Response to COVID-19 vaccine is reduced in patients with inflammatory bowel disease, but improved with additional dose
Abstract
Background
Coronavirus disease 2019 (COVID-19) vaccination is recommended for patients with inflammatory bowel disease (IBD); however, suppressed immune responses have been reported for fully vaccinated patients under immunosuppressive therapy, mainly from Western countries. We prospectively analyzed antibody titers of IBD patients in Asia induced by two-dose and additional dose of messengerRNA COVID-19 vaccine.
Methods
After measuring high-affinity antibody titers, factors associated with antibody titers were identified by multiple regression analyses using the following covariates: sex, age (≥60 or <60 years), disease type (Crohn's disease or ulcerative colitis), vaccine type (BNT162b2 or mRNA-1273), time from second/third vaccination, molecular-targeted agent (anti-tumor necrosis factor [TNF] agents, ustekinumab, vedolizumab, tofacitinib, or no molecular-targeted agents), thiopurine, steroid, and 5-aminosalicylic acid.
Results
Among 409 patients analyzed, mean titer was 1316.7 U/mL (SD, 1799.3); 403 (98.5%) were judged to be seropositive (≥0.8 U/mL), and 389 (95.1%) had neutralizing antibodies (≥15 U/mL). After the third vaccination, mean titer raised up to 21 123.8 U/mL (SD, 23 474.5); all 179 were seropositive, and 178 (99.4%) had neutralizing antibodies. In 248 patients with genetic data, there was no difference in mean titer after two/third doses between carriers and non-carriers of HLA-A24 associated with severe disease during COVID-19 infection. A multiple regression analyses using covariates revealed that older age, vaccine type (BNT162b2), time from second/third dose, anti-TNF agent, tofacitinib, and thiopurine were independently associated with lower antibody titers.
Conclusions
Our findings further support the recommendation for COVID-19 vaccination in patients under immunosuppressive therapy, especially additional third dose for patients receiving anti-TNF agents and/or thiopurine or tofacitinib.
Introduction
Coronavirus disease 2019 (COVID-19; caused by severe acute respiratory syndrome coronavirus-2 [SARS-CoV-2]) vaccination is highly effective and recommended for patients with inflammatory bowel disease (IBD)1, 2; however, the induced immune response may be reduced under some immunosuppressive therapies.3, 4 Notably, suppressed immune responses have been reported for IBD patients treated with anti-tumor necrosis factor (TNF) agents, mainly from Western countries.5-10 Because patients in Asia have different genetic profile from Western countries, such as human leukocyte antigen (HLA) haplotype, it may have some impact on the immune response against SARS-CoV-2.11 Also, even if the immune response is suppressed up to the second dose of vaccination, it may improve with additional third dose; however, there are still few reports on this.
We prospectively analyzed antibody titers induced by messengerRNA (mRNA) COVID-19 vaccines in fully vaccinated Asian patients with IBD under immunosuppressive therapy. We also analyzed the booster effect under immunosuppressive therapy after the third vaccination.
Materials and methods
Subjects
We prospectively analyzed antibody titers in patients with Crohn's disease (CD) or ulcerative colitis (UC) who were treated at the Tohoku University Hospital with one or more of the following drug types: molecular-targeted agent (infliximab, adalimumab, golimumab, ustekinumab, vedolizumab, or tofacitinib), thiopurine (azathioprine or 6-mercaptopurine), steroid (prednisolone), and 5-aminosalicylic acid. The mRNA COVID-19 vaccines (BNT 162b2 [Pfizer-BioNTech]12 or mRNA-1273 [NIH-Moderna]13) were each administered on their approved two-dose vaccination schedule. More than 6 months after the above two-dose vaccination, a third dose (either BNT162b2 or mRNA-1273) was additionally administered. However, for mRNA-1273, only half dose was used as the third vaccination.
The Ethics Committee of Tohoku University Hospital approved the study protocol on 25 May 2020 (No. 2020-1-140). All participants provided written informed consent.
Measurement of antibody titer
High-affinity antibodies, including IgA/IgG/IgM specific for the spike protein of SARS-CoV-2, in serum were quantitatively measured using an Anti-SARS-CoV-2 detection kit (Elecsys Anti-SARS-CoV-2 S RUO, Roche Diagnostics K.K., Tokyo, Japan). Blood samples were collected for antibody titers and other blood tests at regular visits before SARS-CoV-2 vaccination, after the first dose, after the second dose, and after additional third dose. Patients with a history of COVID-19, those with positive pre-vaccination antibody titers indicating a high probability of prior infection, and those without pre-vaccination antibody titer data were excluded. We defined an antibody titer of ≥0.8 U/mL as a positive result and an antibody titer of ≥15 U/mL as indicative of having neutralizing antibodies.
The following information was extracted as the patient clinical characteristics: sex (male or female), age (≥60 or <60 years), disease type (CD or UC), vaccine type (BNT162b2 or mRNA-1273), dates of the first/second/third vaccine administration, post-vaccination adverse reactions (e.g., fever of ≥37.5°C), and type of therapeutics used (molecular-targeted agents, thiopurines, steroids, and 5-aminosalicylic acids). For patients whose HLA information had been previously analyzed, we checked whether they were carriers of the HLA-A24 allele.
Statistical analysis
Data are presented as mean value and standard deviation (SD). Using the Wilcoxon signed-rank test or Steel's multiple comparison test as appropriate, we assessed differences in SARS-CoV-2-specific antibody titers between groups for each clinical characteristic. In addition, factors associated with antibody titers were identified by multiple regression analyses using covariates. Covariates included sex, age (≥60 or <60 years), disease type (CD or UC), vaccine type (BNT162b2 or mRNA-1273), time from second/third vaccination, molecular-targeted agent (anti-TNF agents, ustekinumab, vedolizumab, tofacitinib, or no molecular-targeted agents), thiopurine (with or without), steroid (with or without), and 5-aminosalicylic acid (with or without). These analyses were performed using the JMP Pro Ver. 14 software program (SAS Institute Inc., Cary, NC, USA). A p-value of <0.05 was taken to indicate a statistically significant difference.
Results
Clinical characteristics
Between March 2021 and May 2022, 746 patients with confirmed diagnosis of CD or UC received outpatient or inpatient care at our university hospital, of whom 514 (68.9%) consented to participate in the present study. Of these 514 patients, 105 patients who had no pre-vaccination antibody titer, had a positive pre-vaccination antibody titer indicating pre-vaccination infection, had not received two-dose vaccination, or had no antibody titer measured after two-dose vaccination were excluded, resulting in 409 (79.6%) patients being included in the final analysis.
The clinical characteristics of these 409 patients are presented in Table 1. These patients included 258 men (63.1%) and 151 women (36.9%) with the diagnosis of CD or UC. The mean age was 44.3 years (SD 13.9); 60 (14.7%) patients were 60 years of age or more. Regarding molecular-targeted agents, 146, 58, 36, and 10 patients were treated with anti-TNF agents (61 with infliximab, 78 with adalimumab, and seven with golimumab), ustekinumab, vedolizumab, and tofacitinib, respectively; the remaining 159 received no molecular-targeted agents. Regarding other medications, 125 (30.6%) received thiopurines, and 271 (66.3%) were treated with 5-aminosalicylic acids. Cases with treatment changes between the vaccination schedules were not included.
| Clinical characteristics | N = 409 | |
|---|---|---|
| Sex | ||
| Male | 258 (63.1%) | |
| Female | 151 (36.9%) | |
| Age | Mean (SD) | 44.3 (13.9) |
| <60 years | 349 (85.3%) | |
| ≥60 years | 60 (14.7%) | |
| Disease type | ||
| Crohn's disease | 234 (57.2%) (active disease†: 20.3%) | |
| Ulcerative colitis | 175 (42.8%) (active disease†: 10.9%) | |
| Disease duration | Mean (SD) | 6.4 (1–27) |
| <3 years | 89 (21.8%) | |
| ≥3 years | 320 (78.2%) | |
| Disease location in Crohn's disease | ||
| Ileitis type | 30 (12.8%) | |
| Colitis type | 22 (9.4%) | |
| Ileocolitis type | 182 (77.8%) | |
| Disease extent in ulcerative colitis | ||
| Total colitis | 82 (46.9%) | |
| Left-sided colitis | 70 (40.0%) | |
| Proctitis | 23 (13.1%) | |
| Smoking habit | ||
| Current or past smoker | 132 (32.3%) | |
| Never | 277 (67.7%) | |
| Type of mRNA vaccine | ||
| BNT 162b2 (Pfizer) | 299 (73.1%) | |
| mRNA-1273 (Moderna) | 110 (26.9%) | |
| Molecular-targeted agents used | ||
| Anti-TNF agents | 146 (35.7%) | |
| (Anti-TNF agent plus thiopurines) | (57) | |
| (Anti-TNF agents alone) | (89) | |
| Tofacitinib | 10 (2.4%) | |
| Ustekinumab | 58 (14.2%) | |
| Vedolizumab | 36 (8.8%) | |
| None | 159 (38.9%) | |
| Thiopurines | ||
| Present | 125 (30.6%) | |
| Absent | 284 (69.4%) | |
| Steroids | ||
| Present | 26 (6.4%) | |
| Absent | 383 (93.6%) | |
| 5-Aminosalicylic acids | ||
| Present | 271 (66.3%) | |
| Absent | 138 (33.7%) | |
- SD, standard deviation; TNF, tumor necrosis factor.
- † Active disease is defined as a Crohn's disease activity index (CDAI) of 150 or more for Crohn's disease or clinical activity index (CAI) of 5 or more for ulcerative colitis.
Vaccinations
Two doses of BNT162b2 (Pfizer) and mRNA-1273 (Moderna) vaccines were given to 299 (73.1%) and 110 (26.9%) patients, respectively. Among 409 patients, 179 (43.8%) received additional third dose (either BNT162b2 or mRNA-1273) more than 6 months after the two-dose vaccination. The same vaccine as the first and second vaccines was administered as the third vaccine in 62 (Pfizer–Pfizer) and 41 (Moderna–Moderna) patients. Meanwhile, a different vaccine from the first and second vaccines was given in 71 (Pfizer–Moderna) and five (Moderna–Pfizer) patients.
Antibody titers after the first, second, and third vaccinations
In the 115 (28.1%) patients whose SARS-CoV-2-specific antibody titer was measured between the first and second vaccination, the mean titer was 21.1 U/mL (SD, 55.2) (Fig. 1); 65 (56.5%) of them were considered to be seropositive (≥0.8 U/mL), and only 29 (25.2%) patients had neutralizing antibodies (≥15 U/mL). After the second vaccination, antibody titers were measured on a mean of 38.5 (SD, 31.8) days: within 29 days in 200 (48.9%) patients, after 30–59 days in 140 (34.2%) patients, and after ≥60 days in 69 (16.9%) patients. The mean titer was 1316.7 U/mL (SD, 1799.3) (Fig. 1); 403 (98.5%) patients were judged to be seropositive, and 389 (95.1%) patients had neutralizing antibodies (≥15 U/mL).

After the third vaccination, blood samples were collected on a mean of 36.1 (SD, 22.7) days: within 29 days in 81 (45.3%) patients, after 30–59 days in 72 (40.2%) patients, and after ≥60 days in 26 (14.5%) patients. The mean titer raised up to 21 123.8 U/mL (SD, 23 474.5) (Fig. 1); all 179 (100.0%) patients were judged to be seropositive (≥0.8 U/mL) and 178 (99.4%) patients had neutralizing antibodies (≥15 U/mL).
Antibody titers divided by clinical characteristics
After the second vaccination, the mean antibody titer was lower in male (1218.9 vs 1483.6 U/mL in female) and 60 years or older (1030.5 vs 1365.9 U/mL in <60 years), but not significantly. However, the mean titer was significantly lower in patients with CD (1184.4 U/mL) than in patients with UC (1493.5 U/mL). When divided by the type of vaccination, the mean titer was significantly lower in the Pfizer group (987.6 U/mL) than in the Moderna group (2211.1 U/mL). Additionally, the mean titer was significantly lower when measured at 30–59 days post-vaccination (1019.1 U/mL) and ≥60 days post-vaccination (700.4 U/mL) than when measured within 29 days post-vaccination (1737.6 U/mL).
Following the third vaccination, there were no differens in the mean antibody titers by sex (22 561.1 U/mL in male vs 18 885.6 U/mL in female) or age (21 521.2 U/mL in ≥60 years vs 21 033.9 U/mL in <60 years). However, the mean titer was significantly lower in patients with CD (18 434.9 U/mL) than in patients with UC (24 164.8 U/mL). When divided by the type of vaccination, there was also no significant difference in the mean antibody titers among Pfizer–Pfizer group (21 737.8 U/mL), Pfizer–Moderna group (19 859.5 U/mL), Moderna–Pfizer group (25 146.0 U/mL), and Moderna–Moderna group (21 894.0 U/mL). Compared with antibody titers measured within 29 days post-vaccination (23 756.4 U/mL), those measured at 30–59 days post-vaccination (21,236.3 U/ml) were lower but not significantly, while those measured at ≥60 days post-vaccination (12 610.5 U/mL) were significantly lower.
In the 248 patients for whom HLA-A24 allele data were available, there was no difference in the mean titer between HLA-A24 carriers (1151.3 U/mL) and noncarriers (1168.3 U/mL) after two-dose vaccination. Following the third vaccination, HLA-A24 carriers had lower antibody titers (18 695.6 U/mL) than non-carriers (21 220.4 U/mL), but not significantly.
Antibody titers according to therapeutic agents
After the second vaccination, patients treated with thiopurines (764.6 U/mL) had a significantly lower mean antibody titer than those not receiving these drugs (1559.6 U/mL; Fig. 2a). In 125 patients receiving thiopurines, 109 (87.2%) were judged to have neutralizing antibodies (≥15 U/mL). Similarly, patients treated with molecular-targeted agents (1090.3 U/mL) had a significantly lower mean antibody titer than those not receiving these therapeutics (1672.6 U/mL). The mean titers and the rates of patients having neutralizing antibodies by molecular-targeted agents are as follows: anti-TNF agent plus thiopurines (combination therapy), 395.9 U/mL and 77.2% (44/57); anti-TNF agent alone (monotherapy), 690.8 U/mL and 98.9% (88/89); tofacitinib, 695.2 U/mL and 100.0% (10/10); ustekinumab, 1660.8 U/mL and 94.8% (55/58); vedolizumab, 2367.9 U/mL and 100.0% (36/36); and no molecular-targeted agents, 1672.6 U/mL and 98.1% (156/159), respectively (Fig. 3a and Fig. S1a). In contrast, there was no difference in the mean antibody titers between patients treated with (1309.5 U/mL) and without (1330.8 U/mL) 5-aminosalicylic acids.
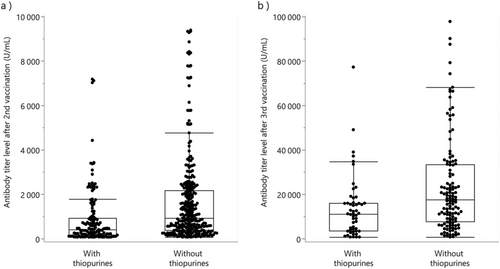
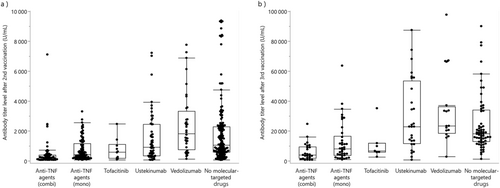
Following the third vaccination, patients treated with thiopurines (13 226.5 U/mL) had a significantly lower mean antibody titer than those not receiving these drugs (24 357.3 U/mL; Fig. 2b). In 52 patients receiving thiopurines, 51 (98.1%) were judged to have neutralizing antibodies (≥15 U/mL). Patients treated with molecular-targeted agents (17 661.3 U/mL) also had a significantly lower mean antibody titer than those not receiving these therapeutics (27 196.5 U/mL) The mean titers and the rates of patients having neutralizing antibodies by molecular-targeted agents are as follows: anti-TNF agent plus thiopurines (combination therapy), 5354.8 U/mL and 96.0% (24/25); anti-TNF agent alone (monotherapy), 11 920.1 U/mL and 100.0% (38/38); tofacitinib, 10 490.0 U/mL and 100.0% (7/7); ustekinumab, 29 694.3 U/mL and 100.0% (28/28); vedolizumab, 32 605.0 U/mL and 100.0% (16/16); and no molecular-targeted agents, 27 196.5 U/mL and 100.0% (65/65), respectively (Fig. 3b and Fig. S1b). Meanwhile, there was no difference in the mean antibody titers between patients treated with (21 414.2 U/mL) and without (20 638.2 U/mL) 5-aminosalicylic acids.
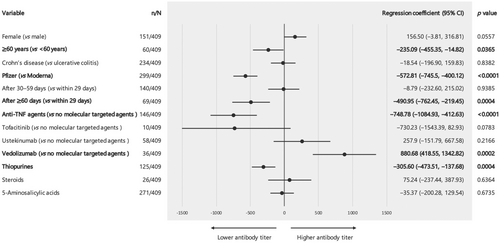
A multiple regression analysis using covariates revealed that older age, vaccine type (Pfizer), time from the second dose (after ≥60 days), molecular-targeted agents (anti-TNF agents), and thiopurines were significantly associated with lower antibody titers after the second vaccination (Fig. 4). Following the third vaccination, time from the third dose (after ≥60 days), molecular-targeted agents (anti-TNF agents and tofacitinib), and thiopurines were also correlated with reduced immune responses. However, vaccine type did not show any association with immune response (Fig. 5).

Post-vaccination adverse reactions
After the first and second vaccinations, 25 patients had no adverse reactions, whereas the remaining 384 (93.9%) had the following adverse reactions: pain at the vaccination site, fatigue, headache, chills, and fever. Similarly, 162 (90.5%) patients had adverse reactions after the third vaccination.
With regard to fever, 177 (43.3%) and 70 (39.1%) patients had a fever of 37.5°C or higher after the first or second and third vaccinations, respectively. After the first or second vaccination, the frequency of fever was significantly higher in patients younger than 60 years and in those given mRNA-1273, but no differences were observed in other clinical characteristics. After the third vaccination, fever was similarly more frequent in patients younger than 60 years, whereas fever was less frequent in those treated with molecular-targeted agents (Table S1).
Patients without fever had lower antibody titers (942.6 U/mL) than those with a fever of ≥37.5°C (1807.0 U/mL) after the first or second two-dose vaccination. Similarly, following the third vaccination, patients without fever had significantly lower antibody titers (16 108.4 U/mL) than those with a fever of ≥37.5°C (28 933.4 U/mL).
Discussion
Of the patients whose antibody titer was measured after the first dose of COVID-19 vaccine, approximately half were judged to be positive for SARS-CoV-2-specific antibody, and only one-fourth had neutralizing antibodies. However, two doses of vaccine induced adequate immunity in the majority of IBD patients. The importance of completing the recommended two-dose vaccination has already been reported.1-3 However, patients receiving immunosuppressive therapeutics, especially anti-TNF agents, showed a low (neutralizing) antibody positivity rate. Also, antibody titers have been reported to decrease over time.4, 14-16 Thus, further studies are warranted to investigate the efficacy of additional third dose. The present study showed that the third vaccination induced sufficient immunity in almost all IBD patients including those receiving anti-TNF agents and that antibody titers increased exceedingly higher than those after two-dose vaccination. Instead of ending with two doses, a third booster dose is strongly recommended.
Compared with mRNA-1273 (Moderna), the antibody titer induced by two-dose BNT162b2 (Pfizer) was significantly lower, which is consistent with the previous report.17 This result may influence clinical decisions regarding which vaccine to choose for additional booster dose. However, following the third dose, there was no significant difference in the mean antibody titers among Pfizer–Pfizer, Pfizer–Moderna, Moderna–Pfizer, and Moderna–Moderna groups. This is because only half a dose of mRNA-1273 was administered when used as the third vaccine. This may also explain why Moderna–Pfizer group showed the highest mean antibody titer.
There have been reports of differences in the clinical characteristics of IBD patients between Asian and Western countries. For example, twice as many male patients with CD are reported as female patients in Asia, whereas female patients with CD are slightly more common in Western countries.18, 19 In addition, the proportion of CD patients with anal lesions is much higher than that in Western countries.18 It is thought to be partly due to differences in genetic profile. In the present study, we analyzed the HLA-A24 allele, which has been reported to be associated with severe disease during COVID19 infection11; however, no significant difference was found between carriers and non-carriers of HLA-A24. Other related genes may exist, or rather differences in genetic profile may not be associated with response to the vaccine.
As in previous reports from Western countries,5-10 we found that antibody titers were significantly lower in patients receiving immunosuppressive therapy, such as molecular-targeted agents and thiopurines. Patients receiving anti-TNF agents (with or without concomitant thiopurine) or tofacitinib had significantly lower antibody titers than those not receiving molecular-targeted agents, which is consistent with a previous report.10 However, overall antibody titer after the third dose was more than 10 times higher than that after the second dose, and almost all patients had neutralizing antibodies, indicating that the third vaccination is strongly recommended, especially for patients receiving anti-TNF agents or tofacitinib. Whereas previous studies reported that thiopurines reduce antibody titers when used in combination with anti-TNF agents but not when used alone,5-10 we found that thiopurine use decreased antibody titer independently of other factors. Because combination therapy with anti-TNF agent and thiopurine not only lowers antibody titers but also increases the risk of severe disease in the case of COVID19 infection20,21 withdrawal of combination therapy may be considered in patients with IBD in remission.
In contrast with anti-TNF agent-treated patients, those receiving ustekinumab (anti-IL-12/23 p40 antibody) or vedolizumab (anti-integrin antibody) did not show reduced immune responses, but rather higher antibody titers than those not receiving molecular-targeted agents. These higher antibody titers may be a consequence of not only higher intestinal selectivity of vedolizumab but also lower rate of concomitant thiopurine use in patients receiving ustekinumab vedolizumab. In fact, rates of concomitant thiopurine use were 39.0%, 24.1%, 16.7%, and 30.2% in patients treated with anti-TNF agents, ustekinumab, vedolizumab, and no molecular-targeted agents, respectively.
The present study had several limitations. First, the timespan between vaccination and antibody titer measurement varied widely. To minimize this influence, a multiple regression analysis was used. Second, the IBD activity status of participants was diverse with some having active disease, which may have also affected the results. Third, only antibody titers were analyzed in this study, and it is unclear regarding the extent to which cellular immune response is induced. In fact, sufficient T-cell responses have been observed after SARS-CoV-2 vaccination in patients with IBD under immunosuppressive therapy.22, 23 Despite these limitations, our findings among IBD patients in Asia reinforce the current evidence regarding mRNA COVID-19 vaccination under immunosuppressive therapy. Because COVID-19 vaccination has been reported not to be associated with IBD exacerbation,24 IBD patients, especially those under immunosuppression, should be recommended to receive at least two doses of COVID-19 vaccine. In patients receiving anti-TNF agent, thiopurine, or tofacitinib, two-dose vaccination may not provide sufficient immunity; therefore, additional third dose is strongly recommended.
Funding
None.
Open Research
Data availability statement
The data underlying this article cannot be shared publicly given the privacy of the individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.




