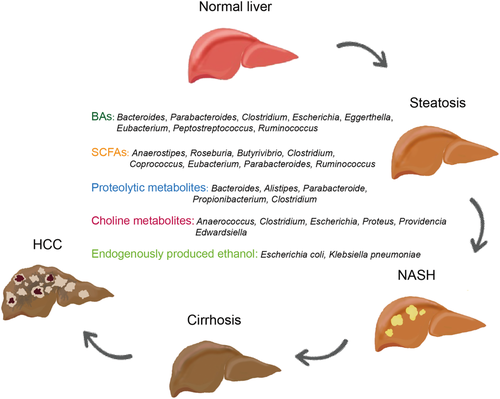
The microbial metabolome in metabolic-associated fatty liver disease
Abstract
Metabolism-associated fatty liver disease (MAFLD) is defined as the presence of excess fat in the liver in the absence of excess alcohol consumption and metabolic dysfunction. It has also been described as the hepatic manifestation of metabolic syndrome. The incidence of MAFLD has been reported to be 43–60% in diabetics, ~90% in patients with hyperlipidemia, and 91% in morbidly obese patients. Risk factors that have been associated with the development of MAFLD include male gender, increasing age, obesity, insulin resistance, diabetes, and hyperlipidemia. All of these risk factors have been linked to alterations of the gut microbiota, that is, gut dysbiosis. MAFLD can progress to non-alcoholic steatohepatitis with the presence of inflammation and ballooning, which can deteriorate into cirrhosis, MAFLD-related hepatocellular carcinoma, and liver failure. In this review, we will be focused on the role of the gut microbial metabolome in the development, progression, and potential treatment of MAFLD.
Introduction
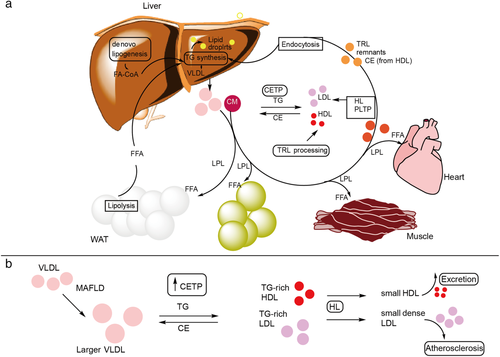
Metabolic-associated fatty liver disease (MAFLD) is characterized by steatosis that results from elevated accumulation of lipids in the liver.1 Initially, lipid accumulation is benign as lipids are stored as the neutral lipids, triglycerides (TGs), and cholesterol esters, but when elevated concentrations of free cholesterol, diaylglycerols, and ceramides occur in the liver, steatohepatitis results.2 Non-neutral lipids such as free cholesterol and ceramide have been shown to trigger and promote cellular stress responses leading to inflammation, cell death, and fibrosis.3, 4 Additionally, diacylglycerols and ceramides have been reported to induce insulin resistance. Patients with MAFLD are often overweight, insulin resistant, and have dyslipidemia characterized by high TGs and lipoprotein patterns similar to that of metabolic syndrome and type 2 diabetes (T2D). Hallmark MAFLD dyslipidemia presents as hypertriglyceridemia due to increased large very low-density lipoprotein particles, increased small, dense low-density lipoprotein (LDL), low high-density lipoprotein (HDL) cholesterol, and an increase in plasma cholesteryl ester transfer protein activity.5, 6 This lipid profile is the result of increased exchange of cholesterol ester and TG molecules between LDL or HDL and TG-rich lipoprotein particles (Fig. 1) governed by cholesteryl ester transfer protein. Ultimately, this exchange leads to a gain of TGs associated with LDL/HDL making them a better substrate for hepatic lipase which, in turn, hydrolyzes TGs off the LDL/HDL particles shrinking them into small, dense LDL and HDL particles. Small, dense LDL particles can pass through endothelia more easily to promote arterial wall plaque formation. Small, dense HDL can be eliminated by the kidney, which partly explains their low levels in MAFLD patients.7 In summary, hepatic fatty acids (FAs) come from four sources: (i) lipolysis of adipose tissue, (ii) dietary ingestion, (iii) endogenous production via de novo lipogenesis (DNL), and (iv) released from hepatic lysosomes by autophagy. In a clinical study of MAFLD patients, it was determined that ~50–60% of TGs in the liver were derived from nonesterified free fatty acids (FFAs) (from lipolysis of adipose tissue and chylomicron fragments), ~19–33% from DNL, and 8–22% from dietary sources. In normal patients, DNL accounts for only about 5% of hepatic TGs indicating that this pathway is upregulated in MAFLD.8, 9
Another factor that T2D, metabolic syndrome, and MAFLD have in common is gut dysbiosis. The gut microbiota have been shown to be important for MAFLD development in both preclinical and clinical studies.10-12 High-fat diet (HFD) is the standard method for inducing obesity, steatosis, and insulin resistance in mice.13 Previous studies have shown that germ-free mice treated with HFD gained less weight, exhibited less glycaemia, insulinemia, better glucose tolerance and insulin sensitivity along with increased FA oxidation, and decreased lipogenesis relative to conventional mice.14, 15 This review addresses just how the gut microbiota impact glucose, insulin, and lipid homeostasis via important microbial metabolites that promote MAFLD.
The role of microbial metabolites in metabolic-associated fatty liver disease
Introduction to bile acids
Bile acids (BAs) are the important host-derived and microbial-modified metabolites that regulate lipid homeostasis.16 Primary BAs are synthesized from cholesterol in the liver mainly through two biosynthetic pathways, the classical and alternative pathways.17 The classical pathway is responsible for about 75% of BA production, initiated by cytochrome P450 family enzyme of cholesterol 7α-hydroxylase (CYP7A1) then via sterol 12α-hydroxylase (CYP8B1) and sterol 27-hydroxylase (CYP27A1) to produce the primary BAs cholic acid (CA) or via CYP27A1 to produce chenodeoxycholic acid (CDCA). The alternative pathway is initiated by CYP27A1 and oxysterol 7α-hydroxylase (CYP7B1) to yield CDCA. The primary BAs conjugated with either glycine or taurine by liver-specific enzymes, bile acid-CoA ligase (BAL) and bile acid-CoA: amino acid N-acyltransferase (BAAT), form conjugated BAs. Conjugated BAs are secreted from the liver into the bile and released into the intestinal lumen to facilitate absorption of nutritional ingredients. In the intestine, the gut microbiota biotransform conjugated BAs into their unconjugated forms through the activity of bile salt hydrolase (BSH). Important gut bacterial genera involved in BSH metabolism include Bacteroides, Parabacteroides, Clostridium, Escherichia, Eggerthella, Eubacterium, Peptostreptococcus, and Ruminococcus. CA or CDCA then becomes metabolized into the secondary BAs DCA or LCA through 7α-dehydroxylation. The other BA metabolisms mediated by bacteria are the dehydrogenation and epimerization of 3-, 7-, and 12-hydroxyl groups. CDCA can be transformed by microbial 7α-hydroxysteroid dehydrogenase into the tertiary BA, ursodeoxycholic acid. The main gut bacterial genera involved in 7α-dehydroxylation reactions include Bacteroides, Eubacterium, and Lactobacillus, which carry out esterification reactions, whereas Clostridium, Fusobacterium, Peptococcus, and Pseudomonas, perform desulfation reactions.16, 18
Abnormal levels of BAs have been found in MAFLD patients, and BA receptors are implicated in the pathogenesis of MAFLD. Major BA-sensing receptors include Farnesoid X receptor (FXR), Takeda G-protein-coupled receptor 5 (TGR5), liver X receptor (LXR), vitamin D receptor, pregnane X receptor, and constitutive androstane receptor.19 Several clinical trials on FXR for the treatment of liver diseases are underway.20 In this review, we will focus on FXR, due to its highest clinical relevance and transformational potential.
Bile acids and Farnesoid X receptor: regulation of glucose and lipid metabolism
Farnesoid X receptor plays a key role in the hepatic lipid metabolism and the interplay of glucose, insulin, and BAs in lipid/glucose metabolism. Both sterol response element binding protein and carbohydrate response element binding protein expression are regulated by the BA sensitive nuclear receptor, FXR via inhibition of LXR.21 In the intestine, FXR is known to target the expression of genes that lead to the synthesis of ceramide the ceramide synthetic enzymes, sphingomyelin phosphodiesterase 3, and serine palmitoyltransferase long-chain base subunit 2.22 Increased ceramide activates three different signaling pathways in the liver, inhibitor of nuclear factor κB kinase subunit β, c-Jun N-terminal kinase, and protein kinase C-ζ that all result in insulin resistance.23 However, a hepatoprotective effect of FXR activation in the ileum is the FXR-induced production of fibroblast growth factor 19/(15 in mice), a hormone that when secreted into the circulation binds to the hepatic receptor fibroblast growth factor receptor 4 along with co-factor, β-Klotho. Fibroblast growth factor 19/15-fibroblast growth factor receptor 4 binding causes a decrease in BA synthesis, activation of ERK1/2 signaling pathways that serve to increase protein (GLUT1), and glycogen synthesis thus increasing glucose uptake and increasing storage of excess glucose as glycogen thus protecting against hyperglycemia and hepatic insulin resistance.24 Ceramides can also be synthesized in the hepatic de novo ceramide synthetic pathway from palmitate, a DNL product.4, 25 Most studies have shown that the increase in ceramides during hepatic steatosis is linked to an enhanced sphingolipid de novo synthesis with an increase in the expression of enzymes, SPT, and CerS (1, 2, 4, & 6).26, 27 A recent study of the liver lipidome in MAFLD patients indicated that the hepatic, de novo ceramide synthetic pathway, as determined from the dihydroceramide concentrations, but not other ceramide synthetic pathways, was increased.28 Hepatic FXR controls this pathway via its control over activation of LXR.
The gut microbiota shape the composition of the BA pool producing the endogenous ligands for FXR and TGR5. Gut microbiota that produce BSH are essential for deconjugation of the primary BAs, glyco-chenodeoxycholic acid (or tauro-chendoxycholic acid [TCDCA]) and glycocholic acid (or taurocholate acid) into CDCA and CA, which must precede subsequent, multiple 7α-dehydroxylation steps to produce the secondary BAs, deoxycholic acid (DCA) and lithocholic acid (LCA). The unconjugated, primary, and secondary BAs have high affinity for FXR, and their affinities have been ranked as CDCA > LCA = DCA > CA.29-31 On the other hand, hepatic reconjugation in the liver of the secondary BAs LCA and DCA to taurolithocholic acid (or glycolithocholic acid) and tauro deoxycholic acid (or glycodeoxycholic acid) gives rise to the most potent ligands for the intestinal BA sensitive G-protein-coupled receptor, TGR5. The rank order of BAs for activating TGR5 was taurolithocholic acid, glycolithocholic acid, LCA, tauro deoxycholic acid, glycodeoxycholic acid, DCA, TCDCA, glyco-chenodeoxycholic acid, CDCA, taurocholate acid, glycocholic acid, and CA.29, 32-34
In a recent study, Huang et al. reported that theabrownin derived from Pu-erh tea hypercholesterolemia altered the gut microbiota by reducing the abundances of BSH-producing bacteria.35 The reduced BSH activity in the gut microbiome resulted in increased conjugated BAs that were found to inhibit the intestinal FXR-FGF15/19 signaling pathway leading to elevated hepatic BA production from cholesterol.35 This study revealed that conjugated BAs served as antagonists for FXR confirming the original observations in a previous work.36 Decreased BSH activity results in increased conjugated BAs, especially TCDCA and TUDCA, in the distal ileum which, in turn, causes inhibition of intestinal FXR signaling. Inhibition of intestinal FXR leads to increased expression of the enzyme CYP7B1, the gatekeeper to the alternative BA synthetic pathway. The overall result is an increased production of CDCA and a shift away from 12-OH-BAs (CA). Increased 12-OH-BAs/non-12-OH-BAs ratios are associated with metabolic disease. The ratio of 12α-hydroxylated (12α-OH) to non-12α-OH-BAs was measured to be the highest in NASH patients.37 Accumulation of free cholesterol in the liver is part of the lipotoxicity associated with MAFLD and its continuance parallels development of NASH and fibrosis.38, 39 An independent study which inhibited BSH activity in mice using caffeic acid biphenyl ester produced similar results including increased conjugated tauro β-muricholic acid, a known intestinal antagonist, decreased expression of FXR target genes, Shp, Fgf15, Osta, and Ostb in the ileum but not in the liver.40
In another recent investigation, hyocholic acid (HCA) species, BAs common in pigs (80%) which do not develop diabetes in spite of their being on HFD were tested for effects in improving glucose homeostasis in pigs, mice, and human enteroendocrine cells.41 Pigs that were fed an intestinal FXR agonist, GW4064, that caused significant suppression of HCA species production, along with a 30% increase in blood glucose levels and 69% decrease in blood glucagon-like peptide-1 (GLP-1) levels. When HCA species were administered, the blood glucose levels decreased and circulating GLP-1 increased, suggesting that glucose homeostasis and GLP-1secretion were regulated by HCA species in pigs. Further in vivo testing of administered HCA species was performed in diabetic mouse models, and results were significantly lower serum glucose levels and increased circulating GLP-1 levels. In human enteroendocrine cells, HCA species outperformed other non-HCA BAs in their ability to stimulate GLP-1 secretion and proglucagon transcription. HCA species were found to be antagonists for intestinal FXR by their ability to reverse the inhibition of proglucagon transcription that leads to decreased GLP-1 production and secretion and agonists for TGR5 by their ability to downregulate the expression of SHP, a downstream target of FXR. CDCA, an FXR agonist, produced opposite effects.41
To summarize, the gut microbiota and their ability to metabolize many BAs and control the BA pool composition have a profound effect on glucose and lipid homeostasis. BAs show some promise as therapeutic agents for MAFLD and other metabolic diseases. It is also evident from the above discussion that we need both conjugated and unconjugated BAs as they are “switches” for fine-tuning BA receptor activity. MAFLD represents an upset in this fine tuning as the balance between Firmicutes and Bacteriodetes are shifted leading to changes in important metabolic pathways.
Short-chain fatty acids and metabolic-associated fatty liver disease
Short-chain fatty acids (SCFAs) are volatile organic compounds that are produced mainly through saccharolytic fermentation of carbohydrates in the small intestine by gut microbes. It has been demonstrated that SCFA production is associated with T2DM.42 Acetic acids, propionic acid, and butyric acid are the three most common SCFAs. The acetic acid production pathway is widely distributed in most enteric bacteria, while the propionic acids, butyric acids production pathways, seem to be more conservative and have higher substrate specificity.43 Ruminococcus bromii can degrade dietary resistant starch and produce formic and acetic acids.44 Recently, Ara et al. reported the acetic acid-producing bacterium Desulfovibrio vulgaris was associated with attenuation of hepatic steatosis in MAFLD mice.43 The bacteria that are associated with propionic acid production include Veillonella parvula, Bacteroides eggerthii, Bacteroides fragilis, R. bromii, and Eubacterium dolichum.45 The butyrate-producing bacteria, Anaerostipes, Roseburia, Butyrivibrio, Clostridium, Coprococcus, Eubacterium, Parabacteroides, and Ruminococcus have been identified by culture-based and sequencing-based approaches.46 Faecalibacterium prausnitzii, which is an anti-inflammation-associated bacterium, produces butyric acid. Butyric acid can promote the maintenance of tight junction barriers and has anti-inflammatory properties.47 Akkermansia municiphila is a mucin-degrading bacterium, which has the ability to produce diverse SCFCs, including butyric, propionic, and acetic acids.48, 49
The receptors for SCFAs include two G-protein-coupled receptors (GPRs): GPR43 (also known as free fatty acid receptor [FFAR] 2) and GPR41 (also known as FFAR3). Figure 2 is a diagrammatic representation of SCFA functions in the intestine, hepatocytes, and white adipose tissue (WAT). GPR43 when activated by either acetate or propionate promotes secretion of the hormone, GLP-1. This hormone is known to increase insulin release and decrease glucagon secretion thus decreasing blood sugar. It also acts to decrease GI motility resulting in increased energy harvest and feelings of satiety.50 GPR41 that is activated by butyrate or propionate increases plasma levels of peptide YY that also has the effect of decreasing gut motility and increasing energy harvest and feelings of satiety.51 Activation of either of these two receptors causes a decrease in the secretion of ghrelin causing decreased appetite.52 However, overnutrition accompanied with increased energy harvest and decreased transit time through the colon can lead to weight gain.
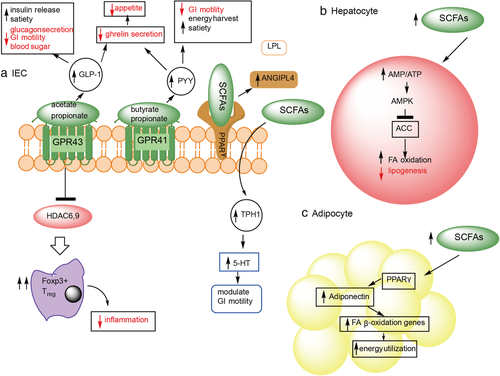
Peroxisome proliferator-activator receptor-γ is highly expressed in the colon and when activated by SCFAs causes increased secretion of angiopoietin-like-4 (ANGPTL4), a circulating inhibitor of lipoprotein lipase. Increased ANGPTL4 causes inhibition of cellular FA uptake, decreased adipose triglyceride accumulation, and less fat storage.53 Confirmation for the effect of ANGPTL4 on lipid storage has come from studies of germ-free ANGPTL4−/− mice, which showed the same degree of adiposity as wild-type controls.14 SCFAs can also directly interact with intestinal enteroendocrine cells to cause increased expression of TRP hydroxylase-1, the rate-limiting enzyme for 5-HT synthesis. 5-HT modulates GI motility and affects energy harvest which, if stored, leads to weight gain.54
In the adipocyte, SCFAs can activate peroxisome proliferator-activator receptor-γ in WAT to cause upregulation of adiponectin. The result is increased expression of FA β-oxidation genes thereby increasing energy utilization and preventing triglyceride accumulation in WAT.55 To summarize, SFCA production by gut microbiota modulates host metabolism by increasing energy harvest, decreasing blood sugar, increasing energy utilization, decreasing appetite and either increasing, or decreasing lipid storage. Overnutrition in combination with increased energy harvest may cause weight gain. Microbiota changes can thus lead to obesity via altered SCFA production.
Short-chain fatty acids also impact the liver by increasing the hepatic ratio AMP/ATP. The resulting increase of AMP-activated protein kinase blocks acetyl-CoA carboxylase causing increased expression of enzymes for FA β-oxidation. The result is decreased de novo hepatic lipogenesis and increased energy utilization.56 This effect affords protection against liver steatosis. Finally, GPR43 mediates Treg cells' IL-10 production to maintain intestinal homeostasis and suppress inflammation. SCFAs may increase colonic GLP-1/peptide-YY secretion independent of FFAR2/FFAR3.57 Behary et al. reported the enrichment of the SCFAs-producer microbes in hepatocellular carcinoma patients, the increased SCFA is associated with the immunosuppressive phenotype (increased regulatory T cells, reduced CD8 + T cells, and reduced antigen-presenting cells).58
Branched-chain and aromatic amino acids and metabolic-associated fatty liver disease
The predominant digestive activities of colonic gut microbes are the fermentation of carbohydrates and proteins. Protein fermentation mainly takes place at the distal colon where the fermentable carbohydrates are exhausted and the pH is close to neutral.59 It has been reported that Bacilli, Streptococci, Propionibacterium, Clostridium, and Bacteroides can degrade proteins,60 among which Bacteroides, Alistipes, and Parabacteroides have been identified as the major proteolytic taxon in the human colon. The proteolytic bacteria are associated with the pro-inflammatory responses and progression to MAFLD.12 In addition to gaseous products (H2S, ammonia, CO2, etc.), metabolites from proteolytic fermentation include derivatives from branched-chain amino acids (BCAAs) and aromatic amino acids (AAAs). The gaseous products, particularly H2S and ammonia, have detrimental effects on epithelial cell health.61 The ratio of plasma BCAAs (valine, leucine, & isoleucine) to AAAs (tyrosine & phenylalanine) has been proposed as a physiological hallmark for evaluating liver metabolism.62, 63 A lower ratio has been reported for liver cirrhosis and hepatocellular carcinoma.64
Research on BCAAs oral supplementation has shown decreased fat accumulation in the liver and attenuation of lipotoxic hepatocellular damage in the cirrhotic liver.65-69 However, several reports revealed that the elevated circulating BCAA was associated with MAFLD and liver injury.70, 71 BCAAs (including valine, leucine, & isoleucine) were found to increase during MAFLD progression as a response to the increased inflammation and oxidative stress.72 Gaggini et al. found that increased plasma BCAAs and AAAs were associated with liver inflammation in MAFLD with more severe liver damage associated with higher levels of BCAAs (isoleucine & valine) and AAA (tyrosine).73 Zhang et al. reported that BCAAs caused liver injury by enhancing adipocyte lipolysis and inhibiting lipogenesis and autophagy under HFD conditions.74
Dodd et al. demonstrated the pathway for reductive AAAs (tryptophan, phenylalanine, and tyrosine) metabolism.75 The species Clostridium sporogenes, Clostridium botulinum, Clostridium cadaveris, and Peptostreptococcus anaerobius CC14N were found to produce indolepropionic acid in the gut. Indolepropionic acid is a metabolic product from tryptophan, which can fortify the intestinal barrier via pregnane X receptor.76
Choline metabolism and metabolic-associated fatty liver disease
Choline deficiency has been associated with MAFLD in both animal models and humans.77 It is an essential nutrient as it is a major methyl donor for the biosynthesis of the important cell membrane lipids, phosphatidylcholine (PC), lysophosphatidylcholine, and sphingomyelin.78, 79 It is also necessary for the synthesis of the neurotransmitter, acetylcholine.79 PC deficiency increases DNL, which causes an increase in TGs. Lack of PC in hepatic lipid droplets reduces their surfactant properties, and larger lipid droplets are formed that are less likely to undergo lipolysis. PC is required for both VLDL synthesis and secretion from the liver.79, 80 PC has also been identified as a cell wall component of ~10–15% of all bacteria.81
On the other hand, dietary choline can be metabolized by gut microbiota to trimethylamine, which can then be further processed by the liver to produce the highly toxic trimethylamine oxide (TMAO).82 The bacterial species, Anaerococcus hydrogenalis, Clostridium asparagiforme, Clostridium hathewayi, C. sporogenes, Escherichia fergusonii, Proteus penneri, Providencia rettgeri, and Edwardsiella tarda, have all been reported to metabolize choline to TMA production.83 Several studies have revealed the association between higher circulating TMAO and MAFLD/NASH.84 A clinical study showed that higher TMAO concentrations were significantly associated with an increased risk of all-cause mortality in patients with MAFLD independent of traditional risk factors.85
Endogenously produced ethanol and metabolic-associated fatty liver disease
Ethanol is constantly produced in the gut via glycolysis and metabolized in the liver by alcohol dehydrogenase under normal conditions. The intestinal microbiota is the major source of endogenous alcohol. Enterobacteriaceae, including Escherichia coli, can produce ethanol through the mixed-acid fermentation pathway. In recent years, evidence has continued to accumulate for the role of endogenous alcohol in MAFLD. Volynets et al. reported the increased blood ethanol levels in patients with MAFLD86; Zhu et al. reported the elevated endogenous alcohol level in the blood of NASH patients, as well as the increased abundance of Escherichia.87 Mitochondrial dysfunction due to impaired lipid metabolism in the liver plays a key role in the development of MAFLD/NASH as it leads to the accumulation of reactive oxygen species, which, in turn, triggers lipid peroxidation and hepatocyte lipoapoptosis.88, 89 Recently, a study demonstrated that a high-alcohol-producing species, Klebsiella pneumonia, caused mitochondrial dysfunction and induced MAFLD. The elevated alcohol was also associated with increased inflammation,90 intestinal permeability,91 and mitochondrial dysfunction,92 resulting in adverse pathological effects in the gut and liver.
Methodologies used in microbial metabolomics
Metabolomics is an important type of analysis that can be used to quantify the metabolites derived from gut microbiota. Chromatography-mass spectrometry (MS) spectroscopy is a widely used technique in host–microbiota studies due to its high sensitivity and high throughput. The detections of BAs, TMAO, amino acids, and lipids are mainly using liquid chromatography-MS, while the volatile metabolites (such as SCFAs) are detected by gas chromatography-MS. Several microbial metabolomics methods have been developed to detect metabolites from certain microbes. Barkal et al. designed an integrated co-culture and extraction platform that can perform cultures of bacteria and further chemical studies. This integrated platform can detect the components of metabolites in bacteria under different culture conditions on a large scale, which is conducive to an in-depth study of the metabolic functions of bacteria.93 Hou et al. developed a method for targeted metabolomics analysis of 11 key gut microbiota, which was useful for studying the mechanisms of important microbiota–host co-metabolites.94 Methodologies have also been developed to analyze a certain class of substances such as BAs,95 SCFAs,96 and BCAAs.97 In recent years, an automated high-throughput metabolite array technology was reported that could rapidly and quantitatively determine 324 metabolites, including abundant microbial metabolites.98 Notably, Zhao et al. proposed a method especially for microbial metabolome, which can detect fatty acid, amino acid, carboxylic acid, hydroxy acid, indoles, phenols, cinnamic acids, keto-acids, sugar acids, and their derivatives in both human and bacteria biological samples.99
Moreover, many bioinformatics analysis methods have been developed for understanding the biomarkers in metabolomics, host–microbe interactions, and associated genomics. Several approaches of machine learning100 and data mining101 have been used in grouping prediction and biomarkers finding in microbial metabolomics-related studies. Liang et al. designed and developed an MS-based untargeted metabolomics data mining software for metabolomics biomarker detection and diverse pathway analysis.102 Correlation analysis is widely used in study association in host–metabolites–microbes.103 Spearman and generalized linear regression model are mainly used for detecting linear correlations, while the method of maximum information coefficient104 is used for finding linear/nonlinear associations between metabolites and microbes. A novel method, generalized correlation analysis for metabolome and microbiome, was developed particularly for metabolome and microbiome data.105 Pedersen et al. developed a framework of integration approach in the microbiome, metabolome, and host physiology phenotype for the identification of potential mechanistic links.98 Besides the data correlation analysis, recently a correlative imaging workflow was presented, which can identify metabolic phenotypes and link the spatial metabolomes to their taxonomic group within host–microbes and microbes–microbe communities.106 Because the distribution of intestinal bacteria in the gut is geographically specific and the liver disease is closely related to the intestinal bacteria translocation, this in situ imaging correlation method has the potential to find more meaningful interactions.
Conclusion
The study of the metabolic disease (MAFLD) crosses many disciplines, and metabolomics is one of them.
Metabolomics allows one to examine the products of metabolism between different treatment groups to distinguish them on the basis of a metabotype rather than a phenotype. An example of this is a comparison of healthy obese individuals and obese, diabetic subjects. Although their body mass index may be comparable, their metabolisms are quite different. The use of metabolomics to study host–gut microbiota metabolic interactions is still relatively new, and many challenges still remain. For example, how to distinguish what is derived from the host and what comes from the microbiota still requires a lot more depth of sequencing of the bacteria, and more knowledge of what the bacteria can produce and contribute to the overall metabolome. In this review, we highlighted gut metabolites (Fig. 3) that are fairly well known, but complete understanding of how they impact MAFLD development and progression is still a topic of current research investigations.