Global prevalence of advanced fibrosis in patients with type 2 diabetes mellitus: a systematic review and meta-analysis
Wasit Wongtrakul and Sorachat Niltwat contributed equally to this work.
Abstract
Background and Aim
Patients with type 2 diabetes mellitus (T2DM) face a heightened susceptibility to advanced fibrosis, a condition linked to adverse clinical outcomes. However, reported data on liver fibrosis severity among individuals with T2DM vary significantly across studies with diverse characteristics. This meta-analysis aimed to estimate the global prevalence of advanced fibrosis among T2DM patients.
Methods
A comprehensive systematic search of the EMBASE and MEDLINE databases from inception to November 2022 was conducted to identify studies assessing advanced fibrosis in individuals with T2DM. Random-effects models were utilized to calculate point estimates of prevalence, accompanied by 95% confidence interval (CI). Meta-regression with subgroup analysis was employed to address heterogeneity.
Results
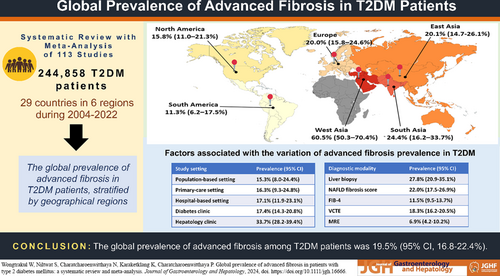
We identified 113 eligible studies involving 244,858 individuals from 29 countries. Globally, the prevalence of advanced fibrosis among T2DM patients was 19.5% (95% CI 16.8–22.4%). Regionally, the prevalence rates were as follows: 60.5% in West Asia (95% CI 50.3–70.4%), 24.4% in South Asia (95% CI 16.2–33.7%), 20.1% in East Asia (95% CI 14.7–26.1%), 20.0% in Europe (95% CI 15.8–24.6%), 15.8% in North America (95% CI 11.0–21.3%), and 11.3% in South America (95% CI 6.2–17.5%). The prevalence of advanced fibrosis varied notably based on the study setting and diagnostic methodology employed. Meta-regression models highlighted that 45.13% of the observed heterogeneity could be attributed to combined diagnostic modality and study setting.
Conclusions
Globally, approximately one fifth of the T2DM population presents advanced fibrosis, with prevalence differing across geographical regions. Our findings underscore the need for effective strategies to alleviate its global burden.
Graphical Abstract
Introduction
Nonalcoholic fatty liver disease (NAFLD) stands as the most prevalent chronic liver condition, affecting up to 65% of people worldwide diagnosed with type 2 diabetes mellitus (T2DM).1, 2 Recently, with the transition from the term NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD) or metabolic dysfunction-associated steatotic liver disease (MASLD), these nomenclatures aim to better capture its essence and connection with metabolic dysfunction.3, 4 T2DM is a major metabolic risk factor for the development and progression of MASLD.5 MASLD can progress from simple steatosis to steatohepatitis, which is accompanied by various degrees of liver fibrosis. Substantial evidence indicates that those with advanced fibrosis are at risk of adverse clinical outcomes and mortality.6, 7 Identifying high-risk individuals becomes pivotal for clinical care and intervention trials. In the absence of approved pharmacotherapy, lifestyle modifications and specialized multidisciplinary care are beneficial for patients with advanced fibrosis. Additionally, a lack of awareness regarding fibrosis staging appears linked to poor adherence to lifestyle changes.8 International guidelines recommend evaluating T2DM individuals with elevated aminotransferases or imaging evidence of liver steatosis for advanced liver fibrosis.9, 10 However, the available evidence supporting such recommendations remains inconclusive due to the uncertain prevalence of advanced fibrosis among patients with T2DM.
Studies have been conducted to examine the severity of liver fibrosis in T2DM patients to identify and offer interventional strategies to individuals who are at risk of unfavorable clinical outcomes. Yet, outcomes have varied across studies with diverse characteristics. Thus, this systematic review and meta-analysis aimed to comprehensively establish the global prevalence of advanced fibrosis among patients with T2DM, stratified by geographical regions. Understanding the worldwide prevalence of advanced fibrosis is crucial for comprehending the significance of this liver condition in diabetes. Such data can potentially influence clinical practice by raising awareness of advanced fibrosis among high-risk populations.
Methods
Search strategy
A systematic literature review of the EMBASE and MEDLINE databases from inception to November 28, 2022, was done to identify all published studies evaluating the prevalence of advanced fibrosis in T2DM patients with and without documented MASLD. The search strategy used terms such as “liver fibrosis,” “nonalcoholic fatty liver disease,” and “type 2 diabetes mellitus” and is described in Data S1. The searches were limited to human studies. This study was done in accordance with PRISMA guidelines and was registered on Open Science Framework, number osf.io/u24vj.
Inclusion and exclusion criteria
To evaluate the prevalence of advanced fibrosis in patients with T2DM, eligible studies had to report the proportion of diabetes patients according to their fibrosis stages. Advanced fibrosis was defined as fibrosis stage 3 or more. The diagnostic modality used to determine advanced fibrosis had to be specified. Any definitions or cutoff values for the diagnosis of liver fibrosis mentioned in the original articles were accepted. The exclusion criteria for the meta-analysis were as follows: conference abstracts, studies involving a pediatric population (<18 years old), studies reporting type 1 diabetes mellitus, or studies not published in English.
Data extraction
W.W. and S.N. independently assessed the eligibility of each study. In the first round of screening, titles and abstracts were reviewed to exclude articles that did not meet the eligible criteria. The second round of screening involved a full-text review to ensure that the eligible studies met all inclusion criteria. Disagreements were resolved through discussion and consultation with N.C., a third senior investigator.
The extracted data included the author, country where the study was conducted, study design, year of publication, number of participants, study setting, recruitment or identification methods for diabetic participants, diagnostic modalities and cutoff values used to identify advanced fibrosis, baseline characteristics of participants, diabetic duration, and glycemic control as determined by hemoglobin A1c (HbA1c). If an article reported multiple prevalence values through different diagnostic modalities, all values were included in our subsequent meta-analysis.
The entire T2DM population was derived from participants in each study that reported the prevalence of advanced fibrosis in all T2DM patients, regardless of documented MASLD status. The T2DM population with MASLD was derived from participants in each study that reported data on advanced fibrosis in T2DM patients with documented MASLD. MASLD was defined as the presence of hepatic steatosis in conjunction with one cardiometabolic risk factor and no significant alcohol consumption.
Statistical analysis
The prevalence of each study was calculated using raw data by dividing the number of patients with advanced fibrosis by the study sample size. The reported prevalence (%) and sample size were used to estimate the number of cases. The quality of the eligible studies was assessed using the Joanna Briggs Institute (JBI) Prevalence Critical Appraisal Tool.11 A pooled prevalence was calculated with the DerSimonian–Laird random-effects model with logit transformation, and a 95% confidence interval (CI) was estimated using the Wilson score method. The Cochran's Q test and I2 statistics were employed to determine statistical heterogeneity. Estimates with a P value lower than 0.10 for the Q-statistic and I2 greater than 50% were considered significant heterogeneity.12
The pooled prevalence of advanced fibrosis in T2DM was also assessed under sub-categorizations of 12 study characteristics: mean age of the sample, body mass index (BMI), geographic regions, country, study population, diagnostic modalities, publication year, study sample size, study setting, diabetic duration, glycemic control, and JBI score. Meta-regression analyses using mixed-effects models were performed to explore the diversity in the results of different studies. The percentage of males, the mean age of the sample, mean BMI, mean diabetes duration, mean HbA1c, diabetic complications, geographic regions, study sample size, diagnostic methods, publication year, and year of start/end data collection, study setting, and JBI score were examined univariately and jointly in a meta-regression model using the backward method.13 Egger's test was used to assess publication bias. All data were analyzed using STATA 14 (StataCorp LP, College Station, Texas, USA).
Results
Identification and selection of studies
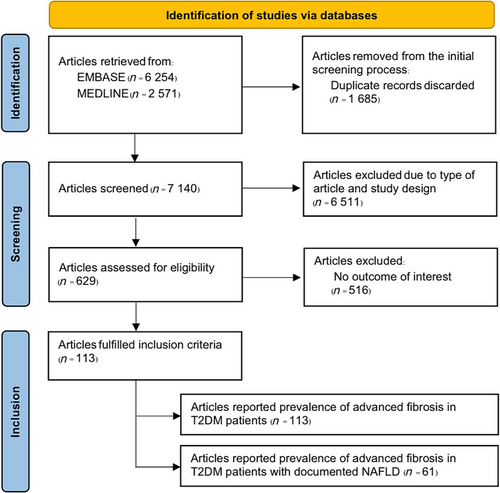
A total of 8825 potentially relevant articles were retrieved, comprising 6254 from EMBASE and 2571 from MEDLINE. After removing 1685 duplicated articles, 7140 articles underwent the first-round review. Subsequently, 6511 titles and abstracts not meeting the eligibility criteria were excluded, resulting in 629 articles for the second round of full-text review. Ultimately, 113 studies fulfilled the inclusion criteria and were included in the analysis. Figure 1 provides an overview of the literature review and study selection process.
Characteristics of the studies
Table 1 summarizes the baseline characteristics of the T2DM population included in this meta-analysis. A total of 113 studies conducted between 2004 and 2022 involving 244 858 patients with T2DM were included. The mean age of the participants was 57.9 years (range 37.4–78.9 years), and the mean BMI was 30.2 kg/m2 (range 24.4–46.9 kg/m2). On average, 49.0% of the T2DM patients were male. Regarding diabetic characteristics at baseline, the mean duration of diabetes was 8.7 years (range 3–16.8 years), and the mean HbA1c was 7.6% (range 6.1–9.9%). One hundred thirteen reports covered six regions of the world: seven from South America, 21 from North America, 31 from Europe, 44 from East Asia, nine from South Asia, and one from West Asia. Thirty-three studies reported multiple values of prevalence through different diagnostic modalities. Data S2 and S3 summarize the characteristics of the study population and diabetic complications, respectively. The prevalence of diabetic complications was as follows: nephropathy (39.5%, range 6–80.6%), neuropathy (31.9%, range 10.6–66.0%), retinopathy (24.3%, 9.7–54.0%), and cardiovascular disease (21.9%, range 0.8–40.4%). Data S4 describes the quality assessment using the JBI Tool.
| Studies, n | Median | Mean | Range | |
|---|---|---|---|---|
| Total n | 113 | 346 | 2167 | 32–121 513 |
| Mean age, years | 110 | 59.0 | 57.9 | 37.4–78.9 |
| Male, % | 111 | 49.7 | 49.0 | 17.0–77.0 |
| Mean BMI, kg/m2 | 106 | 29.4 | 30.2 | 24.4–46.9 |
| Mean hemoglobin A1c, % | 88 | 7.6 | 7.6 | 6.1–9.9 |
| Duration of diabetes, years | 49 | 8.2 | 8.7 | 3–16.8 |
| Publication year | 113 | 2021 | — | 2004–2022 |
| Start data collection, year | 98 | 2015 | — | 1997–2021 |
| End data collection, year | 99 | 2017 | — | 2002–2022 |
Data on the histologic characteristics of MASLD among biopsied patients with T2DM is available from the following 11 countries: USA (seven studies), Brazil (three studies), France (two studies), Spain (two studies), China (two studies), India (two studies), Italy (one study), Australia (one study), Japan (one study), Malaysia (one study), and Turkey (one study) (Data S5). The random-effects analysis yielded a summary proportion of steatosis of 95.5% (95% CI 89.5–99.2%), steatohepatitis of 67.4% (95% CI 57.7–76.4%), hepatic inflammation of 93.1% (95% CI 84.0–98.7%), and hepatocyte ballooning of 75.3% (95% CI 60.5–87.6%), as well as liver fibrosis stages 1, 2, 3, and 4 of 29.9% (95% CI 22.9–37.5%), 13.1% (95% CI 9.2–17.6%), 16.5% (95% CI 11.0–22.9%), and 5.9% (95% CI 2.8–10.0%), respectively.
Prevalence of advanced fibrosis in patients with T2DM according to geographic regions
The global prevalence of advanced fibrosis among the entire T2DM population was 19.5% (95% CI 16.8–22.4%; I2 = 99.84%). Egger's test for the global prevalence of advanced fibrosis did not show evidence of publication bias (P = 0.458). The regional prevalence was 11.3% in South America (95% CI 6.2–17.5%; I2 = 92.17%), 15.8% in North America (95% CI 11.0–21.3%; I2 = 99.95%), 20.0% in Europe (95% CI 15.8–24.6%; I2 = 98.63%), 20.1% in East Asia (95% CI 14.7–26.1%; I2 = 99.74%), 24.4% in South Asia (95% CI 16.2–33.7%; I2 = 95.02%), and 60.5% in West Asia (95% CI 50.3–70.4%) (Table 2 and Fig. S1). Egger's test showed evidence of possible publication bias for the regional prevalence in Europe (P < 0.001) and South Asia (P = 0.029). However, it did not show significant publication bias for East Asia (P = 0.227), North America (P = 0.071), and South America (P = 0.096). Among the regions with at least three studies for each country, South Asia had the highest prevalence of advanced fibrosis among the TDM population, at 24.9% (95% CI 15.7–35.2%; I2 = 95.43%), and South America had the lowest, at 11.0% (95% CI 5.5–18.1%; I2 = 93.21%).
| Number of studies | Total patients | Inter-study range | Prevalence (95% CI) | I2 | |
|---|---|---|---|---|---|
| Mean age, years | |||||
| <50 | 10 | 2021 | 36–821 | 20.7 (15.8–26.0) | 97.48% |
| 50–59 | 57 | 86 345 | 42–47 146 | 20.9 (10.9–33.1) | 99.67% |
| ≥60 | 43 | 149,081 | 47–121,513 | 18.1 (14.6–22.0) | 99.89% |
| Mean BMI | |||||
| <25 kg/m2 | 3 | 8495 | 264–1729 | 13.0 (6.5–21.3) | - |
| 25–30 kg/m2 | 57 | 88 865 | 50–47 146 | 21.3 (16.5–26.5) | 99.67% |
| > 30 kg/m2 | 46 | 20 582 | 36–2940 | 18.5 (15.3–21.9) | 98.29% |
| Mean hemoglobin A1c | |||||
| ≤7% | 17 | 6192 | 73–2,940 | 22.2 (16.1–29.0) | 97.97% |
| >7% | 71 | 170 942 | 42–121 513 | 17.2 (14.2–20.5) | 99.86% |
| Duration of diabetes | |||||
| <10 years | 31 | 23 657 | 32–3861 | 17.2 (10.6–25.1) | 99.96% |
| ≥10 years | 18 | 137 334 | 79–121 513 | 16.6 (11.2–22.9) | 99.61% |
| Diagnosis | |||||
| Liver biopsy | 23 | 5284 | 32–1295 | 27.8 (20.9–35.1) | 96.78% |
| NAFLD fibrosis score | 34 | 122 491 | 47–96 260 | 22.0 (17.5–26.9) | 99.36% |
| FIB-4 | 32 | 142 186 | 85–113 935 | 11.5 (9.5–13.7) | 98.17% |
| APRI | 6 | 125 328 | 100–114 055 | 5.2 (2.4–8.8) | 99.23% |
| VCTE | 50 | 19 237 | 42–1884 | 18.3 (16.2–20.5) | 92.62% |
| MRE | 3 | 289 | 95–98 | 6.9 (4.2–10.2) | — |
| BARD | 5 | 51 258 | 21–47 146 | 75.7 (55.0–91.6) | 99.77% |
| Others | 16 | 135 044 | 104–121 393 | 19.6 (14.4–25.3) | 99.23% |
| Region† | |||||
| East Asia | 44 | 85 509 | 68–47 146 | 20.1 (14.7–26.1) | 99.74% |
| Europe | 31 | 16 837 | 36–4278 | 20.0 (15.8–24.6) | 98.63% |
| North America | 21 | 139 366 | 47–121 513 | 15.8 (11.0–21.3) | 99.95% |
| South America | 7 | 1356 | 47–554 | 11.3 (6.2–17.5) | 92.17% |
| South Asia | 9 | 1717 | 32–531 | 24.4 (16.2–33.7) | 95.02% |
| West Asia | 1 | 73 | 73–73 | 60.5 (50.3–70.4) | — |
| Publication, year | |||||
| <2017 | 15 | 4277 | 32–1884 | 15.0 (10.1–20.7) | 94.71% |
| ≥2017 | 98 | 240 581 | 36–121 513 | 20.1 (17.2–23.1) | 99.86% |
| Sample size | |||||
| ≤1000 participants | 92 | 29 083 | 32–911 | 20.8 (18.4–23.4) | 97.57% |
| >1000 participants | 21 | 215 775 | 1108–121 513 | 14.3 (9.2–20.4) | 99.97% |
| Study setting | |||||
| Population-based setting | 14 | 74 929 | 173–47 146 | 15.3 (8.0–24.4) | 99.89% |
| Primary care setting | 9 | 3825 | 95–1734 | 16.3 (9.3–24.8) | 98.16% |
| Hospital-based setting | 21 | 136 298 | 32–121 513 | 17.1 (11.9–23.1) | 99.95% |
| Diabetes clinic | 51 | 26 310 | 47–2770 | 17.4 (14.3–20.8) | 98.33% |
| Hepatology clinic | 18 | 3496 | 36–523 | 33.7 (28.2–39.4) | 95.95% |
| JBI score | |||||
| ≤6 | 56 | 147 391 | 32–121 513 | 20.0 (16.7–23.5) | 99.85% |
| 7 | 37 | 27 288 | 68–3861 | 20.9 (15.3–27.0) | 99.50% |
| ≥8 | 20 | 70 179 | 272–47 146 | 15.1 (8.0–24.0) | 99.84% |
- † North America (USA); South America (Brazil, Chile); Europe (Croatia, Czech Republic, France, Germany, Greece, Italy, Poland, Portugal, Romania, Spain, Sweden, Turkey); East Asia (Australia, China, Hong Kong, Japan, Korea, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam); South Asia (India, Bangladesh); and West Asia (Lebanon).
- APRI, AST-to-platelet ratio index; BMI, body mass index; CI, confidence interval; FIB-4, fibrosis-4; JBI, Joanna Briggs Institute; MRE, magnetic resonance elastography; NAFLD, nonalcoholic fatty liver disease; T2DM, type 2 diabetes mellitus; VCTE, vibration-controlled transient elastography.
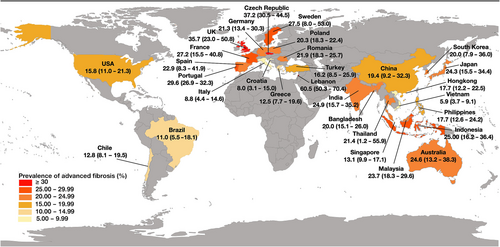
Figure 2 displays the pooled prevalence of advanced fibrosis among the entire T2DM population for all countries with at least one study. The prevalence of advanced fibrosis varied substantially among countries, from 5.9% (Vietnam, 95% CI 3.7–9.1%) to 60.5% (Lebanon, 95% CI 50.3–70.4%). There was high heterogeneity among the results. Among countries with at least three studies, France had the highest prevalence of advanced fibrosis in the T2DM population, at 27.2% (95% CI 15.5–40.8%), and Italy had the lowest, at 8.8% (95% CI 4.4–14.6%).
Meta-regression analyses on the prevalence of advanced fibrosis in patients with T2DM
To assess the robustness of the pooled effects, sensitivity analysis was conducted by performing a series of leave-one-out diagnostic tests that failed to detect outliers (Data S6). A meta-regression analysis was performed to explore the source of heterogeneity (Table 3). Our univariate meta-regression model indicated that mean age, percentage of male patients, mean BMI, publication year, start and end collect year, sample size, diabetic complications, diabetic duration, HbA1c level as indicating glycemic control, and the JBI score were not significantly associated with heterogeneity. The geographic region (R2 = 1.97%, P = 0.037), diagnostic modality (R2 = 39.29%, P < 0.001), and study setting (R2 = 8.86%, P < 0.001) were identified as sources of heterogeneity across studies in the univariate meta-regression analysis. In the multivariable meta-regression analysis, we found that diagnostic modality and study setting were significant predictors, accounting for 45.13% of the heterogeneity.
| Univariate analysis | Multivariate analysis† | ||||
|---|---|---|---|---|---|
| Moderators | OR (95% CI) | P value | R2 (%) | OR (95% CI) | P value |
| Region‡ | 0.037 | 1.97 | |||
| East Asia | Reference | Reference | |||
| Europe | 0.99 (0.94–1.06) | 0.981 | 1.01 (0.96–1.07) | 0.607 | |
| North America | 0.96 (0.89–1.03) | 0.214 | 1.01 (0.94–1.08) | 0.842 | |
| South America | 0.91 (0.80–1.04) | 0.169 | 0.93 (0.84–1.04) | 0.206 | |
| South Asia | 1.04 (0.93–1.16) | 0.481 | 1.06 (0.97–1.16) | 0.203 | |
| West Asia | 1.53 (1.12–2.09) | 0.008 | 1.20 (0.91–1.59) | 0.198 | |
| Mean age | 0.99 (0.99–1.01) | 0.537 | 0.00 | ||
| Male % | 0.99 (0.99–1.00) | 0.435 | 0.00 | ||
| Mean BMI, kg/m2 | 0.99 (0.99–1.01) | 0.999 | 0.00 | ||
| Hemoglobin A1c, % | 1.01 (0.96–1.06) | 0.728 | 0.00 | ||
| Duration of diabetes, years | 1.00 (0.99–1.02) | 0.622 | 0.00 | ||
| Diabetic nephropathy, % | 0.99 (0.99–1.01) | 0.904 | 0.00 | ||
| Diabetic neuropathy, % | 1.00 (0.99–1.01) | 0.998 | 0.00 | ||
| Diabetic retinopathy, % | 1.00 (0.99–1.01) | 0.862 | 0.00 | ||
| Cardiovascular disease, % | 0.99 (0.99–1.00) | 0.693 | 0.00 | ||
| Publication year | 0.99 (0.98–1.01) | 0.854 | 0.00 | ||
| Start data collection, year | 0.99 (0.99–1.01) | 0.339 | 0.28 | ||
| End data collection, year | 1.00 (0.99–1.01) | 0.992 | 0.00 | ||
| Sample size | 0.99 (0.99–1.00) | 0.924 | 0.00 | ||
| Diagnosis | <0.001 | 39.29 | |||
| Liver biopsy | Reference | Reference | |||
| NAFLD fibrosis score | 0.93 (0.86–1.01) | 0.060 | 0.98 (0.95–1.07) | 0.707 | |
| FIB-4 | 0.84 (0.78–0.91) | <0.001 | 0.89 (0.82–0.97) | 0.005 | |
| APRI | 0.78 (0.69–0.88) | <0.001 | 0.83 (0.74–0.94) | 0.004 | |
| VCTE | 0.89 (0.83–0.96) | 0.002 | 0.93 (0.86–0.99) | 0.047 | |
| MRE | 0.79 (0.65–0.96) | 0.015 | 0.81 (0.66–1.04) | 0.054 | |
| BARD | 1.50 (1.30–1.73) | <0.001 | 1.57 (1.35–1.82) | <0.001 | |
| Others | 0.91 (0.83–0.99) | 0.035 | 0.94 (0.85–1.03) | 0.156 | |
| Study setting | <0.001 | 8.86 | |||
| Population-based setting | Reference | Reference | |||
| Primary care setting | 1.01 (0.90–1.14) | 0.813 | 1.05 (0.95–1.18) | 0.333 | |
| Hospital-based setting | 1.02 (0.94–1.11) | 0.664 | 1.02 (0.95–1.09) | 0.646 | |
| Diabetes clinic | 1.03 (0.95–1.11) | 0.504 | 1.03 (0.96–1.10) | 0.475 | |
| Hepatology clinic | 1.18 (1.08–1.30) | <0.001 | 1.15 (1.05–1.25) | 0.002 | |
| JBI score | 0.287 | 0.24 | |||
| ≤6 | Reference | ||||
| 7 | 1.01(0.95–1.07) | 0.844 | |||
| ≥8 | 0.95(0.88–1.02) | 0.151 | |||
- † R2 = 45.13%.
- ‡ North America (USA); South America (Brazil, Chile); Europe (Croatia, Czech Republic, France, Germany, Greece, Italy, Poland, Portugal, Romania, Spain, Sweden, Turkey); East Asia (Australia, China, Hong Kong, Japan, Korea, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam); South Asia (India, Bangladesh); and West Asia (Lebanon).
- APRI, AST-to-platelet ratio index; BMI, body mass index; CI, confidence interval; FIB-4, fibrosis-4; JBI, Joanna Briggs Institute; MRE, magnetic resonance elastography; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; T2DM, type 2 diabetes mellitus; VCTE, vibration-controlled transient elastography.
Prevalence of advanced fibrosis in patients with T2DM by study-level characteristics
Table 2 presents the pooled prevalence of advanced fibrosis in the entire T2DM population stratified by age, BMI, diabetic duration, HbA1c, diagnostic method, region, publication year, sample size, study setting, and JBI scores. High heterogeneity was observed among the results. The prevalence of advanced fibrosis did not significantly differ across age groups. However, it varied based on BMI, with the highest prevalence observed among patients with BMI between 25 and 30 kg/m2 (21.1%) and the lowest in those with BMI less than 25 kg/m2 (13.0%). There were no statistically significant differences in prevalence estimates when stratified by diabetic duration (P = 0.640) and HbA1c levels (P = 0.303). Our analysis also revealed a 34% increase in the global prevalence of advanced fibrosis from 15.0% in studies published before 2017 to 20.1% in those published since 2017 (P = 0.196).
Regarding the study setting, the highest prevalence of advanced fibrosis was observed in hepatology clinics (33.7%), while other settings, including population-based settings, primary care settings, hospital-based settings, and diabetic clinics, showed comparable prevalence rates ranging from 15.3% to 17.4%. Similarly, the global prevalence of advanced fibrosis varied slightly based on the JBI tool, with scores of ≤6, 7, and ≥8 showing prevalence rates of 20.0%, 20.9%, and 15.1%, respectively (P = 0.287).
When assessed by different diagnostic methods, the global prevalence of advanced fibrosis in the entire T2DM population was as follows: 27.8% by liver biopsy (95% CI 20.9–35.1%; I2 = 96.78%), 11.5% by the fibrosis-4 index (FIB-4) (95% CI 9.5–13.7%; I2 = 98.17%), 6.9% by magnetic resonance elastography (MRE) (95% CI 4.2–10.2%), 22.0% by the NAFLD fibrosis score (NFS) (95% CI 17.5–26.9%; I2 = 99.36%), 5.2% by the aspartate aminotransferase-to-platelet ratio index (APRI) (95% CI 2.4–8.8%; I2 = 99.23%), 18.3% by vibration-controlled transient elastography (VCTE) (95% CI 16.2–20.5%), 75.7% by the BARD score (95% CI 55.0–91.6%; I2 = 99.77%), and 19.6% by other scoring systems, including the Enhanced Liver Fibrosis, FibroTest, AST/ALT ratio, Diabetic Fibrosis Score, and Forns Index (95% CI 14.4–25.3%; I2 = 99.23%) (Table 2 and Fig. S2).
The regional prevalence of advanced fibrosis in T2DM stratified by diagnostic modality is presented in Data S7. The sensitivity analysis revealed that the prevalence of advanced fibrosis diagnosed by individual modality varied notably among geographic regions. The regional prevalence of advanced fibrosis among biopsied patients with T2DM was 39.4% in Europe (95% CI 21.6–58.8%), 37.3% in East Asia (95% CI 21.4–54.7%), 26.6% in North America (95% CI 17.4–36.9%), 10.2% in South America (95% CI 0.0–34.8%), and 6.7% in South Asia (95% CI 2.6–12.3%). The regional prevalence of advanced fibrosis as measured by VCTE was 25.7% in South Asia (95% CI 13.9–39.7%), 18.3% in Europe (95% CI 13.7–23.4%), 17.9% in East Asia (95% CI 15.8–20.1%), 15.1% in South America (95% CI 12.0–18.5%), and 11.9% in North America (95% CI 9.1–14.9%). Notably, South Asia had the highest prevalence of advanced fibrosis as evaluated by the FIB-4 or NFS, whereas South America had the lowest prevalence among T2DM patients.
Prevalence of advanced fibrosis in T2DM patients with documented MASLD
A total of 61 studies reported data on advanced fibrosis in 88 942 T2DM patients with documented MASLD. The global prevalence of advanced fibrosis in the T2DM population with MASLD was 23.5% (95% CI 19.3–28.0%; I2 = 99.57%), with regional prevalences of 14.1% in South America (95% CI 4.0–28.8%), 22.0% in North America (95% CI 13.1–32.4%; I2 = 99.49%), 25.1% in Europe (95% CI 17.8–33.3%; I2 = 98.78%), 21.1% in East Asia (95% CI 14.7–28.4%; I2 = 99.73%), and 25.5% in South Asia (95% CI 15.5–37.0%), and 60.5% in West Asia (95% CI 50.3–70.4%).
Discussion
Our meta-analysis revealed that one fifth of the global population with T2DM had advanced fibrosis. These findings have implications for patients receiving diabetes care in these regions and can be used to develop new strategies or to consolidate existing practices in evaluating T2DM patients for advanced fibrosis and referring them to hepatology services.
In 2019, a meta-analysis reported a 22.0% prevalence of advanced fibrosis in biopsied patients with MASLD and T2DM, limited by data from only 439 patients across five countries.2 Another meta-analysis in 2023 found a prevalence of 14.95%, based on a sensitivity analysis of 21 studies with limited liver biopsy and VCTE data.1 Acknowledging the limitations of these studies, particularly in data comprehensiveness and methodology, these shortcomings may have introduced bias and affected the accuracy of the estimates. To address these limitations, our meta-analysis encompasses a larger dataset comprising 113 studies with a total of 244 858 middle-aged patients with T2DM from diverse geographic regions worldwide. Our approach, incorporating both biopsy and various noninvasive modalities, offers a more comprehensive evaluation of advanced fibrosis prevalence in this population. Our findings indicate a global prevalence of advanced fibrosis in patients with T2DM at 19.5%, with a higher prevalence of 23.5% in T2DM patients with documented MASLD. This figure is three times higher than that of the general population with MASLD from North America.14 The synergistic effects of MASLD and T2DM on liver damage and fibrosis progression highlight the importance of risk assessment for advanced fibrosis in T2DM patients, especially considering the global burden of T2DM.15
Since the European Association for the Study of the Liver published the NAFLD Clinical Practice Guideline in 2016,9 there has been a substantial increase in the number of published studies assessing MASLD and liver fibrosis stage in T2DM patients. In this study, we showed that, compared to the 2004–2016 period, the prevalence of advanced fibrosis among patients with T2DM rose 34% in the recent period, 2017–2022, with a prevalence of 20.1%. These data align with predictions about the burden of MASLD-associated cirrhosis by 2030.16
Our study also examined the prevalence of advanced fibrosis in T2DM patients across different geographic regions. We acknowledge the concerns raised regarding the reliance on data from a limited number of studies, particularly from West Asia, which could potentially introduce selection bias and limit the generalizability of the findings to the broader region. To address this issue and ensure the robustness of our analysis, we implemented rigorous criteria, excluding data from countries with fewer than three studies. As a result, we found that South Asia had the highest prevalence of advanced fibrosis in T2DM at 24.9%, while the rest of the world exhibited lower prevalence rates, with South America reporting the lowest prevalence at 11.0%. This observation is consistent with existing literature indicating that South Asians often present with a more unfavorable body fat distribution characterized by central obesity, predisposing them to insulin resistance and metabolic disorders associated with accelerated liver fibrosis progression.17 Contributing factors such as low physical activity, consumption of energy-rich and imbalanced diets, reduced disease awareness, healthcare-seeking behaviors, delayed diagnosis, and social disparities further compound the risk of advanced fibrosis in South Asian populations compared to other ethnic groups.18 Conversely, South America reported the lowest prevalence of advanced fibrosis, based on limited data from seven studies conducted in Brazil and Chile, involving only 1356 patients with T2DM. This observed disparity may stem from under-recognition, under-referral, or genuinely lower prevalence of advanced fibrosis in this region.
Surprisingly, the prevalence of advanced fibrosis in East Asia resembled that of Europe. This similarity could be influenced by increased diagnosis or greater availability of Western diets with energy-dense food consumption in East Asia.19 However, East Asians generally have lower rates of physical inactivity compared to Western populations.20 Ethnic variations in MASLD severity may also stem from genetic differences.21 Therefore, the comparable prevalence of advanced fibrosis between East Asians and Europeans likely involves a complex interplay of diet, physical activity, and genetic factors. Additionally, our study revealed diverse prevalence rates of advanced fibrosis among European countries, possibly attributed to variations in lifestyle and dietary habits. Notably, regions following the Mediterranean diet, such as Italy, Croatia, and Greece, exhibited lower rates of advanced fibrosis among individuals with T2DM compared to other European areas, particularly those in northern France. These findings align with existing research indicating the protective effects of the Mediterranean diet against MASLD severity and progression.22
Our study examined regional variations in the prevalence of advanced fibrosis among T2DM patients, considering factors like diabetic management, study setting, and diagnostic modality. Interestingly, factors related to diabetes management, such as duration of diabetes, glycemic control according to HbA1c levels, and diabetic complications, did not significantly influence advanced fibrosis prevalence, as evaluated by subgroup analysis and meta-regression analysis. Notably, the highest prevalence of advanced fibrosis was observed in hepatology clinics (33.7%), indicating potentially more severe liver disease among patients attending these clinics, possibly due to referral bias or comorbidities requiring specialized care. Conversely, advanced fibrosis prevalence was similar across other settings, including population-based settings, primary care settings, hospital-based settings, and diabetic clinics (ranging from 15.3% to 17.4%), suggesting more homogeneous prevalence rates in these contexts. Regional disparities were evident, with higher prevalence rates in regions where hepatology clinics and liver biopsy were more common, like Europe and North America, compared to regions with limited access to specialized liver care, such as South Asia. Nevertheless, discrepancies in the prevalence of advanced fibrosis between South Asian studies using noninvasive methods versus liver biopsy suggest potential variations in sensitivity and specificity, possibly influenced by metabolic differences, leading to either overestimation or underestimation of prevalence.
This study also examines the impact of different diagnostic modalities on advanced fibrosis prevalence. Meta-regression models revealed that diagnostic modality influences heterogeneity in advanced fibrosis prevalence, with noninvasive diagnostics, except the BARD score, generally reporting lower rates compared to liver biopsy. Elastography-based techniques, like VCTE, showed a prevalence of 18.3% but with significant heterogeneity due to varied diagnostic thresholds. Although MRE outperforms VCTE in detecting advanced fibrosis,23 the limited availability of MRE likely contributed to its lower prevalence. Conversely, blood-based scoring systems reported substantially lower prevalence, possibly due to their limited sensitivity.24, 25 NFS yielded a higher prevalence than FIB-4 and APRI, likely due to diabetes inclusion in its algorithm.26 The BARD score showed notably higher prevalence, reflecting potential overdiagnosis in the T2DM population, as diabetes is one of the parameters included in this score.27 These findings underscore the importance of accurate diagnostic methods, particularly imaging-based approaches, in detecting advanced fibrosis.
This meta-analysis has several strengths. We conducted a thorough literature search spanning two decades, providing a comprehensive overview of the topic globally. Our study expanded on previous meta-analyses1, 2 by incorporating more recent and extensive data, conducting detailed subgroup analyses, and including studies on T2DM patients with and without documented MASLD. Additionally, we utilized meta-regression models to address potential confounders not fully explored in prior analyses due to sample size limitations. Furthermore, our analysis offers epidemiological insights at both country and regional levels, facilitating the development of targeted strategies to address the growing burden of advanced fibrosis in T2DM patients.
Our meta-analysis has limitations inherent to the included studies. High heterogeneity across most subgroup analyses for pooled prevalence can be attributed to variations in ethnicity, sociodemographic factors, characteristics of the study population, study setting, and diagnostic methodology. Despite conducting subgroup analyses to understand heterogeneity sources, it still persisted. We also evaluated the influence of study quality on the variability of the results using the JBI score. Our analysis found no significant difference in the prevalence rates between the quality groups, indicating limited selection bias. However, selection bias may still be a potential limitation that requires further investigation. The lack of data from Africa during our comprehensive systematic review limits the applicability of the results in this region. Finally, evidence of potential publication bias in some subgroups necessitates cautious data interpretation.
In conclusion, our meta-analysis reveals that the global prevalence of advanced fibrosis in T2DM patients is approximately one fifth, with geographic variations. The data underscore the importance of proper noninvasive diagnostics for clinical practice based on resource availability and health system structure. Healthcare professionals should be aware of the high prevalence of advanced fibrosis among T2DM patients. Implementing inclusive screening for high-risk populations would facilitate early referral, specialty consultation, and intensive intervention to mitigate adverse clinical outcomes.
Open Research
Data availability statement
All relevant data are within the paper and its Supporting Information files.