the DG migrate into the inner granule cell layer, differentiate, and become functional granule cells by extending dendrites toward the molecular layer and projecting axons through the hilus toward the CA3 region of the HC. Accumulating evidence suggests that adult hippocampal neurogenesis may play a fundamental role in physiological CNS functions, such as memory consolidation, anxiety regulation, cognitive flexibility, emotional function, and social behavior (Bergmann et al., 2015). With regard to pathological conditions, alteration of adult hippocampal neurogenesis has been described in several rodent neurological disease models (Toda et al., 2019), including ASD as discussed later. Due to technical limitations, it is still not clear how AN contributes to cognitive abilities in humans and if changes are entangled to the pathophysiology of human diseases.
Another neurogenic region in the mammalian brain is the subventricular zone (SVZ) of the lateral ventricles. In this stem cell niche, newborn neuroblasts exit the SVZ via the rostral migratory stream and later develop into neurons of the olfactory bulb (OB), a region that primarily mediates smelling. Interestingly, under pathological conditions, olfactory dysfunction in rodents has been noted in a variety of neurological disease models ranging from neurodevelopmental disorders, including ASD, to neurodegenerative ones (Höglinger et al., 2004; Winner et al., 2011). Likewise, in humans, olfactory impairment is a common feature of many neurological diseases (Khurshid et al., 2019; Kronenbuerger et al., 2018; Sweigert et al., 2020); however, AN in the human OB is supposed to be rudimentary and presumably limited to early childhood (Bergmann et al., 2012). On the other hand, new neurons are generated in the adult human striatum (Ernst et al., 2014), the main input area of the basal ganglia and of particular interest regarding ASD research. However, it is still controversially discussed, if they originate from the SVZ or local neural progenitor cells (NPCs).
To sum up, it is assumed that neurogenesis in adults affects neuroplasticity both under normal physiological and pathological conditions like Alzheimer's disease, depression, or schizophrenia (Anacker & Hen, 2017; Hitti & Siegelbaum, 2014; Moreno-Jiménez et al., 2019; Winner & Winkler, 2015), and therefore, represents an important target for therapeutic intervention. Furthermore, accumulating evidence from animal models suggests that physical and psychological stress can impair the process of AN, which might further augment the symptoms of disorders. But what is the rationale between AN and a neurodevelopmental disorder such as ASD?
The neurobiology of ASD is complex, but emerging research indicates that alterations in AN are associated with ASD in both human patients and mouse models of ASD, suggesting that remodeling neurogenesis may be a novel therapeutic approach for ASD.
Although our knowledge regarding the coherence between ASD with AN and related functions in the human brain is very limited, animal models provide some indications of interconnecting links. Up to now, there are limited treatment options to ameliorate the symptoms associated with ASD, to that effect understanding the common mechanisms underlying the dysregulation of AN in ASD could have a large impact on behavioral interventions and, therefore, help a large population of patients.
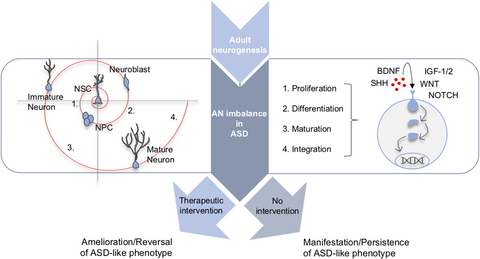
In this review, we, thus, want to juxtapose ASD/AN-overlapping features, in particular with the focus on behavioral phenotypes emerging in ASD found to be AN-dependent as well as molecular mechanisms underlying AN regulation found to be altered in ASD (Figure 1).
2 HOMOLOGIES: SHARED FEATURES AND COMMON REGULATING FACTORS
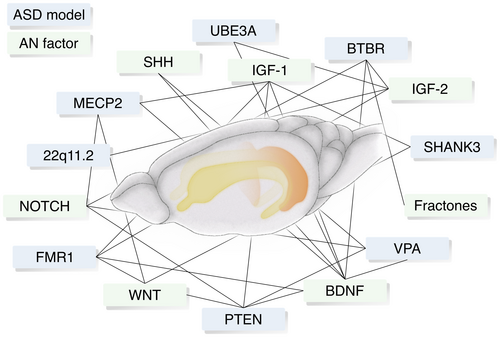
Researchers over the past few years have described several neurogenic effects occurring in various ASD models (Criss) and vice versa, a number of factors have been identified that influence the process of neurogenesis, some of which are interestingly altered in ASD (Cross). In the following two parts of this article, we would like to take a closer look at these two incidents. For a summary of both core ASD-related behavioral phenotypes and major AN-related cellular markers in the models analyzed in this review, refer to Tables 1 and 2.
| ASD-related model | Core ASD-related phenotypes | AN-related cellular markers |
|---|---|---|
| VPA | ||
| FMR1 |
|
|
| BTBR |
|
|
| SHANK3 |
|
|
| PTEN |
|
|
| UBE3a |
|
| AN-related factor | AN-related cellular markers | Core ASD-related phenotypes |
|---|---|---|
| WNT | ||
|
IGF−1 IGF−2 |
|
|
| NOTCH |
|
2.1 Criss: AN alteration in ASD models
2.1.1 Valproic acid
Valproic acid (VPA) is a branched short-chain saturated fatty acid, mainly used as anti-convulsant, mood stabilizer and in the prophylaxis of migraines (López-Muñoz et al., 2012) (Emrich et al., 1980) (Sorensen, 1988).
The multiformity of effects exerted by VPA is grounded on the numerous molecular pathways it affects, such as NMDA-dependent excitatory synaptic response (Gean et al., 1994); GABA production and release (Löscher, 1999); activity of glycogen synthase kinase 3 (GSK) −3 alpha and −3 beta (Chen et al., 2000), ion channels (De Sarro et al., 1992; Tian & Alkadhi, 1994), and histone deacetylase (HDAC) (Göttlicher et al., 2001; Krämer et al., 2003; Phiel et al., 2001). Shortly after the commercialization of VPA, cases of neural tube defects were reported in children born of epileptic mothers treated with VPA (Robert & Guibaud, 1982). Several cohort studies (Jentink et al., 2010) proved the association between application of VPA in the first trimester of pregnancy and various malformations in the newborn. Moreover, maternal use of VPA during pregnancy is associated with a significantly increased risk for ASD in the offspring (Christensen et al., 2013). Alterations of the chromatin remodeling status induced by VPA (reviewed in Diederich et al., 2010) might explain its teratogenic mechanisms.
Rodier and colleagues (Rodier et al., 1996) established for the first time the VPA-induced rodent model of ASD, injecting rat dams with VPA from gestational days 11.5 to 13.5, while the neural tube in the embryos is closing. Histologically, the rats recapitulated features typical of human ASD, such as reduction of Purkinje cells in the cerebellar vermis and reduction of volume of the cerebellar hemispheres (Ingram et al., 2000; Rodier et al., 1997). The behavioral characterization of the rodents exposed in-utero to VPA (Schneider & Przewłocki, 2005) confirmed, among others, the presence of decreased social interaction and the occurrence of repetitive behavior increasing the translational significance of the model. Several in vitro studies further showed that the impact of VPA on neurogenesis and neurodevelopment involves several defined molecules, that is, β-catenin (Jung et al., 2008), HDAC (Hsieh et al., 2004), and GSK-3 beta (Go et al., 2012).
There are surprisingly few papers dealing with the implications of exposure to VPA in pregnancy on AN. Sustained high levels of VPA during pregnancy were shown to affect primarily interneurons, being reelin-, parvalbumin-, and glutamate decarboxylase 67-positive cells reduced in the hilus of the DG. The imbalance could likely explain the increase in granule cell layer neurons reported at post-natal day 77 (Catavero et al., 2018; Watanabe et al., 2017). Similarly, Kinjo and colleagues (Kinjo et al., 2019) reported macroscopic malformations and higher incorporation of bromodeoxyuridine (BrdU) and expression of doublecortin (DCX) in the DG of P30 rats exposed to different concentrations of VPA during pregnancy. The rats showed hyperactivity (augmented total distance covered in the open-field test) and reduced anxiety (longer time spent in the open arm in the elevated plus maze test). Juliandi and colleagues (Juliandi et al., 2015) analyzed adult mice exposed prenatally to VPA both behaviorally and histologically. At an early developmental time point, neurogenesis was increased. Such a phenotype, together with macrocephaly at 2 and 7 days after birth, was similarly shown by another group (Go et al., 2012). The early pro-neurogenic effect led to a partial depletion of the NSC pool in adult mice, which was also accompanied by lower scoring in learning and memory tests compared to wildtypes. Interestingly, voluntary running could restore AN. The beneficial properties of running came along with increased brain-derived neurotrophic factor (BDNF) expression, reduced global and activated microglia in the HC, rescue of neuronal morphology to the values of controls (total dendritic length and dendritic complexity) in the DG, and recovery of impaired inhibition in the mossy fiber pathway (the part of the tri-synaptic pathway connecting the DG and CA3 region of the HC). The curative effects of running on AN in the VPA model were confirmed in later work. Voluntary physical activity could reverse ectopic neuronal migration, seizure susceptibility, and transcriptomic alterations of adult NSCs and NPCs in adult mice (Sakai et al., 2018). The application of an agonist of the liver X receptor in the first 2 weeks of life could restore both behavioral and AN alterations in 8-week-old VPA model mice (Cai et al., 2019). Alternatively, transplantation of mesenchymal stem cells was also an effective measure to rescue impaired AN and behavior in mice exposed prenatally to VPA (Gobshtis et al., 2017). In a similar way, transplantation of human adipose-derived stem cells could also ameliorate social impairment and repetitive behavior and restore the diminished levels of phosphatase and tensin homolog (PTEN) (Ha et al., 2017) in the VPA model.
A model of VPA exposure in pregnancy was recently developed in nonhuman primates (Zhao et al., 2019). Histological and molecular parameters were investigated on full-term aborted fetuses, whereas living offspring was destined for behavioral analysis. Both in the PFC and in the cerebellum, reduced density of neuronal nuclei-positive cells (NeuN+) were observed. Moreover, Ki67 density was also reduced in the cerebellum. Western blot analysis of PFC lysates revealed reduction of neuroblasts, neurons, and glutamate receptors. On the opposite, GABAergic and astrocytic markers were increased. Eye-tracking experiments were also undertaken in animals exposed to VPA during pregnancy. As already shown in human patients with ASD, the monkeys spent watching more time the nonsocial parts of a picture.
Interestingly, Cannabidivarin (CBDV), a nonpsychoactive homolog of cannabidiol (CBD), was shown to rescue both social impairments and increased levels of astrocytes and microglia in a VPA rat model. The effect was specific to HC but not observable in the PFC (Zamberletti, Gabaglio, Woolley-Roberts, et al., 2019).
2.1.2 FMR1/Fragile X
Fragile X syndrome (FXS) is the most commonly inherited form of mental retardation (Hersh et al., 2011). Distinguishing signs are atypical facies, severe testicular hypogonadism with bilateral macro-orchidism, and a variable presentation of intellectual disability and ASD (Hersh et al., 2011; Loesch et al., 2002; Rudelli et al., 1985). The condition owes the denomination to a fragile site located on chromosome X at position q27, corresponding to the gene fragile X mental retardation 1 (Fmr1) (Verkerk et al., 1991). The product of the gene is the fragile x mental retardation protein (FMRP), a protein highly expressed in the brain (Devys et al., 1993). It is located in the cytoplasm, where it binds to mRNA and negatively regulates the translation of several proteins, thereby affecting central nervous system development, neurogenesis, and synaptic plasticity. Its absence determines the activation of several intracellular pathways and increased protein synthesis (Li & Zhao, 2014; Penagarikano et al., 2007). In the central nervous system, FMRP is involved mainly in the control of long-term depression via metabotropic glutamate receptor signaling (Bear et al., 2004), the regulation of scaffold proteins and glutamate receptors in the post-synaptic specialization (Aloisi et al., 2017; Schütt et al., 2009), long-term potentiation in the HC (Yun & Trommer, 2011), amygdala (Zhao et al., 2005), and neocortex (Desai et al., 2006). The molecular alterations related to the absence of FMRP result in a high density of long, thin, and tortuous dendritic spines, which resemble the morphology observed in normal early development both in humans (Hinton et al., 1991; Irwin et al., 2001; Rudelli et al., 1985) and mice (Comery et al., 1997). Dendritic anomalies were also reported in the mitral cells of the OB in a mouse model for FXS (Galvez et al., 2005).
The Fmr1 knockout (B6.129P2-Fmr1tm1Cgr/J) mice, which are completely devoid of FMRP, exhibit learning deficits and hyperactivity, hence, mimicking symptomatic features of human patients and serving as a useful tool for translational research. For more details on the behavioral peculiarities of this model and other Fmr1 models, please refer to the reviews from Kazdoba (Kazdoba et al., 2014) and Dahlhaus (Dahlhaus, 2018).
There is a limited number of studies exploring AN in Fmr1 mutant mice. The impaired glutamatergic transmission observed in the DG of this model might affect AN (Yun & Trommer, 2011). Indeed, NMDA receptor activation in the HC was reported as a central regulatory node of AN (Cameron et al., 1995).
In one of the earliest studies (Eadie et al., 2009), Fmr1 knockout mice were tested for spatial learning and memory in the Morris Water Maze (MWM) and Plus-shaped maze. These tasks, which require the activity of the dorsal HC (for an overview of the functions of HC, please refer to Bannerman et al. (2004)), were not altered. Nonetheless, mutant mice showed a reduction of newborn neurons in the ventral HC and, at the same time, were less prone to display anxiety. Since the ventral HC is responsible for emotional response (Bannerman et al., 2004), these findings seemed to point at a dysfunction of the ventral HC in Fmr1 knockout mice. A successive study (Luo et al., 2010) revealed that FMRP is actively expressed in NPCs and in newly generated neurons, but not astrocytes in the DG of the HC (no anatomical distinction between dorsal and ventral HC was remarked). Contrary to Eadie et al. (2009), the number of BrdU+ cells in Fmr1 knockout animals was not significantly altered 4 weeks after injection in 14-week-old mice. Moreover, both in vitro and in vivo, Fmr1 knockout adult NPCs were still able to differentiate, but were directed toward the astrocytic lineage at the expense of the neuronal one. This effect was partially rescued using an inhibitor of GSK-3 beta, SB-216763, a molecule whose levels are up-regulated in the absence of FMRP, pointing to an involvement of the Wnt3a/β-catenin/Neurog1 pathway in the imbalanced differentiation observed in FMRP-deficient mice. Using the same pharmacological approach, it was possible to revert dendritic pathology, altered AN, and learning deficits in an Fmr1 knockout model (Guo et al., 2012). Excessive dendritic spines reveal a deficit in synapse elimination. The transcription factor myocyte enhancer factor 2 (MEF2) is recruited upon neuronal activity and mediates robust synapse elimination in co-operation with FMRP. As expected, the mechanism is ineffective in Fmr1 knockout mice, but it can be promptly re-induced upon co-expression of wild-type FMRP and MEF2 (Pfeiffer et al., 2010). Activation of MEF2 at the post-synaptic density leads to the recruitment of the ubiquitin E3 ligase murine double minute 2 (Mdm2), enhancing the proteasomal degradation of protocadherin 10 (Tsai et al., 2012), an adhesion molecule involved in ASD (Taylor et al., 2020). The role of the ubiquitin-ligase Mdm2 was further investigated by Li and colleagues (Li et al., 2016), whereby the loss of FMRP led to elevated Mdm2 mRNA and protein through the AKT pathway. The application of the anti-proliferative drug Nutlin-3, a small molecule inhibiting the interaction between Mdm2 and the cell-cycle regulator p53, rescued the decreased neurogenesis and impaired spatial learning behavior.
A deeper understanding of the role of FMRP in AN was achieved by generating and utilizing a cell-specific conditional knockout mouse model, in which FMRP is depleted in adult NSCs (Guo et al., 2011). The model is characterized by an increased number of BrdU+ cells, increased astrocytic and reduced neuronal differentiation, and impaired learning, a function associated with adult hippocampal neurogenesis (Deng et al., 2010). All the mentioned phenotypes were reversed once the expression of FMRP was re-established.
In addition, FMRP appears to play a role in the differentiation of granule cells in the mouse OB, another region where AN takes place (Lledo & Valley, 2016). Furthermore, knocking down FMRP expression through shRNA results in morphological (increase in spine density and length) and functional (increased firing rate) alterations in the granule cells of the OB (Scotto-Lomassese et al., 2011).
2.1.3 BTBR
The black and tan brachyuric (BTBR) mouse is an inbred strain displaying the core behavioral deficits of ASD, meaning impairments of social behavior and communication and occurrence of repetitive behavior (Bolivar et al., 2007; McFarlane et al., 2008; Moy et al., 2007), advancing BTBR as a useful model for scientific investigations.
Besides the main ASD-relevant behaviors, it has been shown that BTBR mice exhibit lower peak heights of sniffing both nonsocial and social odors (Scattoni et al., 2011, 2013; Yang et al., 2012), suggesting an impairment of olfaction and sensory acuity, a characteristic also described in ASD patients. Furthermore, it has been shown that BTBR mice display a worse performance in T- or Y-maze and MWM settings (Karvat & Kimchi, 2012; Moy et al., 2007). Despite some critical opinions, the MWM is still supposed to be the gold standard test of hippocampal function in rodents (Garthe & Kempermann, 2013). Several studies using this test show that mice with reduced hippocampal neurogenesis were impaired in finding the safety platform (Dupret et al., 2008; Garthe et al., 2009; Jessberger et al., 2009), while mice with increased neurogenesis show improved water maze learning (Garthe et al., 2016). Congruently, BTBR mice show reduced levels of neurogenesis in the adult hippocampal SGZ (Stephenson et al., 2011); however, AN in the SVZ and OB of BTBR mice was not explored to date. Interestingly, BTBR mice exhibit various neuroanatomical alterations, including both neurogenic regions, the HC, and the ventricular wall of the lateral ventricles (Mercier et al., 2012). Noteworthy, electrophysiological analysis of BTBR hippocampal slices post-MWM studies revealed that performance differences were accompanied by a significant reduction in BDNF-induced potentiation of synaptic transmission and reduced levels of BDNF expression were detected in the BTBR DG (Stephenson et al., 2011). This indicates a neurogenic correlation as BDNF signaling is critical for neuronal survival, morphogenesis, and plasticity (as discussed later).
In the context of stem cell-based regenerative therapies, it has already been shown that exogenous administration of different types of stem cells could either improve abnormal behavior or correct ASD-like symptoms during childhood (Bradstreet et al., 2014; Lv et al., 2013; Segal-Gavish et al., 2016). Strikingly, a recent study showed that intraventricular transplantation of amniotic epithelial cells into adult BTBR mice could ameliorate social deficits and was accompanied by rescued BDNF levels (Zhang, Cai, et al., 2019). In a similar approach, social deficits and stereotypical behaviors in BTBR mice were alleviated by restoring reduced adult hippocampal neurogenesis through treatment with the synthetic liver x receptor agonist (Cai et al., 2019). Thus, modulation of neurogenesis in adult BTBR mice on the level of NSCs would be an interesting approach in the context of its putative potential to ameliorate ASD-associated behaviors.
2.1.4 SHANK3/Phelan-McDermid syndrome
The SH3 and multiple ankyrin repeat domains protein 3 (Shank3) gene is located on mouse chromosome 15E3 (human location: 22q13.3), has multiple intragenic promoters and several alternative splicing exons, resulting in the generation of several protein isoforms (Jiang & Ehlers, 2013; Monteiro & Feng, 2017; Wang et al., 2014).
SHANK3 is a post-synaptic density (PSD) protein that interacts with a variety of ionotropic and metabotropic glutamate receptors and links them to the actin cytoskeleton (Naisbitt et al., 1999). Mutations in the human SHANK3 gene have been associated with both ASD and Phelan-McDermid syndrome (Durand et al., 2007; Wilson et al., 2003).
The lack of individual sets of Shank3 isoforms contributes to varying neuroanatomical phenotypes among the different Shank3 mutant mouse lines available. Shank3ex4−9 mutants, for example, show reduced GluA1-immunoreactive puncta in the hippocampal region CA1 (Bozdagi et al., 2010), as well as increased perforated synapses at 5 weeks, which do not persist with increasing age (Uppal et al., 2015). Moreover, Shank3ex11 mutants do not show any significant changes in spine number in the CA1 region of the HC or PSD ultrastructure (Schmeisser et al., 2012). Increased dendritic length and complexity but reduced spine density, PSD length, and thickness in striatal medium spiny neurons are characteristics of Shank3ex13−16 mutants (Peça et al., 2011). Decreased spine density, PSD length, and thickness are also observed in the striatum, but not in the HC of the mouse model ablating all Shank3 isoforms (Shank3ex4−22 mutants) (Wang et al., 2016). Importantly, restoration of Shank3 expression in adult Shank3ex13−16 mutants was shown to reverse dendritic spine loss and excitatory synaptic function in the striatum (Mei et al., 2016).
Varying phenotypes between the different mouse lines can also be observed on the behavioral level. In particular, Shank3ex4–9 and Shank3ex11 mutant mice display abnormal social interaction and communication, repetitive behaviors, and learning and memory impairments (Vicidomini et al., 2017; Wang et al., 2011). Shank3ex13−16 mice exhibit repetitive self-grooming and deficits in social interaction (Peça et al., 2011), while Shank3ex21 mice display deficits in HC-dependent spatial learning and memory (Kouser et al., 2013). Similarly, the Shank3ex4−22 model shows increased grooming, increased novelty-induced anxiety with strong escape behavior, impaired HC-dependent memory, and striatal-dependent learning (Drapeau et al., 2018; Wang et al., 2016).
Given that Shank3 disruption can affect synaptic function in the HC, its role in hippocampal AN was investigated in the Shank3ex21 mouse model. Namely, the number of DCX+ immature neurons was unaffected in the dorsal DG, but significantly decreased in the ventral DG of Shank3ex21mice (Cope et al., 2016). The number of cells with radial glial morphology, referred to as neuronal precursor cells, was also decreased in the ventral DG. Considering that both ventral HC and AN have been linked to anxiety and stress regulation (Revest et al., 2009; Wu et al., 2015) as well as social behaviors (Opendak et al., 2016), it is possible that reduced numbers of new neurons may contribute to both social impairment and increased anxiety, a common co-morbidity of ASD (van Steensel et al., 2011).
Interestingly, in Shank3ex4–9 mutants, weaker and fewer synapses between olfactory sensory nerve terminals and OB tufted cells and between olfactory sensory nerve terminals and inhibitory periglomerular cells were detected, leading to deficits in sensory-evoked responses (Geramita et al., 2020). It would, therefore, be of importance to examine whether adult OB neurogenesis plays a role in the olfaction deficits in these mice.
Separately, NSCs isolated from the SVZ of adult Shank3ex11 mutants displayed enhanced progenitor proliferation and differentiated earlier than the wild-type-derived NSCs (Grasselli et al., 2020). In addition, lysosomal and ubiquitin aggregation, including mitochondrial impairment, were detected in astroglial cells, suggesting that glial degeneration likely contributes to neuronal damage in ASD. These in vitro observations point to the fact that Shank3 deficiency could affect the late phases of neurogenesis and/or the survival of mature cells rather than NSC self-renewal.
In an attempt to bridge the species gap and come closer to understanding human pathologies associated with SHANK3 haploinsufficiency, nonhuman primate embryos lacking SHANK3 were created using CRISPR/CAS genome editing (Zhao et al., 2017). After successful pregnancy, one monkey died at birth (p0) and one remained alive. In the wild-type p0 monkey samples, the highest expression of SHANK3 was detected in the PFC. In the PFC of the mutant monkey, fewer neurons and more astrocytes were detected. Additionally, decreased level of DCX was observed in the PFC, but not in the striatum. This study suggests that there might be a brain region-specific neurogenesis defect in SHANK3 mutants during development and further offers a possibility to investigate AN in SHANK3 mutant primates.
2.1.5 PTEN deletion
The tumor suppressor PTEN is a lipid phosphatase regulating the levels of the phospholipid phosphatidylinositol (3,4,5)-trisphosphate, second messenger of the PI3K-AKT pathway (Maehama & Dixon, 1998).
PTEN mutations are associated with autism and extreme macrocephaly (Butler et al., 2005). In the central nervous system, PTEN is involved in the regulation of proliferation, migration, and lamination of NPCs, axon growth cones, dendritic spine density, and synaptic plasticity (Rademacher & Eickholt, 2019). Interestingly, PTEN interacts with several intracellular molecules (e.g., FMRP (Lugo et al., 2013)) and the AN regulating pathways, such as wingless-related integration site (WNT) (Hodges et al., 2018), NOTCH (Jo et al., 2012), and insulin-like growth factor (IGF) (De Paula et al., 2014), discussed later here.
A wide range of mice harboring Pten mutations and recapitulating ASD phenotypes has been generated throughout the years (for a more detailed overview, we suggest Clipperton-Allen & Page, 2020). Newborn cells and NPCs of Pten+/− mice were reduced in the SVZ but increased in the OB, showing a more rapid exit of the newborn cells from the SVZ in the mutant lines. Similarly, a cell culture experiment confirmed that Pten−/− cells migrate and invade more than wild-type cells (Li et al., 2002, 2003).
NSCs derived from the DG of Ptenm3m4 mice exhibited increased proliferation and resistance to neuronal maturation and, hence, an increased ratio of immature neurons to mature ones. Contrarily, astrocytes were significantly increased, a result similar to that already observed in Fmr1 knockout mice (paragraph “FMR/Fragile X”: Eadie et al., 2009; Guo et al., 2011; Li et al., 2016; Luo et al., 2010)) and in a SHANK3-deficient monkey (paragraph “SHANK3/Phelan-McDermid syndrome” (Zhao et al., 2017)). Notably, BDNF treatment partially restored the number of NeuN+ cells (Kang et al., 2020). Nestin-CreER, an inducible mouse line, was used in order to delete PTEN specifically in stem and progenitor cells of the central nervous system after birth. The SVZ of mutant animals was expanded and comprised NeuN+ (missing in the SVZ of control animals) and DCX+ cells. After BrdU injection, expression was detected in the NeuN+ cells of the mutant SVZ, pointing at an involvement of PTEN in the maintenance of this neurogenic niche also in post-natal/adult stage. Interestingly, treatment with Rapamycin, inhibitor of mTORC1, rescued the expansion of the SVZ and the over-expression of DCX in this region (Zhu et al., 2012). In another study, PTEN ablation resulted in increased proliferation in the SGZ in 4- and 7-month-old animals (Amiri et al., 2012). An initial excess of DCX+ cells at 4 months was followed by a sharp depletion of these cells at 7 months, a tendency already observed in the VPA model (Juliandi et al., 2015). The loss of PTEN determined phosphorylation and inactivation of GSK-3 beta. Increased phosphorylated GSK-3 beta was also found during the embryonic development in telencephalon lysates of mice exposed in-utero to VPA (Go et al., 2012). On the opposite, pharmacological inhibition of GSK-3 beta in Fmr1 mutant mice led to an improvement of long-term potentiation (Franklin et al., 2014), amelioration of social- and anxiety-related behaviors (Mines et al., 2010), and the rescue of AN together with an improvement in behavioral tests (delayed nonmatch to place 8-way radial arm maze test and trace conditioning test) (Guo et al., 2012).
2.1.6 Rett syndrome (RTT)
RTT is a developmental disorder often presenting with ASD-like traits (Renieri et al., 2003). RTT-typical symptoms emerge in early post-natal development and manifest throughout life. Patients suffer especially from impaired social communication, respiratory problems, and motor dysfunction ranging from stereotypic hand movements to complete wheelchair dependency (Hagberg, 2002). The prevalence of RTT is estimated as 1 in 10.000 births (Ip et al., 2018). The pathology of RTT is mediated by mutations in the methyl-CpG-binding protein 2 (MECP2) gene, located on the X-chromosome, and thus, inherited predominantly to female offspring. The transcriptional product of the MECP2 gene is a multifunctional protein involved in numerous processes and acts both as a repressor and activator of transcription, thereby, impacting the development of the CNS (Liyanage & Rastegar, 2014).
A recent investigation has shown an increased number of immature neurons within the periglomerular layer of the OB and disrupted neurogenesis in the piriform cortex and olfactory tubercle of male Mecp2y/− mice (Martínez-Rodríguez et al., 2019). Further, an impaired inhibitory/excitatory balance was shown together with impaired activity-dependent refinement of the olfactory circuits in mice lacking MECP2 expression (Degano et al., 2014). Impaired long-range connectivity with the entorhinal cortex, accompanied with poor dendritic development, was also shown to affect newly born neurons derived from the adult DG (Sun et al., 2019). Interestingly, conditional KO of MECP2 in adult mice exhibited hypoactivity, motor dysfunction, and impaired nesting behavior, as well as disrupted learning and memory abilities, as similarly observed in the constitutive KO (Cheval et al., 2012; Du et al., 2016; McGraw et al., 2011). MECP2, by controlling the learning-induced transcriptional response of hippocampal neurons, is also required for long-term memory formation. This evidence further supports the link between MECP2 and cognitive functions (Gulmez Karaca et al., 2019).
2.1.7 UBE3A/Angelman syndrome
Angelman syndrome is a rare genetic disorder marked by severe developmental delay, movement disorder, speech impairment, seizures, microcephalia, and a unique combination of frequent laughter and smiling (Dagli et al., 2012). It is caused by the loss of function of the maternal copy of chromosome 15q11.2-q13, which codes, in neurons including Purkinje cells and mitral cells of the OB, for the protein ubiquitin-protein ligase E3A (UBE3A) (Albrecht et al., 1997; Buiting et al., 2016). Several mouse lines reproducing the genetic alterations of AS were produced. Behaviorally, these lines recapitulate impairments in social communication, motor activity, repetitive behavior, and anxiety (Rotaru et al., 2020). In a mouse model with a maternal null mutation in Ube3a, there was a significant lower fraction of newborn neurons compared to controls at 5 weeks of age (Mardirossian et al., 2009). Following work (Godavarthi et al., 2015) extended the study of AN to later time points (post-natal days 60 and 120). At both time points, mutant mice showed reduced proliferation and a reduced number of DCX+ immature neurons in the HC compared to control mice. Interestingly, chronic injection of Fluoxetine, an antidepressant, partially rescued the impaired proliferation rate. Furthermore, subcutaneous injection of IGF-2 restored cognitive impairments, working memory deficits, repetitive behaviors, and motor dysfunction. Moreover, audiogenic seizures were also significantly reduced upon administration of IGF-2 (Cruz et al., 2021).
2.1.8 22q11.2 deletion/DiGeorge syndrome
22q11.2 deletion syndrome, including the subtypes DiGeorge syndrome or velocardiofacial syndrome, is the most frequent microdeletion syndrome affecting approximately one birth among every 4,000. Typical clinical signs are cardiac anomalies, hypoparathyroidism, immunodeficiency, and pharyngeal dysfunction (Kobrynski & Sullivan, 2007). The prevalence of neuropsychiatric disorders (epilepsy, movement disorders, schizophrenia, ADHD, Parkinson) registered among the individuals affected by this condition is higher than the general population and up to 35% of them received a diagnosis of ASD (Zinkstok et al., 2019).
Since around 2.5–3 Mb containing an elevated number of genes is lost, the efforts of recent works were focused on pinpointing the causal role of each singular gene in the emergence of this complex condition. DGCR-8, essential gene for miRNA biosynthesis, is involved both in the pathogenesis of schizophrenia and ASD (Forsyth et al., 2020). The mouse model deficient for this gene showed reduced proliferation and decreased number of newborn neurons in the DG, but not in the SVZ at 2 months of age. The survival, 4 weeks after BrdU injection, of newly generated cells was reduced compared to controls. After injection of IGF-2, these effects were partially recovered. Interestingly, spatial working memory resulted improved after injection of IGF-2 (Ouchi et al., 2013).
2.2 Cross: ASD features affected by factors regulating AN
2.2.1 BDNF Signaling
During development of the CNS and maintenance of local neuron populations, the neurotrophin BDNF plays a major role (Yeh et al., 2015). The BDNF protein exists in two isoforms with different physiological functions, m-BDNF and pro-BDNF, which bind to the tropomyosin-related kinase B (TrkB) receptor and to the p75 neurotrophin receptor, respectively (Kowianski et al., 2018).
First evidence for the central role of BDNF in supporting the proper function of AN was provided by an in vitro investigation of neurons derived from the adult rat forebrain, which revealed that exposure to BDNF significantly increased their lifespan (Kirschenbaum & Goldman, 1995). Based on this finding, further investigations supported the central role of BDNF in the proper functioning of AN in both mammalian neurogenic niches, the SVZ/OB and the SGZ/HC. Intrahippocampal infusion of BDNF into the DG of rats led to a higher number of new granule neurons (Scharfman et al., 2005), which is consistent with investigations of BDNF exposure to the SVZ, also resulting in an increased production of OB neurons (Benraiss et al., 2001; Zigova et al., 1998). In turn, transgenic Bdnf knockout mice display impaired cell differentiation of granule cells in adult animals (Chan et al., 2008). In addition, strong evidence for the involvement of BDNF in AN was made by Linnarsson and colleagues, demonstrating cell death in the SVZ of Bdnf knockout mice starting 2 weeks after birth (from p13 to p18) (Linnarsson et al., 2000). These results strongly link BDNF to the cell fate of newborn neurons.
BDNF also plays a major role in synaptogenesis and synaptic plasticity (Kowianski et al., 2018). Based on current understanding, impairments of these fundamental processes often result in ASD. Indeed, it was shown that aberrant concentration of BDNF in the brain and blood correlates positively within individuals exhibiting ASD (Nelson et al., 2001; Perry et al., 2001). Furthermore, ASD mouse models, such as Fmr1 and Mecp2 mutants or the BTBR line, strongly link aberrant BDNF expression to the pathology of ASD (Reim & Schmeisser, 2017). First evidence of the connection between BDNF and FMRP, made in 2002 by Castren et al., indicates that TrkB-mediated BDNF signaling decreases the expression of FMRP (Castren et al., 2002). Since then, a strong relationship between FMRP and BDNF/TrkB signaling in regulating NPCs and hippocampal neurons has been established. Findings indicate that absence of FMRP in mice results in reduced BDNF levels in cortical neurons, but increased BDNF levels in hippocampal neurons (Louhivuori et al., 2011). Fmr1 knockout mice also exhibiting Bdnf haploinsufficiency show increased impairments in spatial learning and spontaneous pain behavior (Uutela et al., 2012). Interestingly, these mice display amelioration of aberrant phenotypes, such as locomotor hyperactivity, which is usually seen in the corresponding single mutants. The role of BDNF in Fmr1 knockout mice was further explored by analyzing electrophysiological properties of the mutant hippocampal CA1 region, either in presence or absence of supplemental BDNF. Here, it was shown that infusion of BDNF enables the recovery of long-term potentiation in Fmr1 knockout mice (Lauterborn et al., 2007). Furthermore, UBE3a mice exhibit PSD-95-dependent deficits in BDNF signaling (Cao et al., 2013). Interestingly, in the same mouse model, Jamal et al. could show that altered BDNF levels can be restored by environmental enrichment and, in parallel, these mice display an increased number of GABAergic interneurons in the HC, which was associated with improved behavioral abnormalities (Jamal et al., 2017).
2.2.2 WNT signaling
The WNT pathway is highly conserved from invertebrates to vertebrates with at least 19 genes expressing different isoforms in human. The canonical WNT pathway can be subdivided into two categories based on increasing or decreasing levels of β-catenin, whereas the noncanonical one is characterized by β-catenin-independent signaling.
The first study linking WNT signaling to hippocampal AN showed that blocking WNT signaling in vivo suppresses the number of newborn neurons in the DG of adult rats (Lie et al., 2005). Lie and colleagues could also show that over-expression of WNT3 results in stimulation of WNT/β-catenin signaling, leading to increased hippocampal AN. Since WNT7a is also expressed in the adult neurogenic areas, it was of further interest to analyze its effect on development and AN. Indeed, in vitro investigations using Wnt7a knockout cells, derived from 6-week-old Wnt7a mutant mice, could show that the lack of WNT7a results in strongly reduced self-renewal ability of NSCs (Qu et al., 2013). In addition, WNT7a loss of function was shown to impact neural differentiation toward increased number of astrocytes and decreased number of mature neurons. Interestingly, DCX staining combined with Sholl analysis in Wnt7a knockout mice showed impaired morphology of hippocampal granule cells, such as decreased dendritic length and complexity (Qu et al., 2013). Supporting the importance of WNT7a in terms of neuronal differentiation and maturation, a recent study showed that chronic intrahippocampal infusion of WNT7a leads to both increased arborization of DCX+ hippocampal granule cells and slight increase in the number of newborn cells (Ortiz-Matamoros & Arias, 2019). Ortiz-Matamoros and Arias used the same approach in order to analyze the impact of the noncanonical ligand WNT5a on neurogenesis in the adult HC. Here it was shown that the chronic infusion of WNT5a leads to a 3-fold increase in the number of newborn neurons. In addition, it was also shown that WNT5a leads to altered dendritic arborization. Furthermore, using a lentiviral mediated WNT5a knockdown, a reduced number of newborn neurons and impaired morphological development were observed, linking WNT5a to cell fate and AN (Arredondo, Guerrero, et al., 2020). These results show involvement of both canonical and noncanonical WNT signaling in AN, including differentiation, maturation, and self-renewal (Arredondo et al., 2020).
Comparatively, less is known about altered WNT signaling in the context of ASD pathology than about its influence on AN. However, previously mentioned investigations focusing on WNT and AN already show that mutations concerning WNT ligands not only affect the quantity of newborn cells but also the quality. These deformations, like altered neurite growth and dendritic branching (Ortiz-Matamoros & Arias, 2019; Qu et al., 2013), were also shown to take place in many autistic individuals (Martinez-Cerdeno, 2017). In addition to the impact on neurite development, some WNT ligands also exert influence on β-catenin, which is a central molecule of canonical WNT signaling activation. Interestingly, it was shown that canonical WNT signaling is regulated by chromodomain helicase DNA-binding protein 8 (CHD8), whereby mutations of the CHD8 gene can be found in individuals with ASD (Talkowski et al., 2012). Mouse models exhibiting Chd8 haploinsufficiency exhibit decreased brain size and volume in embryonic and adult stages (Katayama et al., 2016). The studied mice show a range of typical behavioral abnormalities, including aberrant social interactions and increased anxiety. Further studies using a similar model supported the previous findings, showing that Chd8 mutant mice exhibit mild social impairment and an anxiety-like phenotype; however, repetitive behavior could not be detected (Platt et al., 2017). A further strong line of evidence connecting dysfunctional WNT signaling to autistic-like phenotypes can be traced back to the central player of canonical WNT signaling, β-catenin. Previously mentioned WNT ligands WNT3a and WNT7a activate among others β-catenin, a crucial process for not only brain development but also synaptogenesis (Caracci et al., 2016). In a recent investigation, the impact of both increased and decreased β-catenin expression levels in vivo was explored (Alexander et al., 2020). It was shown that increasing expression of β-catenin results in an increase in repetitive behavior and aberrant social interactions. A further study investigating the involvement of β-catenin in ASD was performed using the Shank3ex21 mouse model (Qin et al., 2018). Interestingly, it could be shown that the β-catenin level is redistributed from synapses to the nucleus in cortical slices of Shank3ex21 heterozygous mice. This shift was accompanied by an increased expression of HDAC2. Over-expression of β-catenin within the PFC of wild-type mice results in both increased levels of HDAC2 mRNA and social preference deficits. Apart from that, knockdown of β-catenin within the PFC of Shank3-deficient mice significantly increased social interaction times. Although much more is known about WNT and its influence on neurogenesis and development, central players, such as β-catenin, seem to be crucial in contributing to ASD pathology. More recently, it was shown that MECP2 T158A mice show altered levels of both, GSK-3 beta, and β-catenin phosphorylation, which could be restored by over-expression of WNT6 accompanied by an increase in BDNF and IGF-1 (Hsu et al., 2020). This emphasizes the complex interactions of the various molecules discussed in this review.
2.2.3 IGF signaling
The IGF signaling pathway is a complex and tightly regulated network mediating various cellular activities, including proliferation, differentiation, and survival (O'Kusky & Ye, 2012). The IGF system comprises IGF-1 and IGF-2 and their respective receptors (IGF-1 and IGF-2), as well as six binding proteins, IGF-binding proteins 1–6.
AN is a highly dynamic process, which can be affected by numerous molecules and pathways responding to changes in environmental or physical conditions (Eisinger & Zhao, 2018). Physical activity especially affects AN and induces cell proliferation (van Praag et al., 2005). Interestingly, increased levels of IGF-1, which is mainly expressed in the liver, but also in certain brain areas in the adult, were found in response to physical activity (Carro et al., 2000; Schwarz et al., 1996). Apart from that, IGF-1 exerts influence on BDNF; intracarotid injection results in increased hippocampal levels of BDNF, whereas blocking suppresses the physical activity-mediated increase in BDNF (Carro et al., 2000; Ding et al., 2006). The concomitant expression as well as the previously described direct impact of IGF-1 on BDNF implies IGF-1 as a direct link between physical activity and cell proliferation. As a matter of fact, increased IGF-1 concentration followed by exercise led to accumulation of newborn cells within the HC and increased proliferation due to physical activity could be inhibited by chronic subcutaneous administration of anti-IGF-1 antibody in adult rats (Trejo et al., 2001). Furthermore, application of IGF-1 over a period of 6 days induces neurogenesis in the adult rat HC, and further indicates that IGF-1 promotes survival of NPCs (Aberg et al., 2000). Additionally, IGF-1 deficiency results in a reduced number of newborn neurons. Interestingly, the proliferation ratio as well as the differentiation of NPCs seemed not to be affected, indicating a role of IGF-1 in supporting the survival of newborn cells (Lichtenwalner et al., 2006). Alongside this, it was shown that IGF-2 is highly expressed in the SGZ of the DG, and further characterization revealed a substantial IGF-2 expression in hippocampal NSCs (Bracko et al., 2012). Furthermore, in Bracko et al., it was shown that knockdown of Igf-2 expression results in decreased NSC proliferation in the DG, whereas SVZ-derived NSCs showed neither high levels of IGF-2 nor altered proliferation upon Igf-2 knockdown. However, more recently, Ferrón et al. showed that, in a physiological setting, endothelial-derived IGF-2 contributes to NSC maintenance in the SVZ, but not in the SGZ (Ferrón et al., 2015). The importance of IGF-2 in AN was further supported by the analysis of a conditional, heterozygous Igf-2 knockout mouse, displaying a 90% reduction of Igf-2 mRNA (Ziegler et al., 2019). In this study, it was shown that adult NSCs in both niches require sustained IGF-2 expression and that IGF-2-dependent maintenance of NSCs is needed for learning and memory.
Interestingly, in addition to BDNF, IGF-1 is also related to MECP2 deficiency in RTT females (Chang et al., 2004) (BDNF-MECP2 interconnection, see BDNF paragraph).
Cannabis-derived cannabinoids have neuroactive properties. Experimental research supports the use of CBD in many CNS disorders, though the mechanisms underlying its anticonvulsant and neuroprotective effects remain unclear. Importantly, CBD has been shown to normalize adult neurogenesis (Prenderville et al., 2015). In a pharmaceutical approach, CBDV, Cannabidivarin, was shown to normalize BDNF/IGF-1 levels, rescue time recognition memory deficits, and delay the appearance of other neurological phenotypes in Mecp2 mutant mice (Zamberletti, Gabaglio, Piscitelli, et al., 2019). Other studies have shown that MECP2-deficient mice display an aberrantly high expression of IGF-binding protein 3, thereby inhibiting IGF-1 signaling and thus suggesting a direct mechanistic link of IGF signaling in ASD (Itoh et al., 2007; Tropea et al., 2009). Following this hypothesis, several studies up to now showed that IGF-1 treatment could ameliorate various phenotypes in different ASD mouse models (Reim & Schmeisser, 2017). Hence, administration of NNZ-2566, a synthetic analogue of IGF-1, could rescue hyperactivity and anxiety phenotypes in Fmr1 knockout mice (Deacon et al., 2015) or application of both IGF-1 and the analogue ((1–3) IGF-1) could rescue long-term potentiation, AMPA signaling, and motor function in Shank3ex4−9 mutants (Bozdagi et al., 2013). Furthermore, application of (1–3) IGF-1 to Mecp2 mutant mice partially ameliorates RTT-like phenotypes (Tropea et al., 2009). With regard to another ligand of IGF signaling, Pardo et al. have shown that the hippocampal level of IGF-2 in both Fmr1 knockout mice and siRNA-mediated BDNF knockdown is reduced (Pardo et al., 2017). In both models, cognitive impairments could be reversed upon intranasal IGF-2 administration. In the same context, IGF-2 treatment of BTBR mice resulted in effective recovery of social, cognitive, and repetitive phenotypes (Steinmetz et al., 2018). Summarizing these results, there is a high necessity to further investigate the impact of IGF signaling on ASD phenotypes, especially in the context of putative treatment possibilities.
2.2.4 NOTCH signaling
NOTCH signaling is highly conserved among several species and mediates close-range cell–cell interaction. Canonical NOTCH signaling is characterized by the binding of one of the five canonical NOTCH ligands, from either the DELTA or JAGGED family to the NOTCH extracellular domain, leading to proteolytic cleavage and release of the NOTCH intracellular domain within the receiving cell. Subsequently, the NOTCH intracellular domain translocates to the nucleus and leads to transcriptional repression of proneural genes (Hori et al., 2013). Since NOTCH exerts influence on numerous cellular processes, impairments such as increasing or decreasing levels of NOTCH signaling can be directly linked to various developmental disorders in human.
The NOTCH signaling pathway strongly contributes to proper maintenance of AN within the two neurogenic niches. Increased cleavage of the NOTCH extracellular domain leads to the activation of a molecular cascade that results in discontinued differentiation of NPCs (Gage et al., 2008). The inhibition of determination factors due to NOTCH signaling supports the maintenance of progenitor cells in both proliferating and undifferentiated states. Since NOTCH signaling acts via direct cell–cell interaction, and thereby affects the maturation or differentiation of NPCs, it provides numerous impacts on the development and maintenance of the neurogenic niches in the adult. With regard to NSC fate in the SVZ, JAGGED1 seems to be crucial for NSC maintenance, through the activation of NOTCH1 (Nyfeler et al., 2005). Further investigations also support the importance of proper NOTCH signaling in terms of AN, such as an early cell-cycle exit of NSCs, mediated by the lack of NOTCH1 (Breunig et al., 2007). Additionally, a comprehensive reduction among all cell types during neurogenesis was observed in response to conditional NOTCH1 knockout in the SGZ, particularly the amount of undifferentiated Type-1 NSCs (Ables et al., 2010). NOTCH also interacts with other factors, such as recombining binding protein suppressor of hairless or recombination signal-binding protein for immunoglobulin kappa j region (RBPJ), leading upon activation to the transcription of suppressor genes. The resulting lateral inhibition was shown to be crucial for the maintenance of the NSC population in both embryonic as well as adult neurogenic niches. Mice lacking RBPJ (through conditional knockout) provide transient periods of increased neurogenesis resulting in a depletion of NSCs, indicating the importance of NOTCH signaling for long-term neurogenesis (Imayoshi et al., 2010).
NOTCH signaling is also implicated in affecting the morphology of mature neurons. Namely, up-regulation of NOTCH leads to increased dendritic complexity of hippocampal neurons within the DG, whereas Notch1 knockout mice display reduced dendritic arborization (Breunig et al., 2007). Altered dendritic arborization is also a common morphological feature of ASD and can be observed among FXS and RTT mouse models, mice carrying a Pten knockdown, and BTBR mice (Cheng et al., 2017; Martinez-Cerdeno, 2017). Although NOTCH is well studied in the context of adult and even more in embryonic neurogenesis, less is known about the impact of NOTCH on ASD. However, some findings strongly link NOTCH signaling to the pathology of ASD. Prenatal administration of VPA results in autistic-like behavior and increased NOTCH signaling in offspring is accompanied by elevated spine density in the prefrontal cortex. VPA treatment of rats, together with the NOTCH inhibitor DAPT, was shown to alleviate these phenotypes, indicating a central role of NOTCH signaling in the VPA autism model (Zhang, Xiang, et al., 2019). Further evidence for direct impact of NOTCH signaling on ASD pathology involves MECP2, a major player in RTT. The precise control of the phosphorylation state of MECP2 in neurons is necessary for normal development and function of the brain. In NPCs from the mouse HC, a crucial role for the phosphorylation at serine 421 on MECP2 was observed. (Li et al., 2014). It was shown that this MECP2 phosphorylation might directly regulate transcription of NOTCH ligand and receptors and thereby affect NPC proliferation and differentiation.
2.2.5 Sonic Hedgehog signaling
Sonic hedgehog (SHH) is the best-studied member of the Hedgehog family in mammals. In brief, SHH binding to the membrane-bound receptor PATCHED leads to less suppression of the transmembrane protein Smoothened (SMO). Following the activation of SMO, a downstream pathway is activated, resulting in the expression of target genes, which are implicated in the control of cell proliferation, survival, and self-renewal (Chen et al., 2018).
Indeed, it could be shown that Nestin-Cre-dependent conditional knockout of Smo, which is normally activated upon SHH signaling, results in reduced number of proliferating cells post-natally (Machold et al., 2003). However, mutant mice display normal brain size. These findings strengthen the hypothesis that SHH is involved in maintaining the stem cell population (Machold et al., 2003; Gage et al., 2008). Further studies on SHH showed that application of SHH increased the proliferation of NSCs (Palma et al., 2005). Comparable findings were also made within the stem cell niche of the HC, unmasking SHH as an important player for hippocampal progenitor proliferation (Lai et al., 2003; Machold et al., 2003).
Having a certain impact on the development of the CNS, SHH might also be an interesting factor involved in ASD. Namely, significantly increased levels of SHH were reported in individuals with ASD (Al-Ayadhi, 2012). Interestingly, up-regulation of SHH results in the increased expression of BDNF, which was shown to protect neurons against oxidative stress. In this context, the activation of SHH via up-regulation of BDNF can contribute to neuroprotection. This might be an important mechanism in ASD, since individuals suffering from ASD display increased peroxidation of lipids, which can be directly linked to oxidative stress (Chauhan & Chauhan, 2006; Chauhan et al., 2004). Furthermore, embryonic VPA exposure leads to decreased Shh mRNA expression. In vitro studies could demonstrate that VPA causes a significant decrease of serotonin expression, an effect which could be alleviated by exogenous SHH application (Miyazaki et al., 2005). These findings implicate SHH as a possible target to attenuate the neurotoxic effect of VPA.
2.2.6 Fractones
Mercier and colleagues gave the first description of peculiar structures located in the proximity of the lateral ventricles and named them fractones, after their morphology, which resembles that of fractals. Fractones are laminin-positive, continuous projections of the perivascular basal lamina, which begin in correspondence with perivascular macrophages and extend throughout the subependymal layer, terminating with bulbous terminals at the level of the ependyma.
The several branches detectable throughout the fractone establish contacts with different cell types, such as astrocytes, microglia, ependymocytes, and NSCs, which led to the hypothesis that fractones could act as regulatory milieu for neurogenesis (Mercier et al., 2002). In adult mice, mitotic active cells (BrdU+) co-localize with laminin and N-sulfated heparan sulfate proteoglycan (NS-HPSG), which shows specific binding for growth factors (Douet et al., 2013; Kerever et al., 2007). Interestingly, NS-HPSG was highly detected and associated with cell proliferation in the OB, the rostral migratory stream, the SVZ, and the subcallosal zone, but only weakly in the subgranular layer of the DG (Mercier & Arikawa-Hirasawa, 2012). Age-related reduction of neurogenesis is a typical feature both in mice (Daynac et al., 2016) and in humans (Mathews et al., 2017). Age-related decrease in number and increase in size of fractones were observed (Kerever et al., 2015), pointing to their involvement in the regulation of AN. More recent data (Yamada et al., 2017) proved age-related modifications of 6-O-sulfation, a biochemical modification crucial for the signaling of fibroblast growth factor-2, associated with fractones. Further debate on the topic of fractones (Sato et al., 2019) goes beyond the scopes of this review.
Structural alterations of fractones were extensively investigated in the BTBR model. Together with anatomical abnormalities (e.g., absence of the corpus callosum, reduction of lateral ventricle volume, enlarged arteries, and a continuous falx cerebri), reduction of size and density of fractones in the SVZ and third ventricle, and of the SVZ itself, were observed (Blanchard et al., 2012; Mercier et al., 2011; Meyza et al., 2012). A shrinkage of NS-HPSG associated with fractones in the SVZ, compared to wild-type mice, might possibly explain the alterations of callosal development in BTBR mice (Mercier et al. 2011; Meyza et al., 2012), since NS-HPSG is involved in the development of commissural fibers (Conway et al., 2011; Inatani et al., 2003). In another study using the Cre-Lox approach (Irie et al., 2012), the gene coding for an enzyme that enables the elongation of Heparan sulfate in post-natal neurons was selectively inactivated. On a cytoarchitectonic level, no alterations were found. On a behavioral one, the mice developed impaired social interactions and communication and increased repetitive traits. Following the separation–reunion test, the expression of the immediate early gene c-Fos was significantly reduced both in the medial and basolateral amygdala. On an electrophysiological level, the pyramidal neurons of the basolateral amygdala also showed reduced excitatory transmission. Finally, anxiety behavior, measured in the elevated plus maze, open-field, and light–dark tests, was significantly reduced in the knockout mice and might be acknowledged as an index of amygdalar dysfunction. Considering the extensive body of evidence indicating the importance of fractones and associated NS-HPSG in the maintenance of AN, it would be of greatest interest to investigate the general role of the extracellular matrix and, in particular, fractones in other ASD models, as it could reveal itself as a relevant convergence underlying different genetic and nongenetic causes of ASD.
3 EXISTING AND PUTATIVE THERAPEUTIC APPROACHES FOR ASD ASSOCIATION WITH AN
The following paragraphs deal with existing and putative therapeutic approaches targeting AN in order to improve ASD features in humans. Figure 2 visualizes and summarizes such approaches.
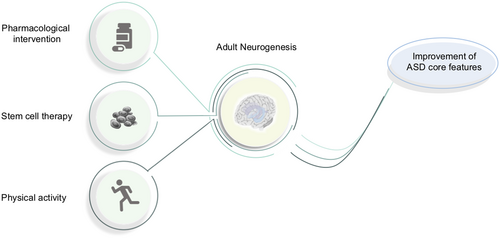
3.1 Physical activity
Evidence of the impact of physical activity on ASD patients is growing. For a comprehensive overview, we suggest Reinders et al. (2019). With regard to rodent studies addressing this correlation, it was already shown that running could restore AN in some ASD models (paragraph “Valproic acid”: Juliandi et al., 2015; paragraph “BTBR”: Karvat & Kimchi, 2012) via IGF signaling (paragraph “IGF signaling”: Carro et al., 2000; Ding et al., 2006; van Praag et al., 2005; Schwarz et al., 1996; Trejo et al., 2001). Due to its relatively easy implementation and the associated avoidance of any pharmacological treatment, the translational approach in this context is of high interest. Following this approach, it has already been shown that children with a diagnosis of ASD exposed to various sports, such as karate (Bahrami et al., 2016), mixed-martial arts (Phung & Goldberg, 2019), and table tennis (Pan et al., 2017), experienced improvement in executive functions, speech, and anxiety. But the question remains, if the physical activity had an impact on neurogenesis. Nevertheless, based on preclinical observations made in children, it would be of huge interest to test the impact of physical activity also in adult patients affected by ASD.
3.2 Pharmacological modulation
3.2.1 IGF-1
As described above, administration of both IGF-1 and a synthetic analogue ameliorates synaptic functionality both in the Shank3ex4−9 mouse model (paragraph “IGF signaling”: Bozdagi et al., 2013), and in a model of RTT (paragraph “IGF signaling”: Tropea et al., 2009). Among the several molecular pathways considered in this review, IGF-1 signaling was the one predominantly targeted in clinical trials so far. Interestingly, the concentration of IGF-1, but not IGF-2, in the cerebrospinal fluid of autistic children was significantly lower than in controls (Riikonen et al., 2006). In a pilot study, IGF-1 was applied to children with Phelan–McDermid syndrome. Compared to placebo, IGF-1-treated children experienced an improvement in both social impairment and restrictive behaviors (Kolevzon et al., 2014). In various studies, the treatment with IGF-1 was applied to patients affected by RTT, but the results are not unequivocal. A single case study proved the efficacy and tolerability of Mecasermin (human recombinant IGF-1) (Pini et al., 2014). The application of the same drug to 10 patients (n = 10) was again successful in rescuing social and cognitive symptoms (Pini et al., 2016), anxiety, and electroencephalographic anomalies (Khwaja et al., 2014). Contrary to these results, Mecasermin did not reveal any significant improvement in a placebo-controlled, double-blinded clinical trial with a larger number of patients (n = 30) (O'Leary et al., 2018).
NNZ-2556 (also known as Trofinetide), an analogue of IGF-1 effective on the FMR1 model (paragraph “IGF signaling”: Deacon et al., 2015), was also tried in human patients. Adolescent and adult male patients affected by FXS experienced benefits from the treatment (Berry-Kravis et al., 2020). Both in adolescent/adult (Glaze et al., 2017) and children/adolescent (Glaze et al., 2019), female patients affected by RTT, NZZ-2556 intervention was safe and clinically relevant compared to placebo. As in the case of physical activity, modulating IGF signaling in adult ASD proved effective in improving the clinical conditions and will deserve further research.
3.2.2 BDNF
Due to its neuroprotective and neuroregenerative properties, BDNF is an attractive therapeutic tool. Effects of BDNF administration were mostly investigated in neurodegenerative diseases, such as amyotrophic lateral sclerosis, Parkinson's, and Alzheimer's. Although treatments were mostly proven to be safe, there was unfortunately not a lot of success in achieving significant efficacy (Ochs et al., 2000). Attempts to administer recombinant BDNF as a therapy have encountered several methodological limitations. One of the main problems for translating BDNF-based therapies into the clinics include delivery to the brain and the short half-life of the recombinant protein. Taking that into consideration, gene therapy is the next strategy for enhancing BDNF expression in CNS. In this context, the first clinical trial to assess adeno-associated virus serotype 2-BDNF delivery to Alzheimer's patients has just started beginning of this year. This approach was already validated in previous clinical trials, where nerve growth factor was used instead of BDNF (Castle et al., 2020) and is based on extensive research in rodents and monkeys (Bishop et al., 2008; Hadaczek et al., 2006).
Nevertheless, physical exercise has been shown to increase BDNF levels, and exercise was employed in many therapeutic approaches, especially with regard to older populations (Hoffmann et al., 2016; Jamali et al., 2020; Ledreux et al., 2019; Veronese et al., 2019). Intriguingly, the down-regulation of BDNF, together with anti-apoptotic signaling, is one of the hallmarks of ASD (Das, 2013; Sheikh et al., 2010). These findings suggest that ASD patients could benefit from elevated BDNF levels, induced either by physical activity and coordinative exercise or by external administration.
3.2.3 GSK-3 beta
We have discussed previously inhibitors of GSK-3 beta as modulators of AN in the context of ASD (paragraph “FMR1/Fragile X”: Guo et al., 2012; Luo et al., 2010; paragraph “PTEN deletion”: Franklin et al., 2014; Mines et al., 2010). A recent clinical trial revealed the clinical safety, tolerability, and efficacy of Tideglusib, an orally administered GSK-3 beta inhibitor, in adolescents with ASD (Anagnostou & Thorpe, 2018). Lithium acts, among several mechanisms, as an inhibitor of GSK-3 beta and is used routinely for the therapy of bipolar disorder. Interestingly, lithium enhances neurogenesis in the SGZ (Chen et al., 2000) and accumulates in neurogenic zones (Zanni et al., 2017).
Several case reports in adult patients affected by Phelan–McDermid syndrome and treated with lithium highlighted improving effects of the clinical outcomes (Egger et al., 2017; Serret et al., 2015). Furthermore, lithium proved also efficacious in children and young adults who had received a diagnosis for ASD (Mintz & Hollenberg, 2019; Siegel et al., 2014).
3.2.4 CBD and CBDV
As previously discussed, CBD administration was linked to enhanced cell survival and development toward the neuronal lineage (paragraph “IGF signaling”: Prenderville et al., 2015) and CBDV was used effectively to treat ASD symptoms acting on AN (paragraph “Valproic acid”: Zamberletti, Gabaglio, Woolley-Roberts, et al., 2019; paragraph “IGF signaling”: Zamberletti, Gabaglio, Piscitelli, et al., 2019). CBD and CBDV have also been administered to ASD patients. In a recent study, it was shown by functional magnetic resonance imaging that CBD significantly alters functional connectivity between the cerebellar vermis and the striatum, two regions typically affected in ASD (Pretzsch, Voinescu, Mendez, et al., 2019). A retrospective analysis on 60 children with ASD treated with CBD-rich cannabis revealed both tolerability and efficacy of the drug. Indeed, following treatment, behavioral outbreaks were much reduced (Aran et al., 2019). A randomized placebo-controlled double-blind trial using two mixtures of CBD and ∆9-tetrahydrocannabinol at different ratios on more than 150 participants aged 5–21 confirmed that CBD is well tolerated. A co-primary outcome (disruptive behavior) and a secondary one (social responsiveness score) were both improved (Aran et al., 2021), even though pre-clinical studies showed that ∆9-tetrahydrocannabinol does not affect AN (Wolf et al., 2010).
CBDV was tested on humans in only one study thus far. It was shown by magnetic resonance spectroscopy that the drug can modify the excitation/inhibition ratio in males with ASD (Pretzsch, Voinescu, Lythgoe, et al., 2019). Several cohorts are currently being recruited for testing of CBD and CBDV in ASD patients.
3.2.5 Stem cell therapy
Research on stem cells and adult neurogenesis has contributed to the development of stem cell therapies. Stem cells possess unique properties of self-renewal and high proliferative capacity, multi-differentiation, and paracrine actions. These paracrine effects might play a major role in stem cell therapy, given they secrete a number of factors which are, among other regenerative processes, crucial for neurogenesis; they include BDNF, IGF-1, and fibroblast growth factor (Baraniak & McDevitt, 2010).
Stem cell therapies have been tested in clinical trials for various diseases, including neurodegenerative diseases, such as Alzheimer's and Parkinson's, but also stroke, spinal cord injuries, and heart disease. The potential for these cell therapies has also been trialed in ASD patients, mainly in children. One of the first studies in this context was conducted in 2013 using autologous bone marrow mononuclear cells, in which 96% patients showed global improvements, including behavioral patterns (66%), social relationships (90.6%), speech, language, and communication (78%) (Sharma et al., 2013). In another study, children with ASD treated with combined transplantation of human umbilical cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells showed significant differences in nonverbal communication and visual, emotional, and intellectual responses (Lv et al., 2013). Both studies demonstrated the safety of the treatment. Some of the later studies were less successful in showing amelioration of symptoms. However, it should be pointed out that in these studies, stem cells or umbilical cord blood were applied intravenously (Chez et al., 2018; Dawson et al., 2020; Riordan et al., 2019). This could be one of the reasons for lower efficacy—as intravenous applications were shown to be less efficient compared to intrathecal (Petrou et al., 2020). Another recent study in which autologous bone marrow mononuclear cells were applied intrathecally demonstrated improvements within 18 months after transplantation in social communication, language, and daily skills, and a remarkable decrease in repetitive behaviors and hyperactivity.
Although there is still space for improvement and the need for standardization regarding appropriate controls, optimal dosage, application means, and the choice of cells, especially in order to compare across studies and their outcomes, the already available therapies exhibit beneficial properties.
So far, stem cell treatment has been applied mainly in children. It would now be of interest to use these therapies also in adults. In one study, a 25-year-old ASD patient received intrathecal autologous bone marrow-derived mononuclear cells and, following 6 months of therapy, the patient showed improvements in concentration, attention, command following, sitting tolerance, social interactions, eye contact, and memory.
Indeed, when discussing clinical applications of stem cells, ethical, legal, and safety issues must be considered. Due to major ethical concerns, the generation and use of human embryonic stem cells (hESCs) for research purposes are mostly prohibited or strictly regulated. In Germany, the Embryo Protection Act prohibits the derivation of hESCs, but under strict and controlled conditions, import and use of embryonic cell lines are possible. On the contrary, researchers in other countries, such as UK, Belgium and Spain, for example, are given more opportunities to work with hESCs. However, other types of stem cells, such as induced pluripotent or mesenchymal stem cells, are considered morally superior, are easier to apply and have shown promising results in clinical applications. Nevertheless, safety issues, such as teratoma formations, unwanted differentiation, potential to promote tumor growth, and metastasis, remain under debate. Thus, further development of differentiation protocols and long-term monitoring of stem cell-treated animal models are needed to optimize and improve stem cell therapies (Vasic et al., 2019; Volarevic et al., 2018).
4 CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Researching ASD and AN pose, using Plutarch's words, “a difficult problem, which gives investigators much trouble.” A great variety of causal factors contributes to the development of the cardinal manifestations of ASD. Some of these factors influence also the formation of neurons in adult age to a greater or lesser extent. Furthermore, mutations of key molecules in the pathways regulating AN appear to lead to symptoms linked to ASD. To cut a long story short, there is accumulating evidence that impaired AN may underlie some of the abnormal behavioral phenotypes seen in ASD, but only little is known about its potential significance in this context. Interventions to regulate neurogenesis and thereby neuroplasticity in adults are, therefore, being evaluated as possible therapeutic strategies.
Indeed, despite the classification of ASD as a neurodevelopmental disorder, it poses a lifelong challenge for both patients and caretakers (Mukaetova-Ladinska et al., 2012; Perkins & Berkman, 2012; Piven et al., 2011). Throughout this review, we have discussed a significant number of studies addressing AN alteration in established ASD models. Interestingly, some ASD-like behavioral traits were partially or completely rescued after intervention on neurogenesis in adult stages (paragraphs “Valproic acid”: Go et al., 2012; Gobshtis et al., 2017; Juliandi et al., 2015, “FMR1/Fragile X”: Guo et al., 2012; Li et al., 2016; Luo et al., 2010, “BTBR”: Zhang, Cai, et al., 2019, “22q11.2 deletion/DiGeorge syndrome”: Ouchi et al., 2013). Moreover, molecular pathways typically related to the regulation and maintenance of the neurogenic niche appear to be affected in a number of ASD models. Hence, reduction of BDNF is registered in the HC of the BTBR model (paragraph “BDNF signaling”: Stephenson et al., 2011) and impairs spatial learning in the Fmr1 model (paragraph “BDNF signaling”: Uutela et al., 2012). Furthermore, GSK-3 beta-WNT-β-catenin signaling is altered in the Fmr1, VPA and Pten models (paragraphs “FMR1/Fragile X”: Guo et al., 2012; Luo et al., 2010, “Valproic acid”: Juliandi et al., 2015, “PTEN deletion”: Amiri et al., 2012) and is regulated by CHD8, whose mutations lead to ASD symptoms (paragraph “WNT signaling”: Katayama et al., 2016; Platt et al., 2017). Additionally, inhibition of the NOTCH pathway in the VPA model has proved effective in rescuing behavior and spine morphology (paragraph “NOTCH signaling”: Zhang, Xiang, et al., 2019) and stimulation of SHH signaling in the VPA model (paragraph “Sonic Hedgehog signaling”: Miyazaki et al., 2005) influenced the morphogenesis of serotonergic neurons both in vivo and in vitro. These representative examples portray the strong interconnections of the molecular pathways related to AN and ASD, but a broader characterization is necessary in order to find possible convergencies/divergencies that could, for instance, explain similar ASD-like symptoms in front of different etiologies. Understanding how much the two phenomena are interdependent, and whether a causal nexus from one to the other exists, will be a crucial objective to pursue in the next years. The following questions are thus, to our perspective, of high interest.
4.1 To what extent can rodent AN be correlated to humans and which role does SVZ/OB neurogenesis play in ASD patients?
Research on AN in ASD has focused mainly on the SGZ of the HC. The rodent SVZ though represents a much larger reserve of adult NSCs, giving rise to neuroblasts, which subsequently migrate via the rostral migratory stream, and eventually reach the OB and become functional neurons involved in olfactory processes (Curtis et al., 2007, 2012). So, what role (if at all) does olfaction play in ASD? Altered olfaction is a clinical hallmark of ASD (Rozenkrantz et al., 2015) and is also found in several other neurological and psychiatric disorders, such as Alzheimer's, Parkinson's (Ubeda-Banon et al., 2020), Huntington's disease, schizophrenia, depression (Negoias et al., 2010), and alcohol abuse (Rupp et al., 2004). Although there is growing evidence that odor processing in ASD shows differential patterns (Sweigert et al., 2020), additional investigation is needed to understand the correlation between ASD symptoms and olfactory dysfunction. Thus, it still remains unclear if olfaction can be regarded as a result of defective neurogenesis in ASD and what role, if any, does it play in its pathogenesis.
Nasal biopsies in patients affected by RTT showed a reduced number of olfactory receptor neurons and an increased number of immature cells, pointing to either a decreased survival of immature differentiated neurons or failure in maturation (Ronnett et al., 2003). Alterations in olfactory circuits were already described above in models for RTT (paragraph “Rett syndrome (RTT): Degano et al., 2014; Martínez-Rodríguez et al., 2019; Sun et al., 2019)”. While approaching other models of ASD, it would be advisable to deepen these aspects.
In humans, AN in the OB seems to play a minor role, if any at all, probably due to the rudimental development of this sense compared to other mammals, such as rodents (Bergmann et al., 2012). The neural precursor cells in the human SVZ are preferentially involved in the new formation of neurons, interneurons, and oligodendroglia in the striatum (Ernst et al., 2014; Inta et al., 2015). A recent paper (Kuo & Liu, 2020) reported an altered microarchitecture of the striatum in ASD patients. In addition, dysfunction of striatal circuits has already been identified as a common hub in ASD mouse models (Fuccillo, 2016). A detailed neuroanatomical understanding of striatal neurogenesis in ASD patients is still lacking and might shed light on the complex genetic etiology of the observed disturbances.
4.2 Do deficits in AN cause ASD symptoms or is ASD leading to altered AN?
In order to break this apparent stalemate, it is decisive to take into consideration all the possible factors concurring to the pathogenesis of ASD. By doing so, it will be possible to understand the role of AN in this complex disorder.
According to the theory of the tetrapartite synapse (Dityatev & Rusakov, 2011), four factors determine its proper physiology: the pre- and post-synaptic elements, glial cells, and the extracellular matrix. Using knockout models for specific pre- and post-synaptic proteins will help to further elucidate the consequences on ASD and AN in the absence of selected molecules. Since we have found only a limited number of papers including both aspects, experimental designs should include the investigation of AN on the microscopic level without overlooking the behavioral characterization. Furthermore, the role of glia, in particular of astrocytes, was highlighted as a peculiarity of altered neurogenesis in the Fmr1, SHANK3, VPA, and Pten models (paragraph “FMR1/Fragile X”: Eadie et al., 2009; Guo et al., 2011; Li et al., 2016; Luo et al., 2010; paragraph “SHANK3/Phelan-McDermid syndrome: Zhao et al., 2017; paragraph “Valproic acid”: Zhao et al., 2019; paragraph “PTEN deletion”: Kang et al., 2020). We therefore reckon that future analysis of AN in ASD models should include an overview of glial populations. Last but not least, the role of the extracellular matrix should be more thoroughly investigated as it might represent a converging point between the pathogenesis of disrupted AN and ASD. The relevance of an intact fractone niche (see above the works of Mercier:Douet et al., 2012; Douet et al., 2013; Kerever et al., 2007; Kerever et al., 2015; Mercier & Arikawa-Hirasawa, 2012; Mercier et al. 2011; Mercier & Douet, 2014; Mercier et al., 2002; paragraph “Fractones”), the implication of perineuronal nets in several mental disorders (for an overview on this topic, please refer to Pantazopoulos & Berretta, 2016), and the involvement of contact molecules like Neuroligins and Neurexins (Südhof, 2008), Ephrins (Wurzman et al., 2015), and Semaphorins (Alto & Terman, 2017) in ASD highlight the importance of an intact communication between the cells with the surrounding environment—meant as both extracellular matrix and neighboring cells—for proper functioning. Investigating these topics in more detail might lead to a better understanding of both ASD and AN.
ACKNOWLEDGMENTS
MJS was supported by the Care for Rare Foundation, the Eva Luise and Horst Köhler Foundation, the Else Kröner Fresenius Foundation (2018_A78), the Volkswagen Foundation (“Experiment” grant 95 235), the BMBF (GeNeRARe; 01GM1519A), and the DFG (CRC1080, B10).
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors would further like to thank Dr. Renée Hartig for commenting on the manuscript and Elisabeth Koster for supporting the creation of the figures.
AUTHOR CONTRIBUTION
MJS and FB designed the manuscript in accordance with all other authors. FB, LN, JM, and VV carried out the literature screening and drafted both text and figures. MJS critically revised and approved the manuscript. All authors agreed to the final version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.