Diet and gut microbiome in fatty liver and its associated liver cancer
Declaration of conflict of interest: None.
Abstract
Non-alcoholic fatty liver disease (NAFLD) is the major cause of chronic liver disease worldwide as a consequence of a sedentary lifestyle and overnutrition. NAFLD could progress to non-alcoholic steatohepatitis (NASH), which may further develop to cirrhosis and hepatocellular carcinoma (HCC). The gut microbiome is one of the central regulators in host metabolism. Diet could change human gut microbiome rapidly and reproducibly and modulate several metabolic pathways. Both diet and gut microbiome dysbiosis are associated with NAFLD and its related HCC (NAFLD-HCC). Dietary cholesterol, fiber, fat, or carbohydrate could change the microbiome composition to contribute to the development of NASH and NAFLD-HCC. Hence, identification of elements of the gut–liver axis that are primarily damaged in NASH and NAFLD-HCC offers new possibility for therapeutic intervention. In this review, the roles of gut microbiome and microbial metabolites in the development and progression of NAFLD and NAFLD-HCC are first discussed. The impacts of different diet compositions including cholesterol, fiber, fat, and sugar on the gut microbiome that leads to predisposition to NASH and NAFLD-HCC are also explored. We summarized the article by discussing potential therapeutic implication of diet and microbiome modulation in fatty liver and liver cancer.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome. In Hong Kong, NAFLD affects more than a quarter of all adults1 and 13.5% of general population could develop NAFLD over a 3- to 5-year period.2 About a quarter of NAFLD patients progress to non-alcoholic steatohepatitis (NASH)/fibrosis in 3 years, while 27% of patients with NASH-associated cirrhosis could develop hepatocellular carcinoma (HCC) (2–3% per year).3 NASH has rapidly become a leading etiology underlying many causes of HCC.4 The proportion of HCC developed from NASH has raised 7.7-fold in the last decade,4 and NASH-associated liver mortality is expected to increase more than double in the next decade due to epidemics of obesity and diabetes.5, 6
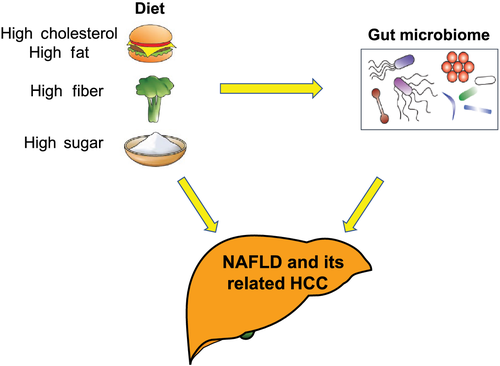
Gut microbiome has crucial physiological roles in host digestion and metabolic processes.7 Recently, investigators have begun to appreciate that the gut microbiome can impact organs beyond the intestine.8 Liver is exposed to gut microbes-derived metabolites through portal vein blood coming from intestine, which accounts for 70% of the whole blood supply of liver, thus showing the crucial correlation between gut and liver.1, 9 Several studies have shown that microbiome could influence susceptibility to many liver diseases, including oxidative liver injury,9 chronic hepatitis B disease,10 steatosis,11 NASH,12, 13 cirrhosis,14 and HCC.15, 16 We previously reported that gut microbiome dysbiosis and alteration of gut metabolites play an important role in NASH and NAFLD-related liver cancer (NAFLD-HCC) induced by high-cholesterol diet.17 Dietary cholesterol led to enrichments of Mucispirillum, Desulfovibrio, Anaerotruncus, and Desulfovibrionaceae during the development of NASH and NAFLD-HCC, while Bifidobacterium and Bacteroides were depleted.17 Apart from cholesterol, dietary fiber, fat, and sugar could also change the microbial diversity and alter microbiome composition. These alterations could contribute to the pathogenesis of chronic inflammatory diseases including NASH and NAFLD-HCC.18 Increased fat consumption has been positively correlated with insulin resistance, postprandial lipid metabolism dysfunction, and even NAFLD development or progression.19 In contrast, intake of vegetables, fruits, and vitamin K could effectively reduce the risk of NAFLD development.20, 21 These findings indicate the importance of a balanced diet to prevent NAFLD development. Here, we discuss how diet composition impacts gut microbial ecology and leads to predisposition to NASH and liver cancer.
Microbiome in non-alcoholic fatty liver disease and non-alcoholic fatty liver disease-hepatocellular carcinoma
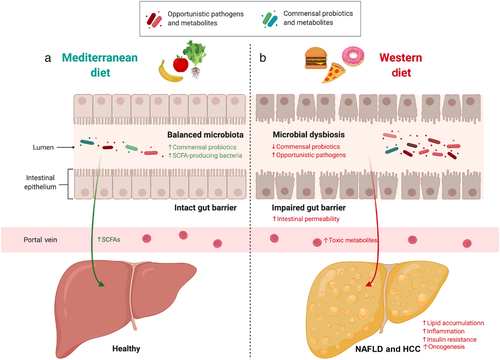
Gut microbiome has been considered as the driving force of both hepatic steatosis and inflammation,22 and gut dysbiosis is involved in the development of multiple liver diseases including simple steatosis, NASH, and NAFLD-HCC. Indeed, patients with steatosis already showed features of gut dysbiosis with lowered microbial richness and enrichments of Acidaminococcus, Escherichia spp., and Bacteroides spp., which were correlative with insulin resistance.11, 23 While NASH patients were reported to have depletions of Faecalibacterium and Anaerosporobacter and enrichments of Parabacteroides.12 Gut dysbiosis is also actively involved in NASH-related cirrhosis and NAFLD-HCC. Patients with NASH-related cirrhosis showed higher abundance of Enterobacteriaceae and Streptococcus and lower abundance of Akkermansia.16 NASH-HCC patients showed increased gut Bacteroides and Ruminococcaceae, while Bifidobacterium was reduced.16 Both Akkermansia and Bifidobacterium play protective roles in liver injury, and their depletions could enhance inflammation in intestine and liver to contribute to the process of NASH-HCC.24, 25 Behary et al. performed metagenomic sequencing analysis and identified enrichments of five bacterial species in the gut of NAFLD-HCC patients (Bacteroides caecimuris, Ruminococcus gnavus, Veillonella parvula, Bacteroides xylanisolvens, and Clostridium bolteae), and all of them are short chain fatty acids (SCFAs)-producing bacteria with immunomodulatory activities. In addition, Bacteroides and Ruminococcus are associated with inflammation and pro-inflammatory markers.16, 26 Therefore, these findings clearly demonstrated that gut microbiome dysbiosis is involved in different stages of NAFLD progression. However, it should be noted that the gut microbiome is influenced by various environmental factors; thus, results among microbial profiling studies can be greatly varied.
Gut barrier dysfunction plays an important mechanistic role in microbiome-driven NASH progression. Microbial commensal is an element of gut barrier that prevents pathogenic microbes enter the portal vein.27 However, gut dysbiosis, inflammation, and tight junction disruption can induce leaky gut.27 Production of toxic metabolites by gut microbes could also impair the gut barrier function. Once the gut barrier is disrupted, bacteria and pathogen-associated molecular patterns (PAMPs) including lipopolysaccharides (LPS) can translocate to liver through the leaky gut, thereby activating pathogen recognition receptors and inducing immune response to promote NASH and HCC progression.27, 28 Indeed, mice with defects in intestinal permeability develop more severe steatohepatitis with more steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis.29 Recent study by Mouries et al. reported that impaired gut barrier function, which allows bacterial translocation to the liver, is an early event in the progression of NASH. Using fecal microbiota transplantation (FMT), they found that transplanting microbiome of high-fat diet fed mice to germ-free recipient mice could induce gut barrier disruption, suggesting that disruption of gut barrier in NASH is driven by gut dysbiosis.30
Besides of gut microbes, bacterial DNA was also detected in liver tissues of NAFLD patients with direct correlation with disease severity.31 In particular, Peptostreptococcaceae, Verrucomicrobia, Actinobacteria, and Gamma proteobacteria in liver tissue were closely associated with severity of NAFLD. These results indicate that translocation of gut bacteria could be detrimental to liver as well as hepatopathogenesis. Nevertheless, investigation of microbes in liver tissues is currently at early stage with inadequate evidence proving their contribution to development of NAFLD, NASH, or other liver diseases. Further studies are therefore needed to clarify whether bacteria could be cultivated from a NASH liver and how intrahepatic bacterial components are involved in the pathophysiology of NASH and NAFLD-HCC.
Microbial metabolites in non-alcoholic fatty liver disease and non-alcoholic fatty liver disease-hepatocellular carcinoma
Apart from gut microbes themselves, another intriguing link between microbiome and NAFLD is the microbial metabolites.32 Several metabolites in gut, circulation, and liver tissues including amino acid, SCFAs, and bile acids have been identified to promote NAFLD and NAFLD-HCC. For instance, non-diabetic obese women with hepatic steatosis were found to have dysregulated aromatic and branched-chain amino acid metabolism.11 N,N,N-trimethyl-5-aminovaleric acid, a metabolite of intestinal microbes metabolized from trimethyllysine, could reduce carnitine synthesis and fatty acid oxidation, thereby exacerbating NAFLD development.32
Short chain fatty acids compose of formate, acetate, propionate, and butyrate. Of note, reports on the abundance and function of SCFAs in NAFLD and its related diseases have been controversial. A study involving 72 biopsy-proven NAFLD and 14 NAFLD-fibrosis patients demonstrated that fecal abundances of SCFAs are significantly different in these two groups: enriched acetate and formate in NAFLD patients, whereas butyrate and propionate were increased in NAFLD-fibrosis group.33 However, another study revealed that fecal formate, acetate, and butyrate were all significantly increased in NAFLD-HCC patients compared with NAFLD-cirrhosis patients and healthy controls.26 Butyrate has been reported to protect against diet-induced NASH development in mice,34 while acetate could induce de novo lipogenesis through activating lipogenic genes.35 Nevertheless, diet and other environmental factors may greatly contribute to the result inconsistency among different results. Further mechanistic studies need to be performed to clarify the role of SCFAs in NAFLD and its related liver disease.
Bile acid is another important metabolite that links the gut microbiome with liver diseases. Bile acid can change its receptor farnesoid X receptor (FXR) to regulate NASH development.36 For instance, a FXR activator obeticholic acid could significantly ameliorate fibrosis and disease severity of NASH patients in a phase 3 clinical trial.37 In general, primary bile acids secreted from liver are unconjugated by gut microbes, and these unconjugated bile acids are reabsorbed to form secondary bile acid and being sent back to liver for detoxification.38 Dysregulated bile acid-microbiome crosstalk impairs this process to induce inflammation and HCC development.38 Gut dysbiosis especially enrichment of Clostridium in obesity and NASH could induce secretion of deoxycholic acid (DCA), a secondary bile acid that is famous to cause DNA damage.39 Increased DCA in liver could also provoke senescence-associated secretory phenotype to promote secretion of various inflammatory and tumor-promoting factors, thus further boosting HCC development.39
Diet modulating gut microbiome to influence non-alcoholic steatohepatitis and non-alcoholic fatty liver disease-hepatocellular carcinoma
Accumulating evidence has showed that gut microbiome could promote Western diet-induced systemic pathologies. In turn, Western-style diet, a dietary combination with high contents of sugar, fat, and cholesterol and low in fiber, could alter the gut microbiome.40 Given the interaction between diet and gut microbiome, they are both crucial factors for the development of NASH and NAFLD-HCC (Fig. 1).
Cholesterol and gut microbiome in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease-hepatocellular carcinoma
Epidemiology statistics have reported the significant positive correlation between cholesterol intake and the risk of cirrhosis and liver cancer.41 Preclinical studies also showed that animals (including mice, rats, and rabbits) with consumption of high-cholesterol diet have increased incidence of fatty liver.42-44 Mechanically, excess cholesterol is usually stored in intracellular oil droplets in the form of cholesteryl esters. At the same time, synthesis of triglycerides, which is conducive to the formation very low-density lipoprotein (VLDL), is also stimulated. However, if VLDL cannot be effectively composed, cholesterol and triglycerides are secreted into the circulation, causing lipids to accumulate in liver cells. Therefore, it is expected that excess intake of cholesterol could increase the chance of fatty liver development. As cholesterol intake is closely associated with elevated risk of NAFLD, reducing the content of cholesterol in diet is extremely important in NAFLD prevention.
In our previous study, for the first time, we showed that dietary cholesterol plays a crucial role in NASH-HCC progression by modulating gut microbiome, of which cholesterol-modulated gut microbiome could induce steatohepatitis and liver cells proliferation in germ-free mice.17 High-cholesterol diet increased abundances of Mucispirillum, Desulfovibrio, Anaerotruncus, and Desulfovibrionaceae, while probiotic Bifidobacterium and Bacteroides were depleted (Table 1). Consistently, high-cholesterol diet increased serum level of taurocholic acid and decreased 3-indolepropionic acid, which enter the liver through portal vein and regulate NAFLD-HCC (Fig. 2). When transplanting feces from high-cholesterol fed mice into germ-free mice, hepatic inflammation, lipid accumulation, and hepatocyte proliferation were successfully triggered (Fig. 2).17 Hence, these results implicate that without an intact gut microbiome, the effect of cholesterol to induce metabolic disease could be different or even totally abolished. In another study by Kubeck et al., they found that germ-free mice receiving cholesterol-rich-based high-fat diet could completely reverse diet-induced obesity, suggesting that dietary cholesterol protects germ-free mice from obesity through the absence of gut microbiome.45 When investigating the metabolic fate of dietary cholesterol, gut Clostridiales was found to have positive correlation with bile acid level.45 Collectively, dietary cholesterol plays a crucial role in NASH and NAFLD-HCC development through modulating gut microbiome.
| Diet | Microbiota alteration | Influence on NAFLD and HCC |
|---|---|---|
| Cholesterol |
Mucispirillum ↑, Desulfovibrio ↑, Anaerotruncus ↑, Desulfovibrionaceae ↑, Bifidobacterium ↓, Bacteroides ↓ |
Increased risk17 |
| Fiber |
Bifidobacteria ↑, Firmicutes/Bacteroides ↑ Proteobacteria ↑, Clostridia spp. ↑, and other fiber-fermenting bacteria ↑ |
|
| Decreased risk46 | ||
| Increased risk47 | ||
| Fat |
Enterobacteriales ↑, Bifidobacterium spp. ↓ Parabacteroides ↑, Isobaculum ↑ Bacteroides spp. ↓, Eu. rectale-Cl. ↓ coccoides group |
|
| Increased risk48, 49 | ||
| Decreased risk50 | ||
| Sugar |
Bacteroides genus ↑ Acetate-producing microbiota ↑ |
Increased risk51, 52 |
- HCC, hepatocellular carcinoma; NAFLD, non-alcoholic fatty liver disease.
On the other hand, gut microbiome could also regulate cholesterol homeostasis. Antibiotic treatment decreases the circulating and hepatic cholesterol levels, indicating the crucial role of gut microbiome in cholesterol metabolism.53 Kenny et al. identified a group of coprostanol-forming microbes, which converse cholesterol to sterol coprostanol and reduce gut and serum cholesterol levels.54 Therefore, cholesterol and gut microbiome interact with each other in the progression of NASH and NAFLD-HCC.
Fiber and gut microbiome in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease-hepatocellular carcinoma
Based on its solubility in water, fiber can be divided into soluble fibers (e.g. pectin and inulin) and insoluble cellulose (e.g. cellulose). Recent evidence revealed that fiber consumption could play a crucial role in NAFLD—not only as a part of balanced diet but also involved in the modulation of gut microbiome. Gut microbes ferment dietary soluble fibers into SCFAs, which are known for having health-promoting effects by maintaining the diversity of gut microbiome and production of beneficial metabolites. In particular, inulin-type fructans prebiotics could increase abundances of probiotic Bifidobacterium and Faecalibacterium in obese women to improve host metabolism.46 In a randomized clinical trial, dietary fiber intake significantly improved hepatic steatosis and liver function by ameliorating intestinal permeability of 32 NAFLD patients.55 A high-fiber diet also enriched beneficial microbes (e.g. Bifidobacteria), depleted potential detrimental microbes, and reduced ratio of Firmicutes/Bacteroides in both humans and mice (Table 1).56 In another cross-sectional study by Daubioul et al., daily ingestion of 16 g of oligofructose improved hepatic enzymes and insulin levels in NASH patients,57 whereas Cantero et al.58 reported that consumption of insoluble fibers (≥ 7.5 g/day) rather than soluble fibers could also ameliorate hepatic steatosis and NAFLD.
However, the effects of fiber consumption on host metabolism could be varied among individuals.59 To address this issue, a preclinical study colonized mice with feces collected from different human subjects with supplementation of any one type of fiber (cellulose, inulin, pectin, or assorted fiber). As expected, mice harboring different human-derived microbiomes resulted in diverse metabolic outcomes even though they were fed with the same dietary fiber. Hence, dietary fiber could benefit certain microbiome but not all microbiome composition.59 Dietary fiber was also found to have disconcerting effect. Singh et al. reported that soluble fiber inulin could induce HCC in a microbiome-dependent manner,47 of which high-fat high-inulin diet led to gut dysbiosis with enrichments of fiber-fermenting bacteria and Proteobacteria (Table 1). Thus, supplementation of foods with high content of fermentable fibers needs to be very careful as it may in turn increase risk of HCC. In general, one-fits-all fiber supplementation is unlikely to have consistent effect among individuals, therefore indicating that clinicians need to consider whether fiber supplementation could optimize treatment outcomes. Another critical issue is that, to date, no studies have shown solid evidence to confirm whether patients with advanced NAFLD could be benefited from high fiber intake.
Fat and gut microbiome in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease-hepatocellular carcinoma
Dietary fats can be divided to saturated fats and unsaturated fats. Recent studies have presented evidence that fats could have diverse effects on NAFLD and NASH. For example, diets enriched with omega-3 polyunsaturated fatty acids (PUFA) prevented the development of insulin resistance,50 ameliorated hepatic steatosis,60 and decreased intrahepatic triglyceride storage61 in obese rodents. However, in human, overconsumption of PUFA and saturated fatty acids could significantly promote fat accumulation in liver,62, 63 while, in contrast, another study involving 355 individuals showed that PUFA supplementation could in turn decrease liver fat accumulation.64 Hence, although preclinical studies have shown benefits of fat consumption to the prevention of fatty liver diseases, more in-depth investigation is necessary to confirm these effects in human.
The crosstalk between dietary fat and gut microbiome has long been of interest. Dietary fat is a modifiable risk factor that may impact metabolic syndromes by altering the microbiome composition. In human, a large-scale dietary intervention study involving 88 subjects provided evidence that high-fat diet could reduce microbial diversity and richness.65 Mechanically, Cani et al. found that administration of high-fat diet significantly reduces abundances of dominant members of the gut microbiome in mice such as Bacteroides spp., Eu. rectale-Cl. coccoides group, and Bifidobacterium and induces insulin resistance in a bacterial LPS-dependent manner.48 In another animal study, mice treated with high-fat diet showed enrichment of Enterobacteriales, which was correlated with the obese phenotype.49 However, supplementing oleic acid-derived compounds to these mice could restore bacterial diversity (increased Enterobacteriales and decreased Bifidobacterium spp.) and counteract microbiome imbalance caused by high-fat diet.49 Similar findings were also reported in other studies,66, 67 suggesting that certain fatty acids could have protective roles against obesity through altering the gut microbiome. Indeed, supplementation of different types of fats can lead to diverse changes in the gut microbiome. A clinical trial was conducted with supplementation of different types of high-fat diet to 25 subjects at risk of metabolic syndrome. Diets rich in monounsaturated fatty acids (e.g. conventional canola oil and oleic canola oil) showed significant enrichment of Parabacteroides, while PUFA-rich diets (a blend of corn/safflower oil or flax/safflower oil) favored high abundance of Isobaculum (Table 1).68 Recent study from our group showed that high-fat diet increased Alistipes sp. Marseille-P5997 and Alistipes sp. 5CPEGH6 accompanied with elevated metabolite lysophosphatidic acid and depleted probiotic Parabacteroides distasonis.69 Of note, there is not much known about the functional and mechanistic roles of these altered gut microbiomes; thus, further in-depth investigation of these microbes in the development of obesity and NAFLD is needed. In summary, extensive study is needed to identify specific types of fat as well as gut microbes that are associated with risk of NAFLD, and reduction in total fat intake is still too early to be the simple solution for NAFLD and NASH patients.
Sugar and gut microbiome in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease-hepatocellular carcinoma
Dietary sugar is usually obtained from sucrose, high-fructose corn syrup, and sugary beverages, which all have been shown to have particular tendency to induce NAFLD in epidemiological70-73 and preclinical studies.74-77 The development of insulin resistance is partly driven by the metabolism of fructose by fructokinase C in liver, while fructose metabolism that takes place in mouse gut could disrupt intestinal barrier to increase permeability,75, 78 thereby contributing to the development of NAFLD. Indeed, when treated mice with antibiotics, NAFLD could be alleviated by reducing endotoxemia.79 Dietary fructose could also re-shape the gut microbial community to favor NAFLD development. Boursier et al. observed that the severity of human NAFLD is associated with the changes in metabolic functions of gut microbiome with increased abundance of Bacteroides in NASH,51 of which Bacteroides was shown to have strong positive correlation with fecal content of raffinose and stachyose (containing glucose and fructose).51 In another study, Zhao and colleagues found that fructose consumption triggers hepatic de novo lipogenesis depending on the microbes-mediated metabolism of fructose to produce acetate, which is then consumed in lipogenic pools of acetyl-CoA (Table 1).52 Gut microbes also produced other SCFAs such as butyrate, which has been confirmed as a contributor to hepatic lipogenesis.80 Taken together, these convincing evidence demonstrated that sugar especially fructose intake increases the risk of NAFLD development. Nevertheless, extensive clinical trials and mechanistic studies are urgently needed to confirm the direct interaction between dietary fructose and gut microbiome.
Diet and microbiome regulation to prevent non-alcoholic fatty liver disease and non-alcoholic fatty liver disease-hepatocellular carcinoma
Although being prevalent, Western diet with high contents of red meat, fat, and fructose favors the development of NAFLD, while the Mediterranean diet, characterized by low consumption of red meat, dairy products, and saturated fats and high intakes of vegetables, fruits, nuts, and fish, is the most recommended dietary pattern for NAFLD prevention. Beneficial bacteria could also directly confer health benefits to host; thus, approaches that aim to change the indigenous gut microbiomes or their derived metabolites have been constantly studied.81 Growing evidence suggested that probiotics especially Lactobacillus could inhibit the development of chronic liver diseases. For example, gut-resident Lactobacillus could activate the transcription factor Nrf2 in liver to protect against oxidative injury.9 Lactococcus lactis subspecies cermoris protected mice from NASH by reducing serum cholesterol level and increasing glucose tolerance.13 While in human, treatment of probiotics formula containing Lactobacillus acidophilus could reduce liver fat accumulation and liver injury in NASH patients.82 Prebiotics is another approach for gut microbiome modification, which has shown its therapeutic potential in NAFLD patients by reducing body mass index, hepatic enzymes, and serum cholesterol level.83 Preclinical mechanistic studies suggested that dietary supplementation of prebiotics could improve NAFLD by modulating gut microbiome and attenuating insulin resistance and liver fat accumulation.84
Both NAFLD and NAFLD-HCC patients display signatures of gut dysbiosis signature with enrichments of opportunistic pathobionts. To deplete these detrimental microbes, FMT has been recently emerged as an alternative therapeutic option, which includes transplantation of feces from a healthy donor to the recipient patient. FMT has achieved a big success against inflammatory bowel disease with receiving FDA approval for treatment of recurrent Clostridium difficile infection. In comparison, much less FMT studies were carried on NAFLD patients. A few evidence showed that FMT could improve the gut microenvironment, reduce intestinal permeability and liver lipid accumulation, and improve insulin resistance.85 In another animal study, transplanting feces obtained from patients with steatosis (grade 3, > 66%) into recipient mice resulted in initiation of hepatic lipid accumulation,11 indicating the importance of a pathological microbiota for NAFLD development and progression. By contrast, transplanting feces of lean human donors increase insulin sensitivity in mice with metabolic syndrome, suggesting the feasibility and potential of FMT against NAFLD.86 Apart of probiotics and FMT, antibiotic therapy may act as a way. But employing antibiotics may lead to alter ranges of microbial species. Yet, whether NAFLD patients could benefit from it needs further evaluation.
Conclusion
In this review, the impacts of different diets on the gut microbiome in NASH and its associated disease progression are explored. Both animal experiments and clinical data have shown different diet-associated signatures of gut microbiome signatures in NASH and NAFLD-HCC, and these signatures could potentially serve as non-invasive diagnostic biomarkers for diagnosis. However, the identified signatures need to be extensively evaluated in large-scale randomized clinical trials to ensure their accuracy. Meanwhile, although accumulated clinical evidence confirmed the association of gut dysbiosis with NAFLD, NASH, and NAFLD-HCC, the actual mechanistic links are far from well established. In summary, the gut microbiome is an essential element involved in NAFLD pathogenesis or disease prevention; hence, direct modulation of gut microbiome by dietary intervention could be an ideal and feasible option to NASH patients for disease management.
Acknowledgments
The project was supported by National Natural Science Foundation of China (No. 82103355), RGC Theme-based Research Scheme Hong Kong (No. T12-703/19R), Health and Medical Research Fund, Hong Kong (No. 08191336), and CUHK direct grant.




