Hepatitis B clinical care provision in pregnancy: A whole-of-population linkage study in Victoria, Australia
Declaration of conflict of interest: The authors have no relevant conflicts of interest to declare.
Ethics approval: Approval was obtained from Melbourne Health (LNR/16/MH/60) and the Australian Institute of Health and Welfare (EO2016/2/258).
Informed consent: A waiver of consent was obtained and no identifying information regarding the study participants was available to the researchers.
Financial support: This study was supported by a Royal Melbourne Hospital Foundation Research Project Grant (PG-014-2016).
Abstract
Background and Aim
Pregnancy is a key setting for engagement in chronic hepatitis B (CHB) care, due to the implications for transmission to the infant and antenatal diagnosis representing an opportunity for ongoing follow-up. This study aimed to identify the coverage and predictors of clinical care for women with CHB during and after pregnancy in a population-level cohort.
Methods
Notified CHB cases in Victoria, Australia, were linked with hospitalizations, medical services, and prescribing data, covering the period 1991–2018. Women with an admission for a live birth were identified and services provided during pregnancy were assessed, including general practitioner (GP) or specialist visits, viral load and serology testing, and antiviral treatment. Viral load and serology testing coverage ware also assessed for the 2-year period following pregnancy. Demographic and clinical predictors of viral load testing during pregnancy were assessed.
Results
A total of 11 015 birth events occurred for 6090 women with CHB. During pregnancy most had a GP consultation (91.6%); however, only 39.5% had viral load testing and 41.4% had a gastroenterology or infectious diseases specialist consultation. Viral load testing and serology testing in the 2 years after pregnancy occurred in approximately half (47.9% and 52.2%, respectively) with increases over time. Viral load testing was more likely in those born overseas, those with more than one previous birth, and those living in Melbourne.
Conclusions
Despite improvements over time, key gaps were identified in the provision of CHB clinical care during and after pregnancy, with implications for ongoing transmission and adverse outcomes.
Introduction
Chronic hepatitis B (CHB) affects over 300 million individuals globally,1 with mother-to-child transmission (MTCT) being a key mode of transmission.2 Interventions such universal antenatal hepatitis B virus (HBV) testing and infant vaccination; antiviral treatment in women with elevated HBV viral load during pregnancy; and administration of hepatitis B immunoglobulin among infants born to these women are effective in reducing the risk of infection.1 Implementation of these interventions has contributed to a profound reduction in the global HBV prevalence among those aged < 51; however, coverage remains incomplete.3
In Australia, these interventions are established recommendations in clinical guidelines.4-6 Given low uptake of ongoing care for people with CHB in Australia,7 diagnosis during pregnancy also represents an opportunity to engage women living with CHB in ongoing care to prevent adverse outcomes such as liver disease and liver cancer.
Available evidence suggests that routine antenatal HBV testing is high in Australia, with clinical audit, survey, and routine reporting data suggesting uptake above 95%.8-11 In contrast, there is evidence of substantial gaps in viral load and HBV e-antigen testing and antiviral treatment, and variation by setting. Clinical audit of two Sydney hospitals during 2003–2006 found that 65% of women had e-antigen testing, but only 7% were referred for specialist care.12 Assessment across three Melbourne hospitals (2006–2011) found only 18% of women had specialist care and 34% had either e-antigen or viral load assessment (though viral load testing improved over time).13 A single-center Melbourne study (2008–2013) found e-antigen was assessed for 15.3%, viral load quantified for 28.4%, and specialist care provided for 55.7% of women.14 In contrast, another single-center Melbourne study (2014–2015) found high uptake of both serology (86.1%) and viral load testing (98.0%).15
Given these findings, population-level assessment of potential variation according to demographic, geographic, clinical and other factors is essential, particularly given the prevalence of CHB and the uptake of regular care are known to vary by region.16 Combining relevant data sources through data linkage provides a unique opportunity to generate estimates of the coverage of key interventions across a whole jurisdiction. Here, we use linked data to quantify the proportion of women receiving CHB clinical care, measured by HBV viral load and serology testing, antiviral treatment, and specialist consultations during and after pregnancy in Victoria, Australia.
Methods
Study design, setting, and participants
A retrospective population-based cohort was constructed using linkage of records derived from four routinely collected datasets, both state and national, ranging from 1991–2018. Individuals with a hepatitis B notification in Victoria, Australia, were linked with their hospital admissions to identify those with a record of having had a live birth. This cohort was then linked to national health insurance (Medicare) data to assess the provision of consultation, testing, and treatment items. These datasets are described in detail below.
Data sources
Since 1991, legislation has required notification by both the diagnosing medical practitioner and testing laboratory of any positive HBV test result (either surface antigen or viral nucleic acid) to the Victorian Government Department of Health.17, 18 Cases are classified as newly acquired (where there is evidence of recent acquisition) or unspecified. Unspecified cases are generally considered (and hereafter referred to as) chronic. Our cohort comprised all female cases of CHB notified in Victoria between 1 January 1991 and 31 December 2016, available from the Public Health Events Surveillance System (PHESS), to which subsequent datasets were linked. Throughout this manuscript, we use the terms women and mothers, and respectfully acknowledge this may encompass non-cisgendered individuals who have experienced pregnancy and live birth.
Births
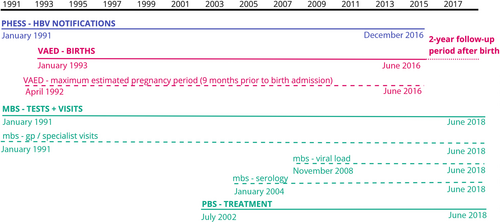
All hospital admissions occurring in public and private hospitals in Victoria are captured in an administrative database, the Victorian Admitted Episodes Dataset (VAED). Notification data were linked to VAED to identify hospital admissions among this cohort for live birth events, using International Classification of Diseases 10th Addition, Australian Modification (ICD-10-AM) codes (see Table S1). As VAED data were only available from July 1993–June 2016, the cohort represents those with a live birth event occurring during this period, although their CHB notification could have occurred earlier (Fig. 1).
Testing and treatment
Australia's universal government-subsidized medical care and testing (Medicare Benefits Schedule, MBS19) and prescription medication (Pharmaceutical Benefits Scheme, PBS20) systems were used as the data sources for relevant testing and treatment items. We identified items for hepatitis serology testing, HBV viral load testing, HBV antiviral treatment, and General Practitioner (GP) and non-GP specialist (hereafter referred to as specialist) consultations (see Supplementary Table 2). Using the MBS speciality variable, we derived a variable denoting specialist consultation if the consultation was provided by an infectious diseases physician or gastroenterologist/hepatologist, reflecting usual management of CHB in Australia.16 MBS data were available from January 1993–June 2018, though HBV viral load testing was only added in November 2008 and serology testing data were only available from 2004 onwards. PBS data were available from January 2002–June 2018 (Fig. 1).
Data linkage and preparation
Deterministic linkage was conducted was conducted using full name, sex, and date of birth by the Centre for Victorian Data Linkage, combining PHESS with the VAED; followed by probabilistic linkage to the MBS and PBS, conducted by the Australian Institute of Health and Welfare. Names were removed and dates were encrypted in the data made available to investigators, with a unique identifier and month and year variables provided for analysis.
Due to the lack of availability of gestational age in the datasets, an approximate pregnancy period was calculated as 9 months prior to the birth event admission date, to distinguish items likely performed during pregnancy rather than as part of regular CHB care.
Clinical care provision
We assessed the receipt during this pregnancy period of HBV viral load testing and hepatitis serology testing, which are both recommended for pregnant women with CHB.4 We also assessed HBV viral load and serology testing in the 2 years after the birth event. The provision of HBV treatment during pregnancy was assessed, and of those the number which were initiated during pregnancy. We also assessed the proportion who had a consultation with a GP, and with a specialist during pregnancy.
The MBS item for “hepatitis serology” includes hepatitis A-E, and so is considered necessary but not sufficient as an indicator of the recommended HBV e-antigen testing during pregnancy. Due to this, and due to the guidelines for treatment only applying to a subset of women during pregnancy, HBV viral load testing during pregnancy was used as the primary outcome of interest and was the subject of multivariable analysis.
Exposures
The proportion of birth events in which these testing and treatment outcomes occurred was considered according to the following characteristics of the mother at the time of the birth event: age, categorized according to the median; region of residence (metropolitan Melbourne vs the rest of Victoria, derived using the designation of the Australian Bureau of Statistics21); country of birth (as first recorded VAED); whether an interpreter was required (recorded in VAED); number of previous birth events (as indicated by VAED); and whether the first notification of CHB occurred during a pregnancy. Local Government Area (LGA) of residence was also assessed for HBV viral load testing where the number of individuals receiving testing in an LGA was ≥ 6. Outcomes were not able to be assessed by Indigenous status due to the low number of cases (n = 25).
Statistical analyses
Demographic and clinical characteristics were described for the first pregnancy only, and all subsequent analyses used the birth event as the unit of measure. We calculated the numbers and proportions of birth events for which there was a record of each clinical item during pregnancy (serology testing, viral load testing, antiviral treatment, GP consultation, specialist consultation); and in the 2 years after pregnancy for HBV viral load and serology testing to assess engagement in ongoing CHB care, for the time period available (Fig. 1).
Crude and adjusted odds ratios were calculated to assess factors associated with having HBV viral load testing during pregnancy using logistic regression models. Candidate models were constructed by including known and plausible confounding factors. To evaluate each factor, univariate analysis was conducted with particular attention paid to the strength of association with the outcome measures of interest.
Data management and analysis were conducted in Microsoft Excel and Stata.
Approvals
Human Research Ethics Committee approval was obtained from Melbourne Health (LNR/16/MH/60) and the Australian Institute of Health and Welfare (EO2016/2/258), including a waiver of individual consent.
Results
Cohort summary
Between 1 July 1993 and 30 June 2016, 11 015 live birth events occurred among 6090 women with a CHB notification. Most had either one (46.7%) or two (35.3%) birth events recorded, and the median age at the time of the first was 29.6 years (Table 1). The cohort was predominantly overseas born (78.8%) and resident in metropolitan Melbourne (84.1%; Table 1). The CHB notification date occurred during the pregnancy period for 2442 individuals, or 40.0% of the cohort.
| Total women | 6090 |
|---|---|
| Median age of mother at time of first birth event (interquartile range) | 29.6 (25.7–33.6) years |
| Total number of birth events recorded for the mother: | Number (proportion of total) |
| 1 | 2844 (46.7%) |
| 2 | 2151 (35.3%) |
| 3 | 716 (11.8%) |
| 4 or more | 379 (6.2%) |
| Region of residence of mother at time of first birth event: | |
| Rest of Victoria | 354 (5.8%) |
| Melbourne | 4769 (84.1%) |
| Not stated | 967 (15.9%) |
| Indigenous status of mother: | |
| Aboriginal and/or Torres Strait Islander | 25 (0.41%) |
| Non-Indigenous | 6032 (99.0%) |
| Not stated | 32 (0.53%) |
| Place of birth of mother: | |
| Born in Australia | 1115 (18.3%) |
| Born overseas | 4798 (78.8%) |
| Not stated | 177 (2.9%) |
| Year of first birth event: | |
| 1993–1998 | 1744 (28.6%) |
| 1999–2004 | 1416 (23.3%) |
| 2005–2010 | 1400 (23.0%) |
| 2011–2016† | 1530 (25.1%) |
- † Data to June 2016 only; see Figure 1.
Clinical care coverage
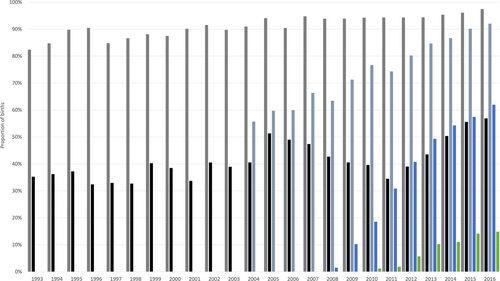
For most birth events there was a GP consultation during pregnancy (10 089 of 11 015, 91.6%), with a gradual increase over time (82.4% in 1993 to 97.5% in 2016; Fig. 2). A specialist consultation occurred for 41.4% (4557 of 11 015). The proportion with a specialist consultation fluctuated over time but was generally higher in more recent years (Fig. 2).
 , GP consultation during pregnancy;
, GP consultation during pregnancy;  , specialist consultation during pregnancy;
, specialist consultation during pregnancy;  , hepatitis serology test during pregnancy;
, hepatitis serology test during pregnancy;  , hepatitis B viral load test during pregnancy;
, hepatitis B viral load test during pregnancy;  , hepatitis B treatment during pregnancy.
, hepatitis B treatment during pregnancy.For birth events occurring when HBV viral load testing was available (2009–2016; n = 4120), 1628 (35.9%) had evidence of testing during pregnancy, increasing from 52 (10.2%) in 2009 to 171 (62.0%) in 2016. HBV viral load testing in the 2 years after the birth event occurred for 1973 (47.9%), and this also increased over time (Fig. 2).
For birth events occurring when serology testing data were available (2004–2016; n = 6446), 4779 (74.1%) women had a hepatitis serology test during pregnancy (Table 2). This proportion increased from 55.7% in 2004 to 92.0% in 2016 (Fig. 2). Testing in the 2 years after the birth event occurred for 4230 (52.5%) women.
| GP consultation during pregnancy | Specialist consultation during pregnancy | Hepatitis serology test during pregnancy | HBV viral load during pregnancy | HBV antiviral treatment during pregnancy | HBV viral load in 2 years after birth event | Hepatitis serology test in 2 years after birth event | |
|---|---|---|---|---|---|---|---|
| Time period available (see Fig. 1) | 1993–2016 | 1993–2016 | 2004–2016 | 2009–2016 | 2003–2016 | 2009–2018 | 2004–2018 |
| Total | 10 094/11015 (91.6%) | 4557/11015 (41.4%) | 4779/6446 (74.1%) | 1628/4120 (39.5%) | 290/6934 (4.2%) | 1973/4120 (47.9%) | 3363/6446 (52.2%) |
| Age category | |||||||
| ≤ 30 years | 6172/6730 (91.7%) | 2545/6730 (37.8%) | 2776/3845 (72.2%) | 891/2354 (37.9%) | 168/4166 (4.0%) | 1104/2354 (46.9%) | 2568/3845 (66.8%) |
| > 30 years | 3917/4266 (91.8%) | 2010/4266 (47.1%) | 1998/2594 (77.0%) | 736/1763 (41.7%) | 122/2761 (4.4%) | 868/1763 (49.2%) | 1659/2594 (64.0%) |
| Region of residence | |||||||
| Rest of Victoria | 761/812 (93.7%) | 303/812 (37.3%) | 391/491 (79.6%) | 81/291 (27.8%) | 10/542 (1.8%) | 86/291 (29.6%) | 217/491 (44.2%) |
| Melbourne | 8340/9031 (92.3%) | 3855/9031 (42.7%) | 1559/5933 (26.3%) | 1545/3816 (40.5%) | 280/6367 (4.4%) | 1881/3816 (49.3%) | 3134/5933 (52.8%) |
| Region of birth | |||||||
| Australia | 2110/2208 (95.6%) | 919/2208 (41.6%) | 808/1120 (72.1%) | 144/614 (23.5%) | 24/1349 (1.8%) | 163/614 (26.5%) | 474/1120 (42.3%) |
| Overseas | 7672/8467 (90.6%) | 3515/8467 (41.5%) | 3868/5184 (74.6%) | 1466/3435 (42.7%) | 262/5881 (4.5%) | 1776/3435 (51.7%) | 2823/5184 (54.5%) |
| Number of birth events prior to this birth event | |||||||
| None | 5444/6090 (89.4%) | 2537/6090 (41.7%) | 2364/3151 (75.0%) | 867/2030 (42.7%) | 164/3622 (4.5%) | 1073/2030 (52.9%) | 1843/3151 (58.5%) |
| 1 | 3054/3246 (94.1%) | 1437/3246 (44.3%) | 1537/2019 (76.1%) | 528/1273 (41.5%) | 93/3224 (2.9%) | 606/1273 (47.6%) | 953/2019 (47.2%) |
| 2 | 1046/1095 (95.5%) | 403/1095 (36.8%) | 555/789 (70.3%) | 156/492 (31.7%) | 23/901 (2.6%) | 185/492 (37.6%) | 345/789 (43.7%) |
| 3 or more | 558/584 (95.5%) | 180/584 (30.8%) | 323/490 (65.9%) | 77/325 (23.7%) | 10/536 (1.9%) | 109/325 (33.5%) | 222/490 (45.3%) |
| Timing of CHB diagnosis | |||||||
| Prior to pregnancy | 6551/6919 (94.7%) | 3094/6919 (44.7%) | 3541/4719 (75.0%) | 1236/3124 (39.6%) | 240/5316 (04.5%) | 1499/3124 (48.0%) | 2430/4719 (51.5%) |
| During pregnancy | 2147/2454 (87.5%) | 929/2454 (37.9%) | 1050/1348 (77.9%) | 373/865 (43.1%) | 50/1562 (3.2%) | 435/865 (50.3%) | 755/1348 (56.0%) |
| Interpreter status | |||||||
| Interpreter not required | 3151/3335 (94.5%) | 1499/3335 (44.9%) | 2511/3255 (77.1%) | 892/2285 (39.0%) | 149/3335 (4.5%) | 1024/2285 (44.8%) | 1647/3255 (50.6%) |
| Interpreter required | 1455/1595 (91.2%) | 685/1595 (42.9%) | 1186/1564 (75.8%) | 529/1125 (47.0%) | 104/1595 (06.5%) | 679/1125 (60.4%) | 979/1564 (62.6%) |
- Note that totals will not add due to those with missing information. Data for interpreter status only available in hospital records from 2003 onwards.
For birth events occurring when antiviral treatment data were available (2003–2016; n = 6934), treatment was provided for 290 (2.9%), increasing from < 1% in 2003 to 14.9% in 2016. For the majority, this represented an initiation during pregnancy (62.4%) rather than ongoing treatment.
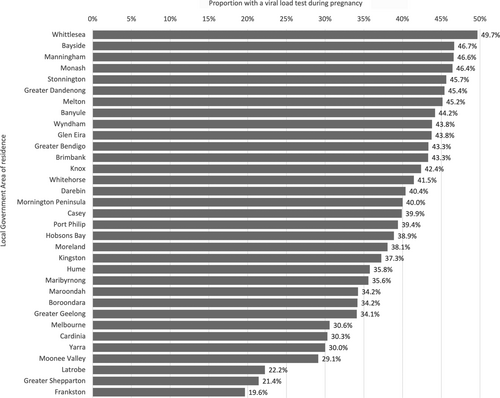
The proportion who had a viral load during pregnancy was higher in women residing in Melbourne than those living in regional Victoria, and those born overseas compared to those born in Australia, as were the proportions who had a viral load in the 2 years following the birth event (Table 2). There was also wide variation by LGA where data were able to be reported, from 19.6% to 49.7% (37 of 62 LGAs) (Fig. 3).
The proportion with a GP consultation was similarly high across groups (> 89% for all categories), and there was limited variation in the proportion with a specialist consultation (30–50% for all categories).
Factors associated with clinical care
Factors associated with an increased odds for having a viral load test during the pregnancy period were residing in metropolitan Melbourne (OR 1.76, 96% CI 1.35–2.30), having been born overseas (OR 2.43, 95% CI 1.99–2.96), and a record indicating a need for an interpreter (OR 1.39, 95% CI 1.20–1.60; Table 3). There was also increased odds of receiving viral load testing during pregnancy in more recent years, and a reduction with a higher number of prior deliveries (Table 3).
| Number of birth events | Number of birth events with an HBV viral load test (proportion) | Univariate odds ratio | 95% CI | Multivariable odds ratio | 95% CI | |
|---|---|---|---|---|---|---|
| Total | 4120 | 1627 (39.5%) | ||||
| Age in years of mother at time of birth event | - | - | 0.99 | 0.98–1.00 | - | - |
| Region of residence | ||||||
| Rest of Victoria | 491 | 81 (16.5%) | Reference | |||
| Melbourne | 5933 | 1545 (26.0%) | 1.76 | 1.35–2.30 | 1.40 | 1.02–1.92 |
| Country of birth of mother | ||||||
| Australia | 1120 | 144 (12.9%) | Reference | |||
| Overseas | 5184 | 1466 (28.3%) | 2.43 | 1.99–2.96 | 1.69 | 1.32–2.17 |
| Number of live births prior to this birth event | ||||||
| None | 3151 | 867 (27.5%) | Reference | |||
| 1 | 2019 | 528 (26.2%) | 0.95 | 0.82–1.10 | 1.02 | 0.85–1.23 |
| 2 | 789 | 156 (19.8%) | 0.62 | 0.51–0.77 | 0.63 | 0.48–0.83 |
| 3 or more | 490 | 77 (15.7%) | 0.42 | 0.32–0.55 | 0.4 | 0.26–0.62 |
| Timing of CHB diagnosis | ||||||
| Prior to pregnancy | 4719 | 892 (27.4%) | Reference | |||
| During pregnancy | 1348 | 529 (33.8%) | 1.01 | 0.89–1.15 | 1.18 | 0.97–1.43 |
| Interpreter requirement reported | ||||||
| Not required | 3255 | 1236 (26.2%) | Reference | |||
| Required | 1564 | 373 (27.7%) | 1.39 | 1.20–1.60 | 1.32 | 1.13–1.56 |
| Year of birth event | ||||||
| 2009 | 508 | 52 (10.2%) | Reference | |||
| 2010 | 523 | 97 (18.5%) | 1.99 | 1.39–2.87 | 1.78 | 1.18–2.67 |
| 2011 | 545 | 168 (30.8%) | 3.90 | 2.78–5.49 | 3.86 | 2.65–5.63 |
| 2012 | 582 | 237 (40.7%) | 6.02 | 4.33–8.39 | 5.32 | 3.68–7.68 |
| 2013 | 586 | 289 (49.3%) | 8.53 | 6.14–11.9 | 8.63 | 5.98–12.5 |
| 2014 | 562 | 305 (54.3%) | 10.4 | 7.47–14.5 | 10.2 | 7.10–14.8 |
| 2015 | 538 | 309 (57.4%) | 11.8 | 8.47–16.5 | 11.3 | 7.79–16.3 |
| 2016 | 276 | 171 (62.0%) | 14.3 | 9.81–20.8 | 14.5 | 9.59–22.0 |
Discussion
This analysis demonstrates considerable improvement in the provision of recommended clinical care for CHB during and after pregnancy in Victoria over time, with increases in the proportion receiving viral load and serology testing, antiviral treatment, and specialist consultations. Those living in metropolitan regions were more likely to receive viral load testing, reflecting other evidence at the population level regarding CHB care uptake.22 Our findings reflect other evidence finding low coverage of comprehensive care for women with CHB during pregnancy12-14; however, the variation according to LGA of residence and increased coverage over time is consistent with higher coverage observed in some centers.15
Identifying and providing management to people living with CHB is a priority in both Australian and global strategies.23, 24 More than one third of the individuals in this cohort were diagnosed during a pregnancy, which highlights the need for a focus on this setting when engaging people with CHB in follow-up care. The demographics of the cohort, reflecting the estimated population living with CHB,22 emphasize the need for care that is linguistically appropriate and culturally safe, particularly as pregnancy can be a time when diagnosis can be distressing and the person may need additional supports.25
These estimates represent the first population-level assessment of CHB clinical care in this population, covering all live births occurring in Victoria over more than two decades. The use of linked data provides the advantage of capturing all facility births occurring in the state (representing > 98% of total births26), removing the biases which occur when assessing only women seen in tertiary centers. Medicare data provide high capture of the provision of testing and treatment, reducing error where reliance is placed on medical records across services. The study also allows for comprehensive assessment of trends over time, including after pregnancy, and the impact of the introduction of new items to the MBS and PBS. The observed variation according to LGA highlights the importance of these state-wide assessments.
However, this type of study does have inherent limitations. Some of these relate to gaps in the granularity of data, for example, the lack of detailed antenatal information such as estimated date of conception. This study used an approximation of the pregnancy period which aimed to increase capture of services potentially provided during that time, which may have led to overestimation of coverage, particularly if gestation was < 40 weeks. Additional data linkage to Victoria's population-wide surveillance system capturing all births (the Victorian Perinatal Data Collection27) would assist with this detail, and allow identification of other potentially relevant factors, including complications during pregnancy or labor and delivery.
Additional gaps include the exclusion of individuals not eligible for Medicare, such as those migrants who are not permanent residents of Australia, which would overestimate overall coverage. However, this is estimated to represent a relatively small proportion of all people living with CHB.28 There will also be under-ascertainment where treatment or testing has been provided outside of Medicare due to restrictions such as the limit on the number of annual viral load tests performed. Antiviral treatment for the prevention of MTCT in the absence of other indications was only added to the PBS in 2020,29 whereas previously treatment in this group may not have been subsidized. However, our findings indicate that prescribing for this indication through the PBS was occurring prior to this. The study was also unable to assess recommended liver function testing, due to the use of non-specific codes in the MBS for this service.
Finally, a major gap in the measurement of antiviral treatment coverage is lack of ability to assess clinical eligibility. Not all pregnant women with CHB require treatment, and the assessment of this relies on the HBV viral load test result, which is not available in the dataset. However, it is possible to compare to population-level treatment eligibility estimates, which range from 20% to 50%13, 14, 30, 31 in Australia; using this baseline the level observed is inadequate, even in the most recent year assessed. The addition of laboratory result data would assist in identifying coverage of antiviral treatment prescribing among the highest risk individuals.
The time delays often involved in data linkage32 mean that there is a need for updated and enduring linkage to assess the current status of the provision of CHB care during pregnancy, and it is likely that coverage has continued to improve since time period examined in this study. Further data would also allow for assessment post-birth for greater than 2 years. However, this analysis forms a baseline for comparison and identifies key priority populations for intervention, and these disparities are unlikely to have fully resolved over time, given ongoing gaps in CHB care provision.22 This type of evidence can inform strategic priorities for CHB in Australia, including new targets in Australia's National Hepatitis B Strategy 2022–2030,33 which aim to ensure comprehensive CHB care during pregnancy. Further, it identifies gaps in coverage of interventions which support the prevention of MTCT for children born in Victoria over the last two decades, and who may have acquired CHB perinatally and require follow-up as adults. This builds on evidence previously identifying gaps in delivery of interventions to infants in Victoria.34
Barriers to the provision of effective CHB care in pregnancy have been identified, with health care providers specifying a lack of staff training and availability of standard policies and procedures, and lack of communication between services as the most common.35 These providers reported high confidence that HBV screening was comprehensive; however, only half were confident that coverage of HBV viral load testing and appropriate antiviral treatment were high, reflecting the findings here. Gaps in systematic referral of pregnant women with CHB to appropriate care, and frequent reliance on verbal reporting of CHB status by the pregnant woman herself have also been identified as issues.35 There is evidence that further training can have positive impacts on confidence in this setting.36 International evidence has shown that an integrated and comprehensive model has a positive effect on the uptake of testing and treatment for hepatitis B in the antenatal setting.37 The embedding of capacity for treatment within obstetric care has also been identified to be effective.38 In both instances, the importance of education and awareness is highlighted as key to effectiveness.
This study provides the first comprehensive assessment of the coverage of CHB clinical care during pregnancy in Victoria, offering insight into key gaps. Although most women were engaged with primary care during pregnancy, at least half did not receive guideline-based care for their CHB. Similar gaps in the period following pregnancy highlights an opportunity for improved engagement in ongoing CHB care. The incorporation of indicators relating to CHB in pregnancy into Australia's National Hepatitis B Strategy 2023–2030 emphasizes the importance of reporting against these key metrics. This will allow a focus on care delivery for all pregnant women living with CHB and their infants, and help ensure progress towards elimination of CHB as a public health concern across Australia.
Open Research
Data availability statement
Data are not publicly available due to restrictions applied by the data custodians to protect confidentiality, due to high granularity of raw data.




