Human intestinal spirochetosis, irritable bowel syndrome, and colonic polyps: A systematic review and meta-analysis
Simon Keely and Nicholas J. Talley share equal contribution.
Declaration of conflict of interest: KF: None. GDE: None. PMN: None. GLB: None. MMW: Grant/research support: Prometheus Laboratories Inc (irritable bowel syndrome [IBS Diagnostic]), Commonwealth Diagnostics International (Biomarkers for FGIDs). SK: Grant/research support: National Health and Medical Research Council (Ideas Grant and Centre for Research Excellence), Viscera Labs (research contract), Microba Life Science (research contract). Consultant/advisory boards: Gossamer Bio (Scientific Advisory Board), Anatara Lifescience (Scientific Advisory Board), Microba Life Science (Consultancy). ECH: None. NJT: HVN National Science Challenge NZ, personal fees from Aviro Health (Digestive health) (2019), Anatara Life Sciences, Brisbane (2019), Allakos (gastric eosinophilic disease) (2021), Bayer [IBS] (2020), Danone (Probiotic) (2018), Planet Innovation (gas capsule IBS) (2020), Takeda, Japan (gastroparesis) (2019), twoXAR (2019) (IBS drugs), Viscera Labs, (USA 2021) (IBS-diarrhea), Dr Falk Pharma (2020) (EoE), Censa, Wellesley MA USA (2019) (diabetic gastroparesis), Cadila PharmIncaceuticals (CME) (2019), Progenity Inc., San Diego (USA 2019) (intestinal capsule), Sanofi-aventis, Sydney (2019) (probiotic), Glutagen (2020) (celiac disease), ARENA Pharmaceuticals (2019) (abdominal pain), IsoThrive (2021) (esophageal microbiome), BluMaiden (2021), Rose Pharma (2021) outside the submitted work; in addition, Dr. Talley has a patent Nepean Dyspepsia Index (NDI) 1998, Biomarkers of IBS licensed, a patent Licensing Questionnaires Talley Bowel Disease Questionnaire licensed to Mayo/Talley, a patent Nestec European Patent licensed, and a patent Singapore Provisional Patent “Microbiota Modulation Of BDNF Tissue Repair Pathway” issued. Committees: Australian Medical Council (AMC) [Council Member]; Australian Telehealth Integration Programme; MBS Review Taskforce; NHMRC Principal Committee (Research Committee) Asia Pacific Association of Medical Journal Editors. Boards: GESA Board Member, Sax Institute, Committees of the Presidents of Medical Colleges. Community group: Advisory Board, IFFGD (International Foundation for Functional GI Disorders). Miscellaneous: Avant Foundation (judging of research grants). Editorial: Medical Journal of Australia (Editor in Chief), Up to Date (Section Editor), Precision and Future Medicine, Sungkyunkwan University School of Medicine, South Korea, Med (Journal of Cell Press). Dr. Talley is supported by funding from the National Health and Medical Research Council (NHMRC) to the Centre for Research Excellence in Digestive Health and he holds an NHMRC Investigator grant.
Abstract
Human colonic spirochetosis (CS) is usually due toBrachyspira pilosicolior Brachyspira aalborgiinfection. While traditionally considered to be commensal bacteria, there are scattered case reports and case series of gastrointestinal (GI) symptoms in CS and reports of colonic polyps with adherent spirochetes. We performed a systematic review and meta-analysis investigating the association between CS and GI symptoms and conditions including the irritable bowel syndrome (IBS) and colonic polyps. Following PRISMA 2020 guidelines, a systematic search of Medline, CINAHL, EMBASE, and Web of Science was performed using specific keywords for CS and GI disease. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using a random-effects model. Of 75 studies identified in the search, 8 case–control studies met the inclusion criteria for meta-analysis and 67 case series studies met the inclusion criteria for pooled prevalence analysis. CS was significantly associated with diarrhea (n = 141/127, cases/controls, OR: 4.19, 95% CI: 1.72–10.21, P = 0.002) and abdominal pain (n = 64/65, OR: 3.66, 95% CI: 1.43–9.35, P = 0.007). CS cases were significantly more likely to have Rome III-diagnosed IBS (n = 79/48, OR: 3.84, 95% CI: 1.44–10.20, P = 0.007), but not colonic polyps (n = 127/843, OR: 8.78, 95% CI: 0.75–103.36, P = 0.084). In conclusion, we found evidence of associations between CS and both diarrhea and IBS, but not colonic polyps. CS is likely underestimated due to suboptimal diagnostic methods and may be an overlooked risk factor for a subset of IBS patients with diarrhea.
Introduction
Although human intestinal spirochetosis was identified in 1967,1 there is still ongoing debate regarding its pathogenic importance in humans.2 Swine and poultry-infecting intestinal spirochetes can induce severe colitis and diarrhea in those animals,3 while species colonizing humans (the intestinal spirochetes Brachyspira pilosicoliand Brachyspira aalborgi) are usually thought to induce few or no symptoms.4 Our understanding of colonic spirochetosis (CS) has been hampered by the difficulty in working with these species. Brachyspira are fastidious, slow-growing, anaerobic bacteria, hard to isolate and to grow in laboratory conditions.5 As a result, the pathogenesis, transmission pattern, and risk factors of CS remain largely unknown. Studies utilizing transmission electron microscopy (TEM) show that spirochete may adhere to the epithelium, as the “head” of the spirochetes anchors between microvilli structures of the intestinal epithelium while the tail end is directed into the colonic lumen.6 Although TEM studies using clinical tissues have found spirochetes present in macrophages7 and close to mast cells,8 the significance of this finding is unclear as there is little evidence of epithelial cell penetration by spirochetes.9
The gold standard diagnosis of CS is based on its characteristic colonization of the epithelial surface, identified by routine histological examination of biopsies taken during colonoscopy.10 Using hematoxylin and eosin (H&E) staining, colonized spirochetes are stained as light purple, while Warthin–Starry silver staining or specific immunohistochemistry (IHC) staining, using cross-reactive anti-Treponema pallidumantibody, better highlight the presence of spirochetes from the background.10 The limitation of histological diagnosis is that successful detection of spirochetes is largely dependent on the location of biopsy sampling and careful pathology examination, and the basophilic brush line from CS can be easily misinterpreted as a normal brush border structure on H&E staining.11 While genomic screening of the gut microbiota is advancing rapidly and it has proven to be a powerful tool to investigate microbiota composition,12 the “conventional” 16S rRNA sequencing approach is unable to detect human colonic spirochetes as the standard 16S rRNA primer sets are incompatible with spirochetes' 16S rRNA region.13 Consequently, there are currently no non-invasive routine diagnostic methods to diagnose human CS.
Recently, interest in CS has increased with reports of a possible association with diarrhea-predominant irritable bowel syndrome (IBS-D).14-16 CS has also been observed with colonic polyps, but an association with adenoma formation is uncertain.17 This systematic review and meta-analysis aimed to determine whether human colonic spirochete infection is associated with specific gastrointestinal (GI) symptoms or GI diseases. We also aimed to identify risk factors associated with the infection and the results of treatment where data were available.
Methods
Search strategy
We followed the PRISMA guidelines for systematic reviews.18 A protocol was developed before initiation of the systematic review (PROSPERO CRD42019124669). Electronic databases including Medline, CINAHL, EMBASE, and Web of Science were searched on June 31, 2021, with limitations set on human studies published between 1967 and the search date. Each database was searched with the same strategy: [spirochetosis OR spirochaetosis OR spirochete OR spirochaete OR spirochaetose OR Brachyspira aalborgiOR Brachyspira pilosicoliOR Serpulina pilosicoli] AND [intestinal disease OR intestinal].
Study selection
After removal of duplicate studies, two independent reviewers (KF and GLB) screened titles and abstracts for relevance to the review topic. Following this, full texts of all remaining studies were assessed for suitability and relevance based on the review inclusion and exclusion criteria. The inclusion criteria were (i) studies in humans with intestinal (colonic) spirochete infection, (ii) case–control studies, case series, or original research studies of GI symptoms in intestinal (colonic) spirochete infection, and (iii) studies in the English language. Exclusion criteria were (i) reviews, (ii) single case reports, (iii) studies with no mention of patient symptoms, (iv) studies where full text was not available, and (v) studies not in the English language.
Data extraction
Data extraction was performed by two independent reviewers (KF and PMN). Disagreements were resolved by consensus. Data information were extracted where available using a standardized data extraction template and included (a) general: publication year, study type, number of cases, sex, age, travel/work/sex activity, and sexuality; (b) comorbidity: GI comorbidity, non-GI comorbidity, and co-infection; (c) GI symptoms: diarrhea, constipation, diarrhea/constipation mixed, abdominal pain, rectal bleeding, blood in stool, mucus in stool, vomiting, weight loss, fever, nausea, anemia or asymptomatic, and physical examination results when reported; (d) colonoscopy findings: normal colonoscopy, abnormal colonoscopy, and degree and location of the abnormalities; (e) histology findings: presence of spirochetes, location of spirochetes infection, mucosal inflammation, crypt changes, and presence of immune cells (plasma cell, lymphocyte, neutrophil, eosinophil, macrophage, and mast cells); (f) species specificity: Brachyspira genus,B. pilosicoli, and B. aalborgi; (g) diagnostic method: histology, PCR, PCR target, PCR material, culture, culture material, and electron microscopy; and (h) treatment and outcomes: type of treatment, symptom relief, bacterial eradication, pathology recovery, and symptom reoccurrence.
Statistical analysis
For case–control studies, pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the risk correlation of CS and gender, GI symptoms, and GI diseases using a random-effects model.19 To test the heterogeneity of the studies included for analysis, Cochran's Q statistic was used, with P < 0.10 indicating significant effects of heterogeneity. Heterogeneity was assessed using the I2 statistic with results of 0–25% (low), 25–75% (moderate), and > 75% (high) levels of heterogeneity.20 Due to the small number of studies included in the meta-analysis, publication bias was not assessed as most methods required at least 10 studies to perform a test.21
For case series studies, the prevalence of sex, a range of GI symptoms, colonoscopy findings, diagnostic methods, mucosal inflammation, and treatment effects in reported CS cases were calculated using pooled prevalence rate (event rate [ER]) and 95% CIs using a random-effects model. Heterogeneity of the studies were evaluated as described above. All analyses were performed with Comprehensive Meta-analysis (version 3.0), Biostat, Englewood, NJ (2014).
Results
Study selection
Of the initial 2157 studies obtained from Medline, EMBASE, Web of Science, and CINAHL, 1079 texts were identified as duplicates. Of the remaining 1078 studies that were screened by title and abstract, 705 were excluded as animal studies, microbiological studies, or incorrect species of bacteria. A total of 373 papers then proceeded to full text screening, and of those, 207 studies were excluded as reviews, lacking patient symptom information, written in non-English language, or had duplicated reports of the same patient cohorts. Ninety-four single case reports were excluded. Three studies were added by hand search. In the end, 75 studies were included for data extraction. Eight case–control studies were included for meta-analysis and 67 case series studies were included for pooled prevalence analysis (Fig. 1).
Meta-analysis of case–control studies
Study characterization
Of the eight case–control studies included (Table 1), in four studies (Walker et al.,14 Goodsall et al.,15 Alsaigh and Fogt,22 and Higashiyama et al.23), cases of CS and controls were identified in pathology databases based on histology findings; in Cooper et al.,24 cases were a cohort of homosexual males identified with CS while controls were male patients without CS; for Esteve et al.,25 cases were patients with chronic diarrhea collected prospectively who were later identified with CS, while controls were asymptomatic patients with colonic biopsies available in a pathology database; in Omori et al.,26 cases were patients with sessile serrated adenoma/polyps (SSA/P) while controls were non-SSA/P patients; and in Jabbar et al.,16 cases were patients with IBS and controls were healthy volunteers. In all studies, CS was confirmed by histological examination.
| Paper | Year | Screening cohort selection criteria | CS patient/screening cohort | Selection criteria for control subjects | CS patient/control cohort | Data for meta-analysis | |||
|---|---|---|---|---|---|---|---|---|---|
| Gender | Symptom | IBS | Polyps | ||||||
| Cooper et al.24 | 1986 | Homosexual men with GI symptoms. | 5/8 | Age matched male patients with available colonic biopsy. | 0/5 | NA | NA | NA | NA |
| Alsaigh and Fogt22 | 2002 | Pathology database. | 15/15 | Age and clinical indication matched patients with biopsy. | 0/30 | Yes | Diarrhea/rectal bleeding | NA | Yes |
| Esteve et al.25 | 2006 | Prospective survey of patients with chronic watery diarrhea and CS patients identified in routine colonoscopy. | 11/11 | Patients with colonic biopsy taken due to rectal bleeding or polyps histology. | 0/100 | Yes | NA | NA | NA |
| Higashiyama et al.23† | 2009 | Pathology database from 2005 to 2008. | 86/86 | Patient with colonic biopsy from 2005 to 2008. | 0/702 | NA | NA | NA | Yes |
| Omori et al.26 | 2014 | Patients with sessile serrated adenoma/polyp identified by histology during 2008–2011. | 10/19 | Patients with biopsy excluding sessile serrated adenoma/polyp and cancer in 2011. | 14/172 | NA | NA | NA | Yes |
| Walker et al.14 | 2015 | Pathology database. | 17/17 | Subjects with colonic biopsy from random population. | 0/17 | NA | Diarrhea/abdominal pain/rectal bleeding | Yes | Yes |
| Goodsall et al.15 | 2017 | Pathology database. | 47/47 | Subjects with colonic biopsy from random population. | 0/48 | NA | Diarrhea/abdominal pain | NA | NA |
| Jabbar et al.16 | 2020 | IBS patients diagnosed by Rome III criteria. | 19/62 | Healthy subjects with colonic biopsy. | 0/31 | Yes | Diarrhea | Yes | NA |
- † The Higashiyama et al. paper is an abstract.
- CS, colonic spirochetosis; GI, gastrointestinal; IBS, irritable bowel syndrome; NA, not applicable.
- In all studies, CS was confirmed by histological examination.
Demographic characterization
Sex
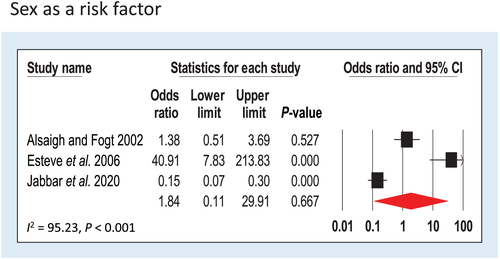
Three studies (Alsaigh and Fogt,22 Esteve et al.,25 and Jabbar et al.16) with no sex restrictions for recruitment were included providing n = 88 cases and n = 161 controls. CS cases were 1.84 times (95% CI = 0.11–29.91, P = 0.667) more likely to be male than female, although this was not significant. Heterogeneity of the studies was high (I2 = 95.23, P < 0.001) (Fig. 2).
Age
Three studies (Alsaigh and Fogt,22 Esteve et al.,25 and Jabbar et al.16) with no age restrictions for recruitment were included providing n = 88 cases and n = 161 controls. The mean age of the cases and controls was 47.1 and 48.2 years (P = 0.94), respectively.
Gastrointestinal symptom in colonic spirochetosis cases
Diarrhea
Four studies assessed diarrhea in association with CS (Alsaigh and Fogt,22 Walker et al.,14 Goodsall et al.,15 and Jabbar et al.16). In total, n = 141 cases and n = 127 controls were included. CS was significantly associated with diarrhea; CS cases were more than three times more likely to have diarrhea compared with controls (OR: 4.19, 95% CI: 1.72–10.21, P = 0.002) (Fig. 3a). Heterogeneity of the studies was moderate (I2 = 27.32, P = 0.25).

Abdominal pain
Two studies assessed abdominal pain in association with CS (Walker et al.14 and Goodsall et al.15). In total, n = 64 cases and n = 65 controls were included. CS cases were almost four times more likely to have abdominal pain (OR: 3.66, 95% CI: 1.43–9.35, P = 0.007) (Fig. 3b). Heterogeneity of the studies was low (I2 = 13.52, P = 0.28).
Rectal bleeding
Two studies examined patients who self-reported symptom of rectal bleeding (Alsaigh and Fogt22 and Walker et al.14). In total, n = 32 cases and n = 47 controls were included. CS cases were twice as likely to experience rectal bleeding (OR: 2.34, 95% CI: 0.36–15.28, P = 0.374) (Fig. 3c), although the reason for bleeding was not specified in these studies and the association was not significant. There was no heterogeneity in the studies (I2 = 0.00, P = 0.44).
Gastrointestinal diseases in colonic spirochetosis cases
Irritable bowel syndrome
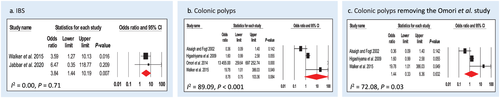
Two studies assessed diagnosis of IBS using Rome III criteria in association with CS (Walker et al.14 and Jabbar et al.16). In total, n = 79 cases and n = 48 controls were included. CS was significantly associated with IBS; CS cases are almost four times (OR: 3.84, 95% CI: 1.44–10.20, P = 0.007) more likely to have a diagnosis of IBS compared with controls (Fig. 4a). There was no heterogeneity in the studies (I2 = 0.00, P = 0.71).
Colonic polyps
Four studies assessed diagnosis of colonic polyps in association with CS (Alsaigh and Fogt,22 Higashiyama et al.,23 Walker et al.,14 and Omori et al.26). In Alsaigh and Fogt, Higashiyama et al., and Walker et al., the subtypes of polyps were not discriminated, while Omori et al. specifically investigated the correlation of CS and SSA/P in patient cohorts. In total, n = 127 cases and n = 843 controls were included. CS cases were almost nine times (OR: 8.78, 95% CI: 0.75–103.36, P = 0.084) more likely to have colonic polyps compared with controls, but this was not a significant finding (Fig. 4b). Heterogeneity of the studies was high (I2 = 89.09, P < 0.001). After the removal of Omori et al., the OR for non-specific polyps dropped to 1.44 (95% CI: 0.33–6.36, P = 0.632), with I2 = 72.08, P = 0.03 (Fig. 4c).
Colonoscopy findings
Only one study (Alsaigh and Fogt22) assessed colonoscopy findings in association with CS, although the definition of normal and abnormal colonoscopy was not specified in the paper. In total, n = 15 cases and n = 30 controls were analyzed. The OR of abnormal visible findings on colonoscopy was 0.87 (95% CI: 0.24–3.10, P = 0.828).
Case series
Pooled prevalence analysis
Sixty-seven case series studies were included for pooled prevalence analysis. Results are shown in Table 2. In reported CS cases, the most common symptoms were diarrhea (39%) and abdominal pain (34%), followed by symptoms of bloating (29%), undefined rectal bleeding (21%), or a finding of blood in the stool (27%). We also observed that nearly half of the CS cases (48%) were reported to be asymptomatic with CS only identified because biopsies were taken during screening or polyp surveillance.
| Event | Pooled study references | Cases | Number of event | Event rate/proportion | Heterogeneity |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Male gender | 1, 4, 6, 8, 9, 17, 28, 29, 48, 60-103 | 2041 | 1409 | 0.68 (95% CI 0.63–0.73) | I2 = 67.24%, P < 0.001 |
| Female gender | 1, 4, 6, 8, 9, 17, 28, 29, 48, 60-103 | 2041 | 632 | 0.32 (95% CI 0.27–0.37) | I2 = 67.24%, P < 0.001 |
| Travel before symptom onset | 29, 61, 66, 70, 81, 83-88, 90, 94, 95 | 105 | 28 | 0.30 (95% CI 0.19–0.45) | I2 = 29.10%, P = 0.14 |
| Childhood sexual abuse | 97 | 8 | 1 | 0.13 (95% CI 0.02–0.54) | I2 = 0.00%, P = 1 |
| Frequent sexual activity | 85 | 4 | 1 | 0.25 (95% CI 0.03–0.76) | I2 = 0.00%, P = 1 |
| Homosexual | 28, 48, 61, 70, 73, 77, 79, 80, 83, 85, 96, 98, 103 | 530 | 158 | 0.44 (95% CI 0.16–0.77) | I2 = 86.83%, P < 0.001 |
| HIV+ | 4, 28, 48, 61-64, 67, 70, 72, 75, 76, 79, 83, 93, 103, 104 | 1369 | 71 | 0.10 (95% CI 0.04–0.21) | I2 = 88.64%, P < 0.001 |
| HIV− | 4, 28, 48, 63, 64, 67, 69-72, 75, 76, 82, 83, 89 | 654 | 586 | 0.89 (95% CI 0.75–0.96) | I2 = 85.58%, P < 0.001 |
| HIV status unknown | 1, 4, 6, 8, 17, 27, 29, 61, 62, 65, 66, 68, 74, 77, 81, 84-92, 94-102 | 429 | 411 | 0.90 (95% CI 0.84–0.93) | I2 = 19.74%, P = 0.16 |
| GI abnormality | |||||
| Colonic polyps | 1, 9, 17, 27, 28, 61-72, 74, 75, 77, 79, 85, 94, 97, 105 | 1094 | 370 | 0.28 (95% CI 0.18–0.40) | I2 = 89.77%, P < 0.001 |
| Diverticular disease | 8, 9, 17, 27, 62-64, 66-69, 85, 105, 106 | 403 | 39 | 0.12 (95% CI 0.07–0.19) | I2 = 47.67%, P = 0.02 |
| Inflammatory bowel disease | 4, 9, 28, 61-63, 65, 66, 72, 75-77, 79, 91, 92, 106 | 924 | 74 | 0.09 (95% CI 0.06–0.13) | I2 = 52.59%, P = 0.007 |
| Cancer | 1, 9, 28, 64-68, 72, 75, 77, 105, 106 | 532 | 70 | 0.24 (95% CI 0.19–0.31) | I2 = 87.85%, P < 0.001 |
| GI symptoms | |||||
| Diarrhea | 1, 4, 8, 9, 29, 60-73, 75-79, 81-97, 103-105, 107 | 1770 | 570 | 0.39 (95% CI 0.33–0.46) | I2 = 74.44%, P < 0.001 |
| Abdominal pain | 1, 4, 6, 8, 9, 29, 60-68, 70-72, 75, 76, 78, 79, 81, 83-88, 90, 92-94, 96, 97, 99, 101, 102, 104, 105 | 1645 | 436 | 0.34 (95% CI 0.26–0.43) | I2 = 83.92%, P < 0.001 |
| Rectal bleeding | 1, 4, 8, 29, 61-70, 72, 75, 77, 82, 84-88, 90, 93-95, 97, 99, 105 | 705 | 114 | 0.21 (95% CI 0.15–0.27) | I2 = 48.94%, P = 0.001 |
| Blood in stool | 4, 8, 27, 28, 66, 75, 76, 79, 82, 85, 87 | 576 | 174 | 0.27 (95% CI 0.20–0.35) | I2 = 62.53%, P = 0.003 |
| Bloating | 29, 62, 88 | 7 | 2 | 0.29 (95% CI 0.07–0.68) | I2 = 0.00%, P = 0.81 |
| Vomiting | 60, 79, 81, 84, 96, 99, 101 | 315 | 28 | 0.17 (95% CI 0.08–0.32) | I2 = 55.47%, P = 0.04 |
| Weight loss | 8, 29, 61, 67, 70, 83, 85, 87, 93, 97, 99, 104 | 527 | 31 | 0.17 (95% CI 0.08–0.32) | I2 = 58.75%, P = 0.005 |
| Anemia | 4, 61, 63, 66, 92, 94 | 112 | 8 | 0.10 (95% CI 0.03–0.26) | I2 = 49.32%, P = 0.08 |
| Mucus in stool | 4, 8, 79, 87, 93, 95, 97 | 232 | 15 | 0.12 (95% CI 0.05–0.27) | I2 = 35.50%, P = 0.17 |
| Asymptomatic | 4, 17, 28, 60, 62, 71, 72, 75, 76, 78, 89, 98, 104 | 1267 | 648 | 0.48 (95% CI 0.34–0.63) | I2 = 90.67%, P < 0.001 |
| Colonoscopy findings | |||||
| Normal colonoscopy | 4, 8, 17, 29, 61, 63, 65, 69, 75, 82, 84-87, 90-92, 94-97, 101, 102 | 189 | 83 | 0.47 (95% CI 0.34–0.61) | I2 = 49.19%, P = 0.007 |
| Abnormal colonoscopy | 4, 8, 17, 27, 29, 61, 63, 65, 69, 75, 82, 85-88, 90-95, 97, 99, 101, 102 | 273 | 109 | 0.45 (95% CI 0.29–0.61) | I2 = 69.34%, P < 0.001 |
| Erythema | 4, 29, 69, 85, 88, 91 | 28 | 17 | 0.33 (95% CI 0.18–0.54) | I2 = 34.34%, P = 0.18 |
| Hyperemia | 61, 97 | 21 | 18 | 0.67 (95% CI 0.01–1.00) | I2 = 89.43%, P < 0.001 |
| Loss of vascular pattern | 69, 82 | 14 | 2 | 0.17 (95% CI 0.01–0.76) | I2 = 61.89%, P = 0.11 |
| Pale mucosa | 85 | 3 | 1 | 0.25 (95% CI 0.03–0.76) | I2 = 0.00%, P = 1.00 |
| Edema | 91, 97 | 7 | 4 | 0.46 (95% CI 0.02–0.97) | I2 = 77.36%, P = 0.04 |
| Erosion | 61, 69, 92 | 21 | 32 | 0.70 (95% CI 0.06–0.99) | I2 = 86.00%, P = 0.001 |
| Ulcer | 63, 87, 92, 93 | 26 | 7 | 0.23 (95% CI 0.06–0.56) | I2 = 52.19%, P = 0.10 |
| Mucosal inflammation | 27, 69, 75, 86, 87, 97 | 48 | 35 | 0.28 (95% CI 0.09–0.60) | I2 = 77.80%, P < 0.001 |
| Blood oozing | 85 | 3 | 1 | 0.25 (95% CI 0.03–0.76) | I2 = 0.00%, P = 1.00 |
| Mucosal inflammation | |||||
| Inflammation presence | 4, 6, 8, 9, 28, 29, 48, 61-64, 66-69, 73, 75, 84, 86-88, 90-97, 99, 100, 102, 105, 106 | 645 | 142 | 0.30 (95% CI 0.21–0.40) | I2 = 62.76%, P < 0.001 |
| Lymphocyte presence | 4, 8, 94, 97 | 40 | 11 | 0.30 (95% CI 0.14–0.53) | I2 = 25.99%, P = 0.25 |
| Eosinophil presence | 62, 84, 86, 87, 97 | 137 | 10 | 0.18 (95% CI 0.03–0.64) | I2 = 80.79%, P < 0.001 |
| Neutrophil presence | 29, 62, 73, 86, 96 | 160 | 14 | 0.18 (95% CI 0.05–0.49) | I2 = 67.55%, P < 0.001 |
| Mast cell presence | 8 | 2 | 2 | 0.83 (95% CI 0.19–0.99) | I2 = 0.00%, P = 1.00 |
| Macrophage presence | 8, 29, 63, 67, 99 | 51 | 10 | 0.45 (95% CI 0.09–0.87) | I2 = 74.63%, P = 0.003 |
| Crypt involvement | 8, 61, 62, 64, 69, 73, 86, 90, 93, 94 | 217 | 27 | 0.20 (95% CI 0.11–0.33) | I2 = 54.40%, P = 0.02 |
| Diagnosis method | |||||
| By histology | 1, 4, 6, 8, 9, 17, 27-29, 48, 60-107 | 2183 | 1854 | 0.92 (95% CI 0.85–0.96) | I2 = 70.44%, P < 0.001 |
| By PCR | 1, 4, 6, 8, 9, 17, 27-29, 48, 60-92, 94-107 | 2104 | 289 | 0.15 (95% CI 0.08–0.25) | I2 = 83.04%, P < 0.001 |
| By culture | 1, 4, 6, 8, 9, 17, 27-29, 48, 60-92, 94-107 | 2104 | 321 | 0.08 (95% CI 0.04–0.14) | I2 = 69.35%, P < 0.001 |
| Species prevalence | |||||
| Brachyspira pilosicolipresence | 28, 29, 61, 63, 64, 67, 70, 75-78, 81, 83, 89-92, 105 | 504 | 175 | 0.20 (95% CI 0.12–0.32) | I2 = 69.32%, P < 0.001 |
| Brachyspira aalborgipresence | 28, 29, 61, 63, 64, 67, 70, 75-78, 81, 83, 89-92, 105 | 504 | 207 | 0.58 (95% CI 0.40–0.74) | I2 = 82.78%, P < 0.001 |
| Metronidazole treatment | |||||
| One course of metronidazole/CS patient | 4, 8, 61, 63, 69, 79, 82-85, 90, 92-97, 99, 102, 103, 107 | 358 | 134 | 0.49 (95% CI 0.34–0.64) | I2 = 60.08%, P < 0.001 |
| Symptom relief/metronidazole-treated patient | 4, 61, 63, 69, 83-85, 90, 92-94, 97, 102, 103, 107 | 65 | 55 | 0.81 (95% CI 0.68–0.90) | I2 = 0.00%, P = 0.95 |
| Bacteria eradication/metronidazole-treated patient | 61, 63, 79, 85, 92, 94, 97, 103, 107 | 61 | 51 | 0.76 (95% CI 0.62–0.86) | I2 = 0.00%, P = 0.55 |
| Pathology recovery/metronidazole-treated patient | 61, 92, 93 | 20 | 19 | 0.84 (95% CI 0.52–0.96) | I2 = 60.08%, P < 0.001 |
| Symptom relapse/metronidazole-treated patient | 4, 63, 84, 85, 90, 93, 94 | 20 | 8 | 0.39 (95% CI 0.20–0.62) | I2 = 60.08%, P < 0.001 |
- CI, confidence interval; CS, colonic spirochetosis; GI, gastrointestinal.
In CS cases, a range of colonic diseases were assessed. Nearly one third had colonic polyps (29%), one quarter had colon cancer (24%), and one in ten had inflammatory bowel disease (9%) or diverticular disease (12%). In CS cases, the proportion of patients with a normal colonoscopy (47%) versus an abnormal colonoscopy (45%) were similar, which was in line with the case–control findings (the remaining 8% were missing data). Among CS cases with abnormal colonoscopy findings, 70% had erosions, 67% had hyperemia, 46% had edema, 33% had erythema, 28% had inflamed mucosa, 25% had pale mucosa, 25% had blood oozing, 23% had ulcers, and 17% had loss of the vascular pattern.
The majority of CS cases were diagnosed by histology (92%), while only 15% had specific PCR tests to confirm the species of infection, and only 8% were diagnosed by successful culture. Within those cases where species differentiation was examined, 20% were infected with B. pilosicoli, 58% were infected with B. aalborgi, and 22% reported undetermined Brachyspira genus.
Metronidazole was the most commonly prescribed treatment for CS. Nearly half of the CS patients (49%) received one course of metronidazole treatment (dose and frequency varied between studies). Among these patients, 81% had reported GI symptom relief (symptom assessment varied between studies), while 79% reported successful bacterial eradication in a follow-up colonoscopy examination. Although most patients (84%) with CS-related pathology experienced recovery after treatment, nearly 40% of these patients also reported symptom relapse between a few days to 15 months after treatment.
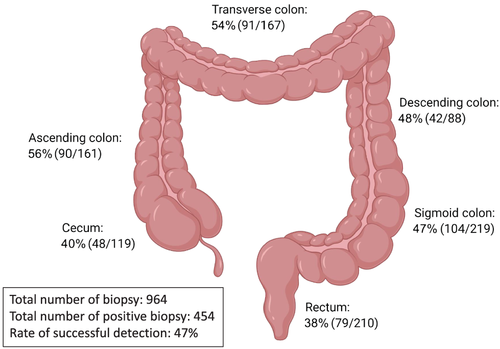
Anatomical locations of spirochetes were extracted from confirmed CS cases who underwent full colonoscopy and had biopsies taken from each section of the colon, or with colonoscopy that specified the biopsy locations.11, 26-41 Biopsies taken from the ascending colon had the highest success rate (56%) for detecting spirochetes from these CS cases, followed by biopsies taken from the transverse colon (54%), descending colon (48%), sigmoid colon (47%), cecum (40%), and rectum (38%). In total, 964 biopsies were taken by colonoscopy in these confirmed CS cases, with 454 biopsies showing presence of spirochetes, providing a 47% successful detection rate (Fig. 5).
Discussion
To our knowledge, this is the first meta-analysis to investigate human colonic spirochetes infection and GI disease. Although spirochetes are generally considered to be commensals and largely ignored, the results of this review have identified a clear association between CS and diarrhea, abdominal pain, and IBS. However, no association between CS and the presence of polyps was identified. Importantly, we found that CS was strongly associated with IBS, a functional GI disorder that is characterized by abdominal pain and a change of bowel habits.42 Although the etiology of IBS is still unclear, there is evidence that suggests GI infections may play a role in the initiation and development of IBS.43 In both studies that directly reported an association of IBS with CS, subtle pathological changes were identified in CS patients, namely, increased eosinophils, mast cells, and lymphoid aggregates in the lamina propria.14, 16 These findings are consistent with low-grade mucosal inflammation that has been observed in other IBS cohorts, although in these studies CS was not evaluated.44-46 IBS patients usually have normal colonoscopy findings and therefore colonic biopsy is not indicated, which may be the reason that a direct involvement of CS in IBS has not previously been widely reported. Given histology is currently the gold standard for diagnosing CS, standardizing biopsy collection from patients with IBS for careful histological evaluation may reveal the true prevalence of CS in IBS cohorts.
In the meta-analysis of case–control studies, we aimed to investigate the association of CS and GI symptoms. Due to the heterogeneity of GI symptoms reported in the available studies, we could only assess diarrhea, abdominal pain, and rectal bleeding by meta-analysis with sufficient sample size. However, the pooled prevalence analysis of case series studies mirrored these findings with diarrhea, followed by abdominal pain, blood in stool, and rectal bleeding, the most commonly reported symptoms with CS. Limitation of the symptom analysis include possible selection bias and reporting bias. CS patients with symptoms may be more likely to seek healthcare and colonoscopy, which could increase the detection of CS compared with asymptomatic patients, and they may also be more likely to be reported and published. Therefore, it is interesting and important that we also assessed and found that 48% of the reported CS cases were asymptomatic and had undergone colonoscopy for polyp surveillance or population screening. It is plausible to assume this number may be higher in general population. Unfortunately, none of the included studies had characterized these cases in detail, and as a result, we were unable to delineate risk factors that may differentiate asymptomatic versus symptomatic disease in CS cases. Future studies are needed to comprehensively assess GI symptoms associated with CS with a thorough and standardized symptom evaluation including abdominal pain, diarrhea, rectal bleeding, blood in stool, weight loss, vomiting, bloating, mucus in stool, and anemia. Population studies are urgently needed to determine an accurate infection rate, symptom, and pathology profile of CS.
We also investigated the relationship between sex and CS, as sex differences have been reported in other infectious GI diseases.47 CS was initially believed to be a sexually transmitted disease, with early work focusing predominantly on homosexual male cohorts.48 Therefore, CS has largely been regarded as a male dominant disease. In the pooled prevalence analysis of case series, we found that the prevalence of male gender in current reported CS cases was 68%. However, the meta-analysis showed that the OR of a male CS patient is only 1.84 compared with female; the difference was not statistically significant. Randomized population study like Walker et al.14 reported that there was only a slight increase in the likelihood of being male (OR: 1.13) in CS patients. This likely reflects selection bias as past studies have focused on certain patient groups (i.e. male homosexuals). Sex differences in IBS subtypes have not been extensively studied, but previous meta-analyses suggest that IBS-D is more common in men over women,49 although whether this is associated with a potentially higher rate of CS is unknown. Alternatively, spirochete infection in males may induce more severe symptoms than in females, as sex differences in infection are known to exist49 and therefore lead to more male patients seeking care for infection.
There was no significant association between CS and colonic polyps. A sensitivity analysis showed that Omori et al.26 was the source of the increased OR and the major contributor to the high heterogeneity of the analysis. Upon the removal of this study, the association of CS and colonic polyps was reduced from an OR of 8.78 to an OR of 1.44, although neither OR was significant. Interestingly, Omori et al. looked specifically at patients with SSA/P, while the remaining studies did not distinguish the polyp pathology subtypes. SSA/P is significantly associated with increased cancer incidence (OR: 1.77 vs OR: 1) and mortality (OR: 1.74 vs OR: 1) compared with a matched cohort at 10-year follow-up.50 The case series study by Young et al.,51 which was similar to Omori et al., found that 28% (26/93) of patients with SSA/P had CS presence in their GI tract in an Australian population, while 2/4 patients with tubulovillous adenomas and 5/8 patients with colonic resection (reason unspecified) had CS. At the same time, we found that in the pooled prevalence analysis, 28% of patients with CS had colonic polyps, and notably 24% had colorectal cancer. This evidence suggests an association between CS and colonic polyps is possible, notably SSA/P and colorectal cancer. It was not possible to infer any causal relationship of CS and polyps with the available data. Firstly, patients with colon cancer and polyps are much more likely to receive surveillance colonoscopy, so there is a risk of detection bias driving the effect estimates for CS. Furthermore, it is known that colonic polyps and cancers exhibit altered mucosal microbiota,52-54 and this may include an increase in spirochetes as a consequence of changes to the ecological niche. Given the clinical importance of colonic polyps and cancer, further research to clarify if there is an association between CS with polyps and cancer is warranted.
Only one case–control study assessed colonoscopy findings, indicating that this is a neglected topic and as such no association between CS and abnormal colonoscopy could be made. Given that the current gold standard for diagnosing CS is by histological examination of biopsies taken during colonoscopy, it remains important to characterize macroscopic features of CS infection in order to better inform endoscopists when to take targeted biopsies for CS. A published Digestive Diseases Week abstract23 reported that red spots or hyperemia and a rough surface with loss of vascular pattern were features of CS at colonoscopy examination. Given that histology is still the gold standard for diagnosing CS, studies for the associations of CS and colonoscopy abnormalities are warranted.
Anatomical locations of spirochetes were assessed in the current case series study analysis. The result showed that the ascending colon and transverse colon had slightly higher positive rates of detecting spirochetes. However, within the confirmed CS cases, the ratio of positive biopsies to the total number of biopsies taken was only 0.47. This suggests that CS infection is patchy and that the current gold standard of diagnosis may be missing more than half of CS patients. 16S rRNA sequencing using stool samples would be an ideal screening method of intestinal microbiota components including spirochetes; however, 16S rRNA sequencing using common primer sets is unable to detect Brachyspira genus.13 Specific PCR primers for Brachyspira genus have been developed by some groups, as well as species-specific primers to B. pilosicoliand B. aalborgi,55, 56 and these approaches can be utilized for screening CS in stool samples. However, this requires a rather complicated protocol of stool sample collection to avoid environmental contamination and DNA extraction, so most clinical facilities would not be able to perform the test. Thus, more sensitive, specific, and non-invasive routine diagnostic methods of CS are urgently needed, for example, a serological test.
One important question that we could not address in our meta-analysis was whether there is a difference in pathogenicity between the two currently isolated species of human intestinal spirochetes, as none of the case–control studies and few case studies distinguished between the two CS species. As many studies used formalin-fixed paraffin-embedded tissue, the quality of DNA isolated from these tissues may be insufficient for subsequent PCR analysis in differentiating between these two species. Furthermore, the presence of yet to be isolated spirochetes species (e.g. Brachyspira hominis) may also contribute to this unclassified group of CS infection.55 Interestingly, we noticed in early studies using culture methods, B. pilosicoliwas believed to be the main species in human CS as it was easier to isolate, required a shorter incubation period, and was not as nutrient-demanding as B. aalborgi. However, subsequent studies refuted this observation, and by PCR, B. aalborgiis more commonly reported in literature. Some studies16, 57 have shown that B. pilosicoliand B. aalborgilive in different niches in the human intestinal tract, with B. pilosicolimore “mucus-associated” while B. aalborgiwas more “membrane-associated”; however, its correlation with symptoms, risk factors, or treatment response are still unclear. Thus, the pathological differences between B. pilosicoliand B. aalborgiare not fully investigated, and potential bias in their prevalence due to methodological limitations in identifying Brachyspira spp. need to be taken into account. More studies are needed to investigate specific factors such as the variation in colonic spirochete species colonization and population diversity, the host immune response, as well as lifestyle and dietary factors that may influence spirochetosis pathology and disease outcome.58
Finally, we characterized the efficacy of metronidazole treatment in reducing CS-associated GI symptoms to provide indirect evidence in support of the bacteria playing a pathogenic role and improve clinical guidance for treating CS. Despite being at the early stages of understanding CS, many antibiotics have been explored as a treatment for CS and the current consensus is to use metronidazole as standard.59 Yet we found a proportion of metronidazole-treated patients would report symptom relapses at follow-up. Whether this is due to re-infection from environmental sources of spirochetes or unsuccessful eradication of the primary infection is still largely unknown. Recently, Jabbar et al.16 found that while the majority of spirochetes were eliminated with metronidazole treatment, some translocated from the colon surface to the colonic crypts and continued to reside within goblet cell granules. This may enable their continued survival despite antibiotic treatment and explain why CS recurs in many patients. A better understanding of spirochete antibiotic sensitivity profiles is therefore required to provide safer and more effective treatment approaches for CS. Eradication of the bacteria and therapeutic effects of antibiotic treatment should be evaluated through careful pathological assessment over an expanded period of time.
Overall, the limitations of our analysis include a relatively small sample size, and the variability between studies and therefore interpretation of the data, especially related to GI symptoms, must be validated directly. The strengths of this meta-analysis include the comprehensive literature search strategy used to identify studies and the detailed review of each manuscript to obtain complete symptoms, pathology, and treatment data for analysis. In conclusion, patients with CS have a higher risk of a diagnosis of IBS, consistent with the increased risk of experiencing diarrhea and abdominal pain. Importantly, this may occur in the absence of abnormal endoscopy findings. CS may therefore represent a treatable infectious etiology for a proportion of IBS patients, and further study of their role in this condition is warranted.
Acknowledgment
Open access publishing facilitated by The University of Newcastle, as part of the Wiley - The University of Newcastle agreement via the Council of Australian University Librarians.




