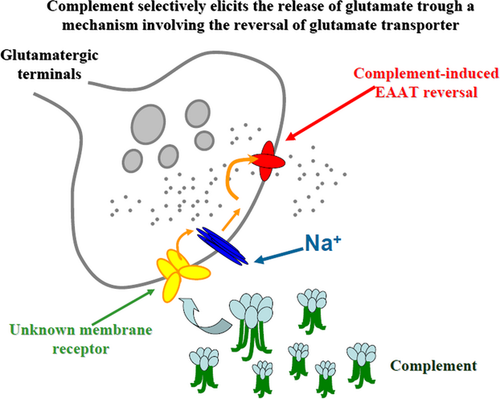
Complement selectively elicits glutamate release from nerve endings in different regions of mammal central nervous system
Abstract
Our study was aimed at investigating whether complement, a complex of soluble and membrane-associated serum proteins, could, in addition to its well-documented post-synaptic activity, also pre-synaptically affect the release of classic neurotransmitters in central nervous system (CNS). Complement (dilution 1 : 10 to 1 : 10000) elicited the release of preloaded [3H]-d-aspartate ([3H]d-ASP) and endogenous glutamate from mouse cortical synaptosomes in a dilution-dependent manner. It also evoked [3H]d-ASP release from mouse hippocampal, cerebellar, and spinal cord synaptosomes, as well as from rat and human cortical nerve endings, but left unaltered the release of GABA, [3H]noradrenaline or [3H]acetylcholine. Lowering external Na+ (from 140 to 40 mM) or Ca2+ (from 1.2 to 0.1 mM) ions prevented the 1 : 300 complement-evoked [3H]d-ASP release from mouse cortical synaptosomes. Complement-induced releasing effect was unaltered in synaptosomes entrapped with the Ca2+ ions chelator 1,2-bis-(2-aminophenoxy) ethane-N,N,N’,N’, tetra-acetic acid or with pertussis toxin. Nifedipine,/ω-conotoxin GVIA/ω-conotoxin MVIIC mixture as well as the vesicular ATPase blocker bafilomycin A1 were also inefficacious. The excitatory amino acid transporter blocker DL-threo-ß-benzyloxyaspartic acid, on the contrary, reduced the complement-evoked releasing effect in a concentration-dependent manner. We concluded that complement-induced releasing activity is restricted to glutamatergic nerve endings, where it was accounted for by carrier-mediated release. Our observations afford new insights into the molecular events accounting for immune and CNS crosstalk.
We investigated whether complement, a complex of soluble and membrane-associated serum proteins, could pre-synaptically affect the release of classic neurotransmitters in the central nervous system (CNS). Our data provide evidence that complement-induced releasing activity is restricted to glutamatergic nerve endings, where it was accounted for by carrier-mediated release. Our observations add new insights to the knowledge of the molecular events accounting for immune and CNS crosstalk. EAAT = excitatory amino acid transporter.
Abbreviations used
-
- [3H]Ch
-
- [3H]choline
-
- [3H]d-ASP
-
- [3H]-daspartate
-
- [3H]NA
-
- [3H]noradrenaline
-
- 3,5-DHPG
-
- (RS)-3,5-Dihydroxyphenylglycine
-
- Ach
-
- acetylcholine
-
- AMPA
-
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
-
- AOAA
-
- amino oxiacetic acid
-
- BAPTA
-
- 1,2-bis-(2-aminophenoxy) ethane-N,N,N’,N’, tetra-acetic acid
-
- CNS
-
- central nervous system
-
- DL-TBOA
-
- DL-threo-ß-benzyloxyaspartic acid
-
- EAAT
-
- excitatory amino acid transporter
-
- EGTA
-
- glycol-bis(2-aminomethylether)-N,N,N’,N’-tetra-acetic acid
-
- f.c.
-
- final concentration
-
- GABA
-
- gamma-aminobutyric acid
-
- GBR 12909
-
- 1-(2-(Bis-(4-fluorophenyl)methoxy)ethyl)-4-(3-phenylpropyl)piperazine dihydrochloride
-
- PTx
-
- pertussis toxin
-
- Tris
-
- tris-(hydroxymethyl)-amino methane
-
- VSCC
-
- voltage-sensitive calcium channel
Complement is a complex of soluble and membrane-associated serum proteins that is expressed in mammals throughout the body, including the CNS (Levistrauss and Mallat 1987; Speth et al. 2002; Stevens et al. 2007; Woodruff et al. 2010). Upon activation by soluble factors, complement plays a key role in controlling the innate immune system, participating to the clearance of pathogens and apoptotic cells (Frank and Fries 1991; Botto et al. 1998; Duvall et al. 2010).
Beside these classic actions, recent observations evidentiate the role of complement as modulator of non-immunological events including synaptic plasticity and neurodevelopment in the CNS (Rus et al. 2006; Alexander et al. 2008; Boulanger 2009; Shinjyo et al. 2009; Forgeaud and Boulanger 2010). In particular, complement was shown to accumulate by axonal transport in the pre-synaptic component of active synapses from which it could be actively released (Hirai et al. 2005; Forgeaud and Boulanger 2007). Once in the synaptic cleft, complement could be tethered by membrane receptors (i.e., the pentraxin receptors, Xu et al. 2003) to exert synaptic refinement action(s) post-synaptically (Stevens et al. 2007). These post-synaptic effects have been proposed to have a role in remodeling synaptic structures during development as well as in neurodegenerative processes (Forgeaud and Boulanger 2007). Inasmuch, previous studies have shown that complement can modulate the excitatory transmission either by controlling glutamate uptake capability (Persson et al. 2009), or by favoring the clustering of glutamate receptors [i.e., α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subtypes] at synaptic plasma membranes (Sia et al. 2007; Perry and O'Connor 2008 and references therein). Interestingly, complement synaptic storage was found to be largely increased in pathologic conditions, i.e., in central ischemia (Arumugam et al. 2004; Rahpeymai et al. 2006; Woodruff et al. 2006), which is consistent with the hypothesis that this complex has a role in excitotoxicity and neurodegenerative processes (Barnum 1999; Gasque 2004; Lucas et al. 2006; Rus et al. 2006). Given these observations, we questioned whether, beside the so far documented post-synaptic activities, complement also could act pre-synaptically, i.e., by influencing the releasing capability of nerve terminals. To test this hypothesis, we investigated whether the complement could affect the release of classic neurotransmitters from isolated nerve terminals (referred here as synaptosomes) freshly prepared from different CNS regions of adult mice. Our results demonstrate that complement acts at glutamatergic terminals, eliciting glutamate outflow through a mechanism that relies on membrane depolarization and glutamate transporter inversion. We propose that complement-evoked releasing effect is selectively restricted to glutamatergic axon terminals since GABA, noradrenaline, and acetylcholine releases were unmodified upon exposure to complement. Our data also suggest that this event is a generalized and widespread phenomenon in mammals, as it could be observed in nerve terminals originating from different regions of CNS (namely, the cortex, the hippocampus, the cerebellum, and the spinal cord) and it could be replicated in both rat and human cortical nerve terminals. These findings may improve our understanding of the functional crosstalk between neuronal and immune systems and could add new insights to the role of complement in excitotoxicity in the CNS of mammals.
Materials and methods
Mouse and rat brain tissue samples
Adult male rats (Sprague–Dawley, 200–250 g) and mice (C57BL/6, 20–25 g) were obtained from Charles River (Calco, Italy) and were housed in the animal facility of Department of Pharmacy, Section of Pharmacology and Toxicology (authorization n. 484 of 2004, June, 8th). The animals were housed at constant temperature (22 ± 1°C) and relative humidity (50%) under a regular light–dark schedule (light 7.00 am–7.00 pm). Food and water were freely available. The animals were killed by decapitation and the cortex, the spinal cord, the cerebellum, and the hippocampus were rapidly removed. Purified isolated nerve endings (synaptosomes) were prepared within minutes. All the experimental procedures described here were in accordance with the European legislation (European Communities Council Directive of 24 November 1986, 86/609/EEC) and the ARRIVE guidelines, and they were approved by the Italian Ministry of Health (protocol number n. 50/2011-B and 23808). Experiments were performed following the Guidelines for Animal Care and Use of the National Institutes of Health.
Human brain tissue samples
Samples of human cerebral cortex were obtained from informed and consenting patients undergoing neurosurgery to reach deeply seated tumors. The samples represented parts of frontal, parietal, and temporal lobes obtained from three women and five men (ages 27–58 years, for pre-medication and anesthesia details see Musante et al. 2008). Immediately after removal, the tissues were placed in a physiological salt solution at 2–4°C and purified synaptosomal fractions were prepared within 30 min. The protocol was approved by the local ethical committee.
Preparation of synaptosomes
Purified synaptosomes were prepared according to Dunkley et al. (1986), with some modifications (see Di Prisco et al. 2012). In a set of experiments, the tissue was homogenized in buffered sucrose containing 1 mM 1,2-bis-(2-aminophenoxy) ethane-N,N,N’,N’, tetra-acetic acid (BAPTA) or 5 nM pertussis toxin (PTx), to entrap these agents into subsequently isolated synaptosomes, (Raiteri et al. 2000; Musante et al. 2008). The synaptosomal pellets were resuspended in a physiological solution having the following composition (mM): NaCl, 140; KCl, 3; MgSO4, 1.2; CaCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 5; HEPES, 10; glucose, 10; pH 7.2-7.4. Synaptosomal protein contents were determined according to Bradford (1976).
Release experiments
Human, mouse, or rat synaptosomes were labeled with [3H]d-aspartate ([3H]d-ASP, cortical synaptosomes, f.c.: 50 nM; spinal cord synaptosomes, f.c.: 20 nM; cerebellar synaptosomes, f.c.: 50 nM; hippocampal synaptosomes, f.c.: 50 nM), with [3H]choline (cortical synaptosomes, f.c.: 23 nM; spinal cord synaptosomes, f.c.: 46 nM; cerebellar synaptosomes, f.c.: 46 nM; hippocampal synaptosomes, f.c.: 46 nM), with [3H]noradrenaline ([3H]NA, cortical synaptosomes, f.c.: 52 nM; spinal cord synaptosomes, f.c.: 52 nM; cerebellar synaptosomes, f.c.: 52 nM; hippocampal synaptosomes, f.c.: 52 nM), or with [3H]GABA, cortical synaptosomes, f.c.: 34 nM; spinal cord synaptosomes, f.c.: 34 nM; cerebellar synaptosomes, f.c.: 34 nM; hippocampal synaptosomes, f.c.: 34 nM). In other experiments, when the release of endogenous aspartate, glutamate, or GABA had to be monitored, synaptosomes were incubated without radioactive tracers. Incubation was performed at 37°C, for 15 min, in a rotary water bath. Incubation with [3H]NA was performed in the presence of 6-nitroquipazine (0.1 μM) and GBR 12909 (0.1 μM) to avoid false labeling with [3H]NA of serotonergic and dopaminergic nerve terminals, respectively (Grilli et al. 2009). Aminooxyacetic acid (50 mM) was added to the superfusion medium in the experiments dedicated to investigate the impact of complement on endogenous GABA or pre-loaded [3H]GABA outflow (Summa et al. 2013).
After the labeling period, identical portions of the synaptosomal suspensions were layered on microporous filters at the bottom of parallel chambers in a Superfusion System (Raiteri et al. 1974; Ugo Basile, Comerio, Varese, Italy), and maintained at 37°C. The apparatus consists of 20 identical units of superfusion, where synaptosomes are plated under moderate vacuum on microporous filters that are located on a filter holder of porous glass and then are superfused at 0.5 ml/min with a standard physiological solution for a total period of 45 min. Within each experiment, experimental condition was run in triplicate to mitigate variability.
To study the spontaneous release of neurotransmitter(s), synaptosomes were first equilibrated during 36 min of superfusion and then three consecutive 3-min fractions (termed b1–b3) were collected. Rabbit (lyophilized, Low Tox) complement or (S)AMPA was introduced at the end of the first fraction collected (basal release, b1; t = 39 min) for 90 s. In a set of experiments aimed at investigating the impact of homologous complements on mouse and rat cortical synaptosomes, mouse and rat complements substituted for rabbit complement, to analyze their capability to elicit glutamate release. In these experiments, mouse or rat synaptosomes pre-loaded with [3H]d-ASP were exposed at t = 39 min for 90 s at mouse or rat complement, respectively. When studying the Na+ dependence, the concentration of Na+ in the medium was lowered to 40 mM and NaCl was substituted with an equi-osmotic concentration of N-methyl-D-glucamine (Musante et al. 2011). When evaluating the Ca2+ dependency of the complement-induced [3H]d-ASP release, the superfusion medium was replaced, starting from t = 20 min, with a medium containing 0.1 mM Ca2+ and 500 μM EGTA. The role of external Ca2+ ions was also investigated by chelating intraterminal Ca2+ ions with entrapped BAPTA or by analyzing the impact of voltage-sensitive calcium channel (VSCC) blockers, namely, the L-type Ca2+ channel blocker nifedipine (f.c. 1 μM) and the N and P/Q type channel blockers ω-conotoxin GVIA and ω-conotoxinMVIIC (f.c. 100 nMbfor both toxins) on the rabbit complement-induced release of pre-loaded [3H]d-ASP. Nifedipine and conotoxins were added together 4 min before complement. When studying the vesicular origin of complement-evoked neurotransmitter release, synaptosomes were pre-incubated with the vesicular ATPase blocker bafilomycin A1 (Pittaluga et al. 2005), final concentration 0.1 μM, for 15 min at 37°C and then incubation was prosecuted by adding [3H]d-ASP as previously described. The possible involvement of PTx-sensitive, G protein coupled-mediated mechanism was investigated by quantifying the effect of rabbit complement in Ptx-entrapped mouse cortical nerve endings. When indicated, the glutamate transporter blocker DL-threo-ß-benzyloxyaspartic acid (DL-TBOA) was present starting from 8 min before complement stimulus.
When indicated, the effect of high KCl on the release of pre-loaded [3H]d-ASP was investigated in control, PTx or BAPTA entrapped cortical synaptosomes. In these cases, synaptosomes were transiently (90 s) exposed, at t = 39 min, to high KCl containing medium (12, NaCl substituting for an equimolar concentration of KCl) in the absence or in the presence of the mGlu1/5 receptor agonist 3,5-DHPG. Fractions were collected according to the following scheme: two 3-min fractions (basal release), one before (t = 36–39 min) and one after (t = 45–48 min) a 6-min fraction (t = 39–45 min; evoked release).
Fractions collected and superfused synaptosomes were counted for radioactivity or analyzed for the endogenous amino acid content. The amount of radioactivity released into each superfusate sample was expressed as a percentage of the total synaptosomal radioactivity content at the start of the fraction collected (fractional efflux). When studying the effect of complement on the spontaneous release of glutamate, results are expressed as percentage of increase over basal release. In this case; drug effects were evaluated from the ratio between the percentage of release in the b3 fraction (corresponding to the maximal complement-induced increase in neurotransmitter release) and that in the b1 fraction (corresponding to the neurotransmitter release immediately before stimulus). This ratio was compared with the corresponding ratio obtained under control conditions (no drug added). The efficiency of synaptosomal labeling was quantified as dpm content in the first fraction collected and amounted, respectively, to: cortical synaptosomes [3H]d-ASP = 2568 ± 141, [3H]choline = 8575 ± 906, [3H]NA = 1724 ± 285, [3H]GABA = 3529 ± 51; spinal cord synaptosomes [3H]d-ASP = 3869 ± 425, [3H]choline = 6392 ± 520, [3H]NA = 2171 ± 104, [3H]GABA = 1124 ± 64; cerebellar synaptosomes [3H]d-ASP = 2145 ± 233, [3H]choline = 4487 ± 496, [3H]NA = 1868 ± 206, [3H]GABA = 3355 ± 332; hippocampal synaptosomes [3H]d-ASP = 3402 ± 520, [3H]choline = 3043 ± 758, [3H]NA = 1320 ± 152, [3H]GABA = 2825 ± 460 (data expressed as media ± SEM from three experiments run in triplicate).
Endogenous amino acid determination
Collected fractions were analyzed for the endogenous neurotransmitter content. Endogenous glutamate, aspartate, and GABA were measured by high-pressure liquid chromatography analysis, after pre-column derivatization with o-phthalaldehyde and separation on a C18 reverse-phase chromatographic column (10 × 4.6 mm, 3 μm; at 30°C; Chrompack, Middelburg, The Netherlands) coupled with fluorimetric detection (excitation wavelength, 350 nm; emission wavelength, 450 nm, Musante et al. 2010; Nasca et al. 2013). Homoserine was used as internal standard. The amount of endogenous amino acid content into each fractions collected was expressed as picomoles of amino acid for milligram of synaptosomal proteins. The spontaneous and complement-evoked release of endogenous neurotransmitters were expressed as total amino acid content in the b1 to the b3 fractions collected. The effects of complement on the spontaneous release of endogenous transmitters were also expressed as percentage of change and were evaluated as a ratio between the total amino acid content released in the presence and in the absence of complement.
Statistical analyses
Analysis of variance was performed by anova followed by Dunnett's test or Newman–Keuls multiple comparisons test as appropriate; direct comparisons were performed by applying Student's t-test. Data were considered significant for p < 0.05 at least.
Drugs
[2,3-3H]d-aspartate ([3H]d-ASP, specific activity 10.0 Ci/mmol), [7,3H]noradrenaline, levo ([3H]NA, specific activity 38.0 Ci/mmol), [3H]choline (specific activity 85.5 Ci/mmol), [3H] gamma-aminobutyric acid ([3H]GABA, specific activity 35.0 Ci/mmol) were acquired from Perkin Elmer (Boston, MA, USA). BAPTA was from Fluka Biochemika (Milan, Italy). Bafilomycin A1, 1-(2-(Bis-(4-fluorophenyl)methoxy)ethyl)-4-(3-phenylpropyl)piperazine dihydrochloride (GBR 12909) and DL-threo-ß-benzyloxyaspartic acid (DL-TBOA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionate [(S)AMPA)], nifedipine, ω-conotoxin GVIA and ω-conotoxin MVIIC were purchased from Tocris Bioscience (Bristol, UK). (RS)-3,5-Dihydroxyphenylglycine (3,5-DHPG) was from Ascent Scientific (Weston Super-Mare, UK) pertussis toxin and O-(carboxymethyl) hydroxylamine hemihydrochloride (AOAA) was obtained from Sigma (Milan, Italy). 6-nitroquipazine maleate was donated from Duphar, Amsterdam, the Netherlands. The standard rabbit complement that was derived from a pooled normal rabbit serum (see for further details Xiong and McNamara 2002) was purchased from Cederlane (Ontario, Canada). The complement is lyophilized and can be reconstructed by resuspending it in distilled water; prior activation was not required to observe complement-induced effect. Mouse and rat complements were from fresh clotted, whole blood collected, respectively, from mixed breed, mixed sex non –Swiss albino mice, and Sprague–Dawley rats (Equitech-Bio, Kerville, Texas, USA).
Results
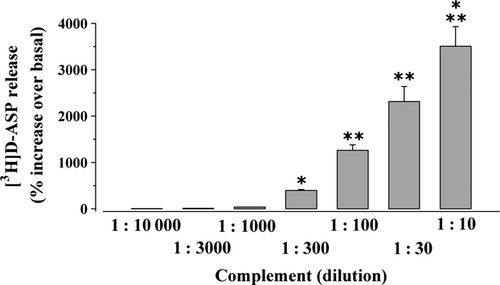
The effect of rabbit complement (on the release of glutamate from mouse cortical nerve endings) was investigated by monitoring the release of pre-loaded [3H]d-ASP, an unmetabolizable analogue of glutamate routinely used in release studies as a marker for the endogenous excitatory amino acid transmitter from isolated nerve terminals (we refer to as synaptosomes, Grilli et al. 2004; Di Prisco et al. 2012). Rabbit complement, added to the superfusion medium at increasing dilutions ranging from 1:10 to 1 : 10000, evoked in a concentration-dependent fashion the release of [3H]d-ASP from mouse cortical nerve endings (Fig. 1). Complement heat-shock denaturation significantly prevented rabbit complement-induced releasing effect (1 : 300 rabbit complement: 312.81 ± 35.86; 1 : 300 heat-shocked rabbit complement: 125.37 ± 23.12, results expressed as percent increase over basal release, p < 0.05 vs. control, n = 3 experiments run in triplicate), compatible with the idea that the integrity of all the soluble proteins involved is required to assure the releasing effect.
Whether the release of [3H]d-ASP represents a reliable measure of endogenous glutamate outflow was investigated by quantifying the impact of complement (1 : 300) on the release of endogenous glutamate from cortical synaptosomes. Results in Table 1 clearly indicate that rabbit complement also elicited a marked, significant release of this endogenous amino acid, allowing to conclude that the use of tritium is an appropriate approach to monitor glutamate release. Notably, rabbit complement also elicited, although less efficaciously, aspartate release. The possibility that aspartate and glutamate colocalize in the same nerve terminal has been a matter of debate. The present results seem compatible with the thesis that the two amino acids colocalize in the same terminals, and that the releasing stimulus drive the exit of both molecules.
| Spontaneous release(pmoles/mg protein) | Complement-induced release(1 : 300)(pmoles/mg protein) | Percent increase over spontaneous release | |
|---|---|---|---|
| Aspartate | 151.55 ± 13.38 | 239.2 ± 23.10* | 57.83 ± 11.45% |
| Glutamate | 304.16 ± 28.46 | 776.48 ± 34.11** | 155.29 ± 22.95% |
| GABA | 208.09 ± 27.06 | 216.41 ± 39.88 | 4.00 ± 2.96% |
- *p < 0.05 versus respective control, **p < 0.01 versus respective control.
- Mouse cortical synaptosomes were superfused as previously described and three 3-min fractions were collected starting from t = 36 min of superfusion (basal release). When indicated, synaptosomes were transiently (90 s) exposed to rabbit complement (dilution 1 : 300) from t = 39 min. Results are expressed as picomoles per milligram of proteins as well as percent increase over basal release. Data represent the media ± SEM of four experiments run in triplicate (three superfusion chambers of each experimental condition).
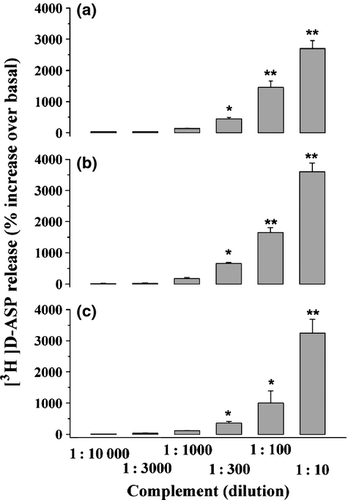
The releasing effect elicited by rabbit complement was not restricted to the cortex, but it appeared to be a widespread phenomenon that can take place in several CNS regions. In particular, Fig. 2 shows that rabbit complement (1 : 10 to 1 : 10000) elicited the release of pre-loaded [3H]d-ASP from superfused synaptosomes isolated from the hippocampus (Fig. 2a), the cerebellum (Fig. 2b), and the spinal cord (Fig. 2c) of adult mice. In all these CNS regions, rabbit complement efficiently evoked tritium outflow in a concentration-dependent manner, displaying a potency similar to that observed in the cortex. Inasmuch, the releasing effect is not species specific, as suggested by the finding that rabbit complement can evoke the release of pre-loaded [3H]d-ASP in both human (Fig. 3a) and rat cortical glutamatergic nerve endings (Fig. 3b). In particular, rabbit complement released pre-loaded [3H]d-ASP from rat cortical synaptosomes with a potency and an efficiency similar to those observed in mouse cortical nerve endings, but it was less potent and efficacious in releasing [3H]d-ASP from human cortical synaptosomes. The origin of the human cortical specimens (oncologic patients undergoing neurosurgery to removing deep-located tumors) might account for these apparent discrepancy (see the Discussion). Unexpectedly, rabbit complement cannot affect the spontaneous release of endogenous GABA (Table 1), of [3H]GABA, of [3H]NA, and of [3H]ACh (Table 2) in synaptosomal preparations isolated from all the CNS regions so far investigated (i.e., the cortex, the hippocampus, the cerebellum, and the spinal cord).
| Complement-induced neurotransmitter release (% increase over basal release) | ||||
|---|---|---|---|---|
| Cortex | Hippocampus | Cerebellum | Spinal cord | |
| [3H]Noradrenaline | 5.95 ± 1.66 | 1.37 ± 2.04 | 3.75 ± 2.90 | 1.85 ± 0.91 |
| [3H]Acetylcholine | 7.72 ± 3.34 | 6.13 ± 6.38 | 5.32 ± 4.46 | 3.39 ± 2.74 |
| [3H]GABA | 3.50 ± 1.25 | 1.71 ± 2.04 | 4.15 ± 0.52 | 4.03 ± 1.33 |
- Mouse synaptosomes isolated from different CNS areas (namely, the cortex, the hippocampus, the cerebellum, and the spinal cord) were pre-loaded with radioactive tracer and then superfused as described previously. When indicated, synaptosomes were transiently (90 s) exposed to rabbit complement (dilution 1 : 300) from t = 39 min. Three 3-min fractions were collected starting from t = 36 min of superfusion (basal release). Results are expressed as percent increase over basal release and were evaluated from the ratio between the percentage of release in the fraction where the maximum effect was observed (the third fraction collected, b3) and that in the first fraction (b1). This ratio was compared with the corresponding ratio obtained under control conditions (no drug added). Data represent the media ± SEM of four experiments run in triplicate (three superfusion chambers of each experimental condition).
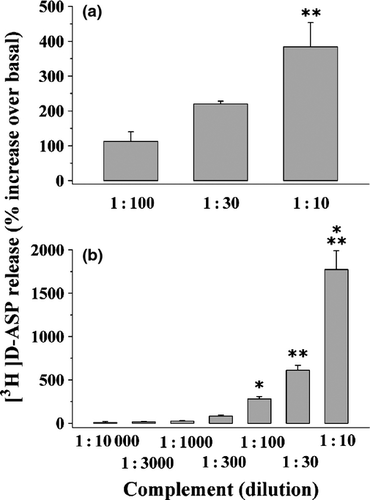
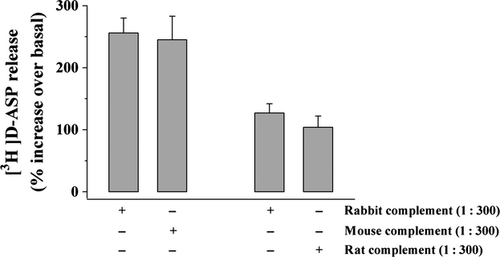
To ascertain whether cross-species reactivity could underlying the effects so far described, we investigated whether mouse and rat complements could mimic rabbit complement in controlling glutamate release from mouse and rat cortical synaptosomes, respectively. The data depicted in Fig. 4 clearly demonstrated that the release of [3H]d-ASP elicited by mouse complement (1 : 300) from mouse cortical synaptosomes was comparable to that evoked by rabbit complement in the same synaptosomal preparation. Similarly, rat complement mimicked rabbit complement in evoking the release of [3H]d-ASP from rat cortical synaptosomes.
Experiments were then performed to investigate the molecular event(s) at the basis of complement-evoked glutamate outflow. To this aim, the role of mono and divalent cations in the 1 : 300 rabbit complement-induced releasing effects was firstly investigated. Figure 5 shows that the 1 : 300 rabbit complement-induced release of [3H]d-ASP from mouse cortical nerve endings was significantly prevented (−72.48 ± 11.75, data expressed as percent inhibition of 1 : 300 complement-induced outflow) when external Na+ ions were diminished from 140 to 40 mM (Musante et al. 2011) as well as when the external concentration of Ca2+ ions in the superfusion medium was drastically reduced (from 1.2 to 0.1 mM, −61.37 ± 7.83, data expressed as percent inhibition of 1 : 300 complement-induced outflow, Fig. 5). Blocking intraterminal Ca2+ ion availability with the entrapped Ca2+ chelator BAPTA, however, did not cause significant changes to rabbit complement-induced release of tritium (Fig. 5), but it prevented the 12 mM KCl-evoked release of pre-loaded [3H]d-ASP (Fig. 6). Inasmuch, the L-type Ca2+ channel blocker nifedipine (1 μM) concomitantly added with the N- and P/Q-type channel blocker ω-conotoxin GVIA(100 nM) and ω-conotoxin MVIIC (100 nM) failed to affect the 1 : 300 rabbit complement-induced release of [3H]d-ASP from mouse cortical synaptosomes (Fig. 5). At the concentration applied, VSCC blockers inactive on their own (data not shown) were previously shown to prevent the 12 mM KCl-evoked overflow of neurotransmitters from mouse cortical synaptosomes (Gemignani et al. 2004; Satoh et al. 2011). Altogether these observations could shed some doubts on the exocytotic-like origin of the complement-evoked glutamate release. Accordingly, pre-incubation of mouse cortical synaptosomes with bafilomycin A1, a well-known selective vesicular ATPase blocker that impedes the vesicular uptake of neurotransmitter, slightly, although not significantly, altered the rabbit complement-mediated releasing effect, leading us to exclude the vesicular origin of the glutamate release so far described. Notably, at the concentration applied bafilomycin A1 almost totally prevented the 50 μM (S)AMPA-evoked exocytotic-like release of [3H]d-ASP from mouse cortical synaptosomes (Fig. 6, but see also Pittaluga et al. 2005). Bafilomycin A1-pre-treatment as well as entrapped BAPTA failed to affect on their own the spontaneous release of [3H]d-ASP from cortical synaptosomes (not shown, but see also Musante et al. 2008).

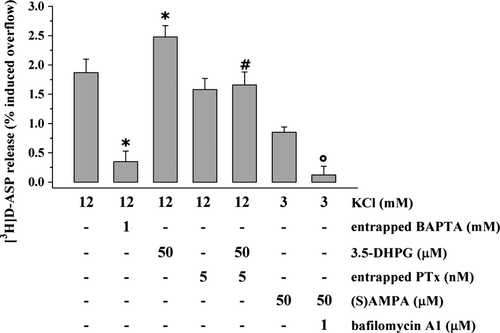
Finally, rabbit complement-induced releasing effect was unaltered in mouse cortical synaptosomes toxified with the PTx, suggesting that PTx-sensitive, G protein-coupled receptor (GPCR)–mediated events were not involved (Fig. 5). Notably, in PTx toxified synaptosomal preparation, the Group I metabotropic glutamate receptor agonist, the compound 3,5-DHPG (50 μM) failed to facilitate the 12 mM KCl-evoked glutamate exocytosis (Fig. 6, but see also Musante et al. 2008).
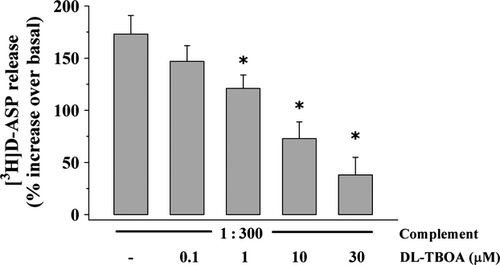
The impact of the broad glutamate transporter blocker, the compound DL-TBOA on the rabbit complement-evoked release of glutamate from mouse cortical synaptosomes was then studied. Figure 7 shows that DL-TBOA (10 μM) significantly prevented the release of tritium elicited by rabbit complement in a concentration-dependent manner. Notably, at the higher concentration applied (30 μM), DL-TBOA failed to affect on its own the spontaneous release of pre-loaded [3H]d-ASP (not shown).
Discussion
The first striking observation of the present work is that rabbit complement (from here on we refer to as complement) alone selectively elicits the release of glutamate from superfused nerve terminals isolated from distinct regions of mammal CNS. Our findings represent therefore a clear demonstration of a pre-synaptic complement-mediated control of central neurochemical transmission, also unveiling an unexpected new mechanism of interaction between the immune and the CNS.
We propose the pre-synaptic nature of complement-mediated effect on the basis of the experimental approach used to study this event, i.e., the up-down superfusion of a thin layer of purified isolated nerve terminals. This technique, which has been proposed as a method of choice to detect pre-synaptic mechanisms of control of neurotransmitter release (Raiteri and Raiteri 2000), has the major advantage that the continuous up-down superfusion prevents indirect effects as a result of endogenous compounds released from one particle and acting at adjacent particles. For instance, if present, antibodies or immune competent macromolecules (including those whose production is stimulated by complement itself) are immediately removed so that they can hardly elicit immune-mediated responses, including complement activation through the classic pathway (Monk et al. 2007; Veerhuis et al. 2011). Inasmuch, the synaptosomal preparations lack the cellular elements that could be involved in immune-mediated effects including microglial cells and macrophages (Pattarini et al. 1998). To conclude, under the in vitro conditions used in this work, the effects observed would be more easily attributed to complement acting at selected receptor structure(s)/protein(s) located pre-synaptically on the plasma membranes of nerve terminals, also consistent with the finding that complement heat-shock denaturation significantly prevented this event. Interestingly, the releasing effect was restricted to a defined subpopulation of nerve terminals, the glutamatergic one, as suggested by the finding that complement was devoid of efficacy in releasing other classic neurotransmitters including GABA, noradrenaline, and acetylcholine. Although differential expression of complement-regulating proteins might account for the different susceptibility of distinct neuron subpopulations (Singhrao et al. 2000), these observations might suggest the specificity and the selectivity of complement-induced releasing effect and preclude the involvement of aspecific complement-induced lytic processes which, because of their aspecificity, should be expected to take place in all the synaptosomal subpopulations (Raiteri et al. 1972; Raiteri and Levi 1973; Docherty et al. 1983). Interestingly, Docherty et al. (1983) demonstrated that GABA release from rat cortical synaptosomes occurred when nerve terminals were exposed to complement (1 : 10), provided that an anti-serum to pure glutamate decarboxylase was added concomitantly, since, they observed, complement alone was unable to release GABA. Here, we demonstrated that complement can elicit on its own the release of glutamate in the absence of exogenous anti-sera/antibodies added to the superfusion medium. Whether a spontaneous activation of complement hydrolysis (the so-called alternative pathway of activation, Veerhuis et al. 2011) could be involved in the effect so far described remains to be established. Future studies are required to address this issue.
Whatever the molecular events involved in the effect so far described, our observations indicate that complement selectively controls excitatory transmission in the CNS, and that this central neuronal pathway mainly mediates the central effects complement exerts both in healthy (i.e., synaptic plasticity and neurodegenerative processes; Boulanger 2009) as well as in pathological conditions (cerebral ischemia, multiple sclerosis, Parkinson's disease, Alzheimer's disease; Barnum 1999; Tahtouh et al. 2009).
Although complement-mediated releasing effect seems to be restricted to glutamatergic nerve terminals, this appears to be a widespread phenomenon that can take place in different CNS regions and in the CNS of different mammals, including humans. As a confounding factor, complement-evoked release of glutamate from human nerve terminals differs from that observed in rodents, both in efficacy and potency. Indeed, higher concentrations of complement (1 : 300–1 : 10) were required to elicit a significant release of radioactive tracer, which is lower than the effects observed in mouse and rat cortical nerve endings. The origin of the human tissue specimens could in part account for this apparent discrepancy. As already stated in the Result Section, human tissues were removed during neurosurgery to reach deep-locate brain tumors, and thus originated from patients suffering from pathological conditions, often involving central sterile inflammation and immune-mediated responses. These pathological conditions might favor the onset of confounding adaptative processes that could emerge as change in release capability as well as reduced sensitivity to proinflammatory immune molecules, including complement. The results obtained with our animal model are therefore particularly relevant as they allow to unveil the role of complement as a modulator of central neurotransmission in healthy conditions.
It is known that glutamate outflow from nerve terminals can occur through different mechanisms, including the classic Ca2+-dependent exocytosis and glutamate exit because of transporter reversal. In an attempt to shed light on the molecular events accounting for complement-evoked glutamate release, we first analyzed whether the Ca2+-dependent, exocytotic-like mechanism of exit could account for the effect(s) so far described. A drastic loss of function of complement was observed when synaptosomes were superfused with a Ca2+-free medium. This result could suggest that complement might elicit the glutamate release from the vesicular pool in a Ca2+-dependent, exocytotic-like manner. Opposite to our expectations, however, complement-induced releasing effect was not modified in synaptosomes where cytosolic Ca2+ ion bioavailability was drastically reduced by entrapping the selective calcium chelator BAPTA, nor blocking VSCCs altered this event. Inasmuch, complement still was efficacious in releasing glutamate in synaptosomes where the vesicular storage of the radioactive tracer was minimized by bafilomycin A1. Altogether, these observations seemed compatible with the thesis that complement-induced glutamate release does not involve a Ca2+-dependent process, nor that it mainly originates from the vesicular store.
Beside its ability to allow Ca2+ influx, complement had been shown to mobilize Ca2+ ions from intraterminal stores through a cascade of events also involving PTx-sensitive, G protein-coupled membrane receptors (Norgauer et al. 1993). Whether such an event also occurs in CNS glutamatergic nerve terminals remains to be established. Nonetheless, our data clearly indicate that toxifying synaptosomes with PTx had no effect on complement-induced releasing effect, leading us to conclude that PTx-sensitive mechanisms do not participate to the complement-evoked releasing effect here described. As Ca2+ ions are essential to catalyze complement activation, we suggest that the loss of efficacy of complement in Ca2+-free medium could be best interpreted as the consequence of a reduced activation of complement itself, instead of the Ca2+ dependence of the releasing effect.
The Ca2+-independent outflow of glutamate elicited by complement was largely impeded by the excitatory amino acid transporter blocker DL-TBOA, suggesting that it was accounted for by a carrier-mediated release (Levi and Raiteri 1993). Such an event could be favored by a precocious complement-induced local depolarization of synaptosomal plasma membranes that in turn could permit carrier reversal. Accordingly, the drastic reduction of [Na+] in the superfusion medium significantly reduced complement-induced releasing effect. It is well known that [3H]d-ASP has a preferential cytosolic compartmentalization, whereas endogenous glutamate preferentially locates in the vesicles than in the cytosol. As complement-induced effect also could be observed when studying the release of endogenous glutamate, one might speculate that complement could favor glutamate exit from vesicles into the cytosol, subsequently allowing its outflow by transporter reversal. Notably, carrier-mediated glutamate release takes place in pathological conditions associated with CNS injury, including stroke and ischemia. Interestingly, complement was proposed to play a role in cerebral ischemic injury; moreover, complement antagonist(s) and /or blockers were shown to ameliorate ischemia-induced neurodegeneration (Rahpeymai et al. 2006; Arumugam et al. 2009).
To conclude, our results clearly demonstrate that complement can control excitatory neurotransmission in mammal CNS. At low doses, complement can evoke a quasi-physiological release of glutamate, which in turn may have a role in regenerative processes as well as in synaptic plasticity and neuronal modeling. Higher concentrations of complement were required to cause a massive release of glutamate that could participate in neurodegenerative processes often observed in neurological diseases and in demyelinating disorders, including multiple sclerosis. Notably, these higher complement concentrations seem compatible with the peripheral level of the immune complex observed in some conditions associated with acute brain damage, when the blood–brain barrier integrity is disrupted and the influx of peripheral proteins, including the complement ones, into the CNS is temporary allowed (Veerhuis et al. 2011). The aberrant glutamate outflow released in CNS in these conditions also could have a role in the recruitment of proinflammatory cells from the periphery to the CNS, then worsening central neuroinflammatory processes and demyelination (Sarchielli et al. 2007).
Acknowledgments and conflict of interest disclosure
This work was supported by grants from Ministero Italiano dell'Istruzione, dell'Università e della Ricerca Scientifica (Projects n. 2009P7WHNR_003) and from FISM – “Fondazione Italiana Sclerosi Multipla” (cod. 2008/R/21). We wish to thank Dr Silvia E. Smith, Ph.D. (Department of Biological Sciences and Institute for Bioinformatics and Evolutionary Studies, University of Idaho) for reviewing the manuscript and Maura Agate for editorial assistance.
The authors have no conflict of interest to declare.