Phase 1 study on the safety and efficacy of E6011, antifractalkine antibody, in patients with Crohn's disease
Declaration of conflict of interest: K. Matsuoka has received research support and lecture/consulting fees from AbbVie, Sekisui Medical, Thermo Fisher Scientific, Alfresa Pharma, EA Pharma, Mitsubishi Tanabe Pharma, Mochida, Kyorin, Kissei, Astellas Pharma, JIMRO, Daiichi Sankyo, Janssen, Pfizer Japan, Takeda, Asahi Kasei Medical, Zeria, Gilead Sciences, Miyarisan, Nippon Kayaku, Celltrion Healthcare, Eli Lilly Japan, Allergan Japan, Shionogi; M. Naganuma has received research support and lecture/consulting fees from EA Pharma, Zeria, Mitsubishi Tanabe Pharma, Kyorin, JIMRO, Mochida; T. Hibi has received research support and lecture/consulting fees from EA Pharma, AbbVie, JIMRO, Zeria, Otsuka, Mitsubishi Tanabe Pharma, Kyorin, Janssen, Mochida, Takeda, Gilead Sciences, Celltrion Healthcare, Nippon Kayaku, Kissei, Miyarisan, Ferring, Eli Lilly Japan, Pfizer Japan, Nichi-Iko, Nippon Kayaku; H. Tsubouchi has nothing to disclose; K. Oketani and T. Katsurabara are employees of EA Pharma; S. Hojo and O. Takenaka are employees of Eisai; T. Kawano and T. Imai are employees of KAN Research Institute. T. Katsurabara, O. Takenaka, and T. Imai are stockholders of Eisai; T. Kanai has received research support and lecture/consulting fees from Zeria, Takeda, EA Pharma, Astellas Pharma, Miyarisan, Aska, Mochida, AstraZeneca, Mitsubishi Tanabe Pharma, Pfizer Japan, Kyorin, Mylan, Ono, Eli Lilly Japan, Chugai, Janssen, Bristol-Myers Squibb, Sumitomo Dainippon, Pola Pharma, MSD, Otsuka Pharmaceutical Factory, Daiichi Sankyo, Yakult, Kyowa Hakko Kirin, AbbVie, Nippon Kayaku, Eisai, JIMRO, Tsumura, Taiho, Otsuka, Fujifilm RI Pharma, Japan Blood Products Organization, UCB Japan, EN Otsuka, Ezaki Glico, EIDIA.
Author contribution: Katsuyoshi Matsuoka and Makoto Naganuma contributed to the study design and provided critical revision of the manuscript; Kiyoshi Oketani and Toshinori Katsurabara designed the study and drafted the manuscript; Seiichiro Hojo and Osamu Takenaka performed the analysis and interpretation of the data; Tetsu Kawano and Toshio Imai contributed to the study concept and provided critical revision of the manuscript; and Toshifumi Hibi, Hirohito Tsubouchi, and Takanori Kanai contributed to the study design, provided critical revision of the manuscript, and supervised the study.
Financial support: This study was supported by Eisai Co., Ltd. and EA Pharma Co., Ltd., Tokyo, Japan.
Abstract
Background and Aim
E6011 is a humanized monoclonal antibody targeting fractalkine (FKN), a CX3C chemokine, which regulates leukocyte trafficking during inflammation. We evaluated the safety and pharmacokinetic profile of E6011 in patients with Crohn's disease (CD) and also performed preliminary pharmacodynamic (PD) and efficacy assessments.
Methods
This study included a 12-week multiple ascending dose (MAD) phase (2, 5, 10, and 15 mg/kg intravenously every 2 weeks, n = 6, 8, 7, and 7, respectively) and a 40-week Extension phase (n = 12) at the same dose as the MAD phase. Serum E6011, serum total FKN (free soluble FKN and E6011-FKN complex) as a PD marker and CD activity index were evaluated. The primary outcome was safety assessment in the MAD phase.
Results
Twenty-seven (96%) of 28 patients had previously been treated with anti-tumor necrosis factor α agents. During the MAD phase, adverse events (AEs) occurred in 18 (64%). The most common AE was nasopharyngitis (five patients, 18%). No severe AEs occurred. Serious AEs occurred in three patients, progression of CD in two, and anemia in one. Serum E6011 concentrations increased dose-dependently after infusion and reached a plateau around 4–6 weeks. Serum total FKN rose simultaneously. Five (18%) patients developed anti-E6011 antibodies during the study. Overall, clinical response and clinical remission were observed at Week 12 in 40% (10/25) and 16% (4/25) of active CD patients, respectively.
Conclusion
E6011 was well-tolerated and might be effective in CD patients. These findings need to be clarified in a randomized controlled study.
Introduction
Crohn's disease (CD) is a chronic inflammatory disorder that mainly affects the ileum and proximal colon, characterized by recurrent cycles of inflammation leading to the development of strictures and intestinal fistulas.1 While the causes of CD have not been fully elucidated, dysregulation of the cooperation between the innate and adaptive immune systems, which promote tolerance toward commensal flora, is reportedly involved in the pathogenesis.2 Anti-tumor necrosis factor (TNF) α therapy has resulted in a paradigm shift in the management of CD, improving outcomes and reducing intestinal resection rates.3, 4 On the other hand, loss of responsiveness after anti-TNFα agent treatment has been reported.5 Moreover, a risk of increased susceptibility to infections and a possible propensity for malignancy have limited the long-term use of these drugs.6 Therefore, personalized treatment with anti-TNFα agents by monitoring the drug concentration has been recently recommended for optimizing their dose regimen.7, 8 In addition, to meet the medical needs of patients with refractory CD, several drugs are being developed with a focus on the adhesion molecules involved in leukocyte trafficking9 or on pathways newly-identified as being involved in the pathogenesis of CD.10
Trafficking of leukocytes such as neutrophils, monocytes, and T cells, through activated venular walls into the inflamed tissues of the digestive tract is highly regulated by various adhesion molecules and chemokines on the endothelial cells acting in the individual tethering, rolling, arrest, and transmigration steps.11-13 Fractalkine (FKN) is a transmembrane CX3CL1 chemokine that can be cleaved by specific proteases.14, 15 It is expressed on several cell types including epithelial and endothelial cells and is induced by inflammatory stimuli such as interleukin-1, TNFα and interferon-γ.16 A soluble form of FKN (sFKN) induces leukocyte chemotaxis.14 In particular, membrane-bound FKN (mFKN) on activated endothelial cells can serve as an adhesion molecule by binding to its receptor (CX3CR1) on leukocytes, such as T cells, natural killer cells, and monocytes.17-19 The FKN/CX3CR1 axis may contribute to the pathogenesis of inflammatory bowel disease,20, 21 as well as several inflammatory/autoimmune diseases.22, 23
E6011 is a newly developed humanized monoclonal antibody that binds to FKN with high specificity and affinity. The results of a previous phase 1 study, administering a single ascending dose of E6011 to healthy subjects, indicated that intravenous (IV) infusion of E6011 at doses from 0.0006 to 10 mg/kg was safe and well-tolerated.24 The pharmacokinetic (PK) profile of E6011 was revealed to be nonlinear, with an increase in exposure as the dose was increased. The present phase 1 study was designed as a multicenter, open-label, multiple ascending dose (MAD) evaluation of the safety, tolerability, PK, and immunogenicity of repeated IV infusion of E6011 in patients with CD. Exploratory assessments for pharmacodynamics (PD) and efficacy of E6011 were also performed.
Methods
Patients
We recruited patients from 22 centers in Japan from April 2014 to November 2017. Inclusion criteria were as follows: 20–64 years of age, diagnosed with CD according to the Japanese diagnostic criteria, which are mainly based on the major colonoscopic findings of longitudinal ulcers and cobblestone appearance,25 mild–severe disease activity according to a Crohn's disease activity index (CDAI) of 150–450, and an insufficient response to conventional therapies including anti-TNFα agents. We set the inclusion criteria for disease severity as mild to severe because the main purpose of this phase 1 study was to evaluate the safety of repeated IV doses of E6011 in CD patients. Patients were excluded from this study if they had been diagnosed with ulcerative colitis or were intolerant to anti-TNFα agents. Other exclusion criteria were as follows: ileostomy, short bowel syndrome or symptomatic strictures, history of bowel resection within 24 weeks before enrollment, positive for Clostridium difficile, immunodeficiency, or tuberculosis.
Written informed consent was obtained from all patients before participation. The Institutional Review Boards of all participating centers approved the study protocol. This study was conducted in accordance with the Good Clinical Practice Guideline and the Declaration of Helsinki and registered with Clinical Trials. gov, NCT02039063.
Study design and assessments
This was a phase 1, open-label, multicenter, MAD study involving patients with mild to moderate CD. The patients received IV E6011 4 mg/kg as a loading dose at Week 0 followed by 2 mg/kg every 2 weeks (Cohort 1, n = 6); 10 mg/kg as a loading dose at Week 0 followed by 5 mg/kg every 2 weeks (Cohort 2, n = 8); 10 mg/kg at Weeks 0, 1, and 2, then every 2 weeks (Cohort 3, n = 7) or 15 mg/kg at Weeks 0, 1, and 2; and then every 2 weeks (Cohort 4, n = 7) for up to 12 weeks. If the patients showed a clinical response (CR70) or clinical remission at Week 12, those in Cohorts 1, 2, 3, and 4 had the option of continuing to receive 2, 5, 10, and 15 mg/kg, respectively, every 2 weeks for up to 40 weeks in the Extension phase. (Fig. S1). During the study, patients were allowed concomitant use at stable doses of oral 5-ASA, oral steroids (≤ 40 mg/day of prednisolone or prednisolone equivalent), azathioprine, 6-mercaptopurine, methotrexate, and enteral nutrition (≤ 1200 kcal/day). Total parenteral nutrition, cytapheresis, cyclosporine, tacrolimus, infliximab, and adalimumab were prohibited. The initial maximum dose was 10 mg/kg (Cohort 3) based on the findings of a previous phase 1 study,22 and then, the 15 mg/kg (Cohort 4) dose was added after reviewing the safety data of Cohorts 1 to 3. Safety data for each Cohort were evaluated prior to escalation to the next cohort. All patients were tested for the presence of JC virus antibodies and assessed for symptoms of progressive multifocal leukoencephalopathy (PML) during the study and by interview for 2 years after receiving the last dose of E6011.
The primary outcome was safety assessment in the MAD phase. Adverse events (AEs) were summarized according to the Medical Dictionary for Regulatory Activities, Version 20.1, in the safety population who had received at least one dose of E6011. Serum E6011 and anti-E6011 antibody concentrations were determined by a validated electrochemiluminescence immunoassay.26 Isotypes of the anti-E6011 antibodies were analyzed by a validated surface plasmon resonance assay on a Biacore instrument, using anti-human IgG and anti-human immunoglobulin E monoclonal antibodies. Neutralizing activity of the antibodies was measured by the validated chemotaxis assay using B300-19 cells expressing human CX3CR1. Serum total FKN (sum of free sFKN and E6011-sFKN complex) concentrations were determined as a PD marker using a validated enzyme immunoassay. A post hoc exploratory simulation of free mFKN and sFKN levels after E6011 administration was performed using a two-target, target-mediated drug disposition model27 with a quasi-steady-state approximation28, 29 (Fig. S2). CDAI was evaluated during the MAD and Extension phases. CR70 and CR100 were defined as a decrease in CDAI by ≥ 70 and ≥ 100 points at Week 12 from baseline, respectively. Clinical remission was defined as a CDAI < 150 at Week 12.
Statistical analyses
Data were summarized using descriptive statistics by cohort for the safety, PK, PD, and efficacy assessments at each visit. The continuous variables were summarized as statistical means with standard deviation, and the categorical variables were summarized as frequencies and percentages. Efficacy was analyzed in patients who received at least one dose and had a CDAI of ≥ 220 at baseline. Any patient who discontinued the study before Week 12 was imputed as a nonresponder. The sample size of this study was chosen empirically to assure the primary outcome of safety and the secondary outcome of PK with reference to the phase 1 study.22 Statistical analyses were performed using SAS® Version 9.3 (SAS Institute, Cary, North Carolina, USA).
Results
Patient disposition and baseline characteristics
In the MAD phase, 28 patients in total were consecutively enrolled and received E6011 in Cohort 1 (n = 6), 2 (n = 8), 3 (n = 7), and 4 (n = 7) (Fig. S1). Among these 28, nine (32%) withdrew from the treatment during the MAD phase. The primary reason for discontinuation was lack of improvement. After completing the MAD phase, 12 (43%) entered the Extension phase (Fig. S1). Among the 12 patients, seven (58%) completed the treatment. Baseline characteristics differed minimally among the cohorts (Table 1), although the rate of concomitant enteral nutrition was higher in Cohort 3 and baseline CDAI was higher in Cohort 4 than the other cohorts. The mean CDAI at baseline in all patients was 295.1. Three patients (11%), one each from Cohorts 1, 2, and 3, had mild CDAI (CDAI < 220). Almost all (96%) of the patients had previously received one or two anti-TNFα agents.
| characteristics | Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Total |
|---|---|---|---|---|---|
| n = 6 | n = 8 | n = 7 | n = 7 | n = 28 | |
| Age, years, mean ± SD | 31 ± 7 | 38 ± 12 | 40 ± 15 | 37 ± 8 | 37 ± 11 |
| Sex, male, n (%) | 5 (83) | 7 (88) | 5 (71) | 4 (57) | 21 (75) |
| BMI, kg/m2, mean ± SD | 19.7 ± 5.1 | 20.2 ± 3.4 | 21.1 ± 1.6 | 19.6 ± 2.3 | 20.2 ± 3.2 |
| Weight, kg, mean ± SD | 55.5 ± 18.2 | 57.4 ± 10.2 | 58.6 ± 9.0 | 52.9 ± 9.9 | 56.2 ± 11.5 |
| History of prior surgery, n (%) | 4 (67) | 5 (63) | 6 (86) | 4 (57) | 19 (68) |
| Baseline CDAI, mean ± SD | 290.8 ± 70.3 | 293.4 ± 76.7 | 259.7 ± 60.2 | 336.3 ± 75.8 | 295.1 ± 72.7 |
| Baseline CDAI ≥ 220, n (%) | 5 (83) | 7 (88) | 6 (86) | 7 (100) | 25 (89) |
| CRP, mg/dL, mean ± SD | 1.5 ± 1.1 | 1.9 ± 1.1 | 2.5 ± 2.9 | 2.6 ± 3.8 | 2.1 ± 2.4 |
| Location of disease, n (%) | |||||
| Anus/perianal | 3 (50) | 5 (63) | 3 (43) | 5 (71) | 16 (57) |
| Rectum | 2 (33) | 8 (100) | 2 (29) | 6 (86) | 18 (64) |
| Colon | 3 (50) | 6 (75) | 4 (57) | 6 (86) | 19 (68) |
| Ileum | 6 (100) | 8 (100) | 7 (100) | 5 (71) | 26 (93) |
| Jejunum | 0 | 3 (38) | 1 (14) | 1 (14) | 5 (18) |
| Upper gastrointestinal | 2 (33) | 2 (25) | 0 | 0 | 4 (14) |
| Concomitant medications, n (%) | |||||
| Aminosalicylates | 5 (83) | 8 (100) | 7 (100) | 7 (100) | 27 (96) |
| Corticosteroids | 0 | 0 | 0 | 0 | 0 |
| Thiopurines | 5 (83) | 4 (50) | 5 (71) | 4 (57) | 18 (64) |
| Antibiotics | 1 (17) | 1 (13) | 2 (29) | 2 (29) | 6 (21) |
| Enteral nutrition | 4 (67) | 3 (38) | 7 (100) | 2 (29) | 16 (57) |
| Prior anti-TNFα therapy, n (%) | |||||
| None | 0 | 1 (13) | 0 | 0 | 1 (4) |
| One drug | 2 (33) | 5 (63) | 3 (43) | 2 (29) | 12 (43) |
| Two drugs | 4 (67) | 2 (25) | 4 (57) | 5 (71) | 15 (54) |
- Data were presented as n (%) or mean ± SD. Cohort 1, 4 mg/kg loading dose at Week 0, then 2 mg/kg every 2 weeks; Cohort 2, 10 mg/kg loading dose, then 5 mg/kg every 2 weeks; Cohort 3, 10 mg/kg at weeks 0, 1, and 2, then every 2 weeks; Cohort 4, 15 mg/kg at weeks 0, 1, and 2, then every 2 weeks.
- BMI, body mass index; CDAI, Crohn's disease activity index; CRP, C-reactive protein; SD, standard deviation; TNFα, tumor necrosis factor α.
Safety
During the MAD phase, 18 patients (64%) reported an AE (Table 2). The most common AE was nasopharyngitis (18%), followed by nausea (7%), headache (7%), progression of CD (7%), and anal abscess (7%). All AEs were mild or moderate. Three patients (11%) experienced serious AEs: progression of CD in one patient each in Cohorts 2 and 3, and anemia in one in Cohort 3. All serious AEs were moderate and were not considered to be related to the study drug. AEs leading to discontinuation of the drugs occurred in two patients (7%): anemia and progression of CD in one patient each in Cohort 3. During the Extension phase, a serious AE, that is, a miscarriage in the partner of one male patient, was reported in Cohort 1, but no other clinically important AEs were noted. (Table S1).
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Total | |
|---|---|---|---|---|---|
| n = 6 | n = 8 | n = 7 | n = 7 | n = 28 | |
| Any AE | 5 (83) | 5 (63) | 6 (86) | 2 (29) | 18 (64) |
| Treatment-related AE | 3 (50) | 1 (13) | 1 (14) | 0 | 5 (18) |
| Severe AEs | 0 | 0 | 0 | 0 | 0 |
| Serious AEs | 0 | 1 (13) | 2 (29) | 0 | 3 (11) |
| AEs leading to study drug discontinuation | 0 | 0 | 2 (29) | 0 | 2 (7) |
| AEs occurring in ≥ 2 of patients | |||||
| Nasopharyngitis | 1 (17) | 3 (38) | 1 (14) | 0 | 5 (18) |
| Nausea | 1 (17) | 1 (13) | 1 (14) | 0 | 2 (7) |
| Headache | 2 (33) | 0 | 0 | 0 | 2 (7) |
| Progression of Crohn's disease | 0 | 1 (13) | 1 (14) | 0 | 2 (7) |
| Anal abscess | 1 (17) | 1 (13) | 0 | 0 | 2 (7) |
- Data were presented as n (%) of AE: adverse events. The doses of Cohorts 1–4 are described in the notes of Table 1. AEs were events with onset dates on or after the start of treatment and up to 70 days after the last dose date, or reemerging AEs, or worsening in severity. Counts and percentages are of patients, not events. A subject with two or more AEs was counted once.
Pharmacokinetics and pharmacodynamics
Serum E6011 concentrations rose dose-dependently after infusion and reached a plateau around 4–6 weeks (Fig. 1a). Five (18%) of 28 patients developed anti-E6011 antibodies during the 52 weeks of the study: Two patients from the lowest dose cohort (2-mg/kg infusion) were in the MAD stage, and one patient from the same cohort was in the extension stage. One patient from the 5-mg/kg cohort and one from the 10-mg/kg cohort were in the MAD stage. Among them, two in Cohort 1 showed a marked decrease in serum E6011 levels. However, serum anti-E6011 antibody levels in the patients remained very low, and no neutralizing activities were observed. Other patients who were positive for anti-E6011 antibodies in Cohorts 1, 2, and 3 showed no apparent changes in serum E6011 concentration. Serum total FKN levels increased simultaneously after administration of E6011. The level was sustained throughout treatment and seemed to reach a similar plateau in Cohorts 3 and 4 (Fig. 1b). A preliminary simulation using a PK/PD model revealed that E6011 might affect the binding occupancy of mFKN and the free sFKN level in patients (Fig. S3).
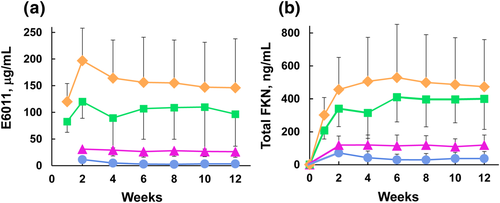
 , Cohort 1 (n = 3–5);
, Cohort 1 (n = 3–5);  , Cohort 2 (n = 6–7);
, Cohort 2 (n = 6–7);  , Cohort 3 (n = 5–7);
, Cohort 3 (n = 5–7);  , Cohort 4 (n = 4–6). FKN, fractalkine. [Color figure can be viewed at wileyonlinelibrary.com].
, Cohort 4 (n = 4–6). FKN, fractalkine. [Color figure can be viewed at wileyonlinelibrary.com].Efficacy
During the MAD phase, the CDAI score decreased in one, two, four, and three patients in Cohorts 1, 2, 3, and 4, respectively, and clinical remission was observed in three and one patient in Cohorts 3 and 4, respectively (Table 3). Overall, CR70, CR100, and clinical remission at Week 12 were observed in 10 (40%), 9 (36%), and 4 (16%) of 25 patients, respectively (Table 3). In the Extension phase, 1 of 1 in the 2-mg/kg group showed CR100, 1 of 3 in the 5-mg/kg group showed clinical remission, 2 of 5 in the 10-mg/kg group showed clinical remission, and 1 of 3 showed CR70 at Week 52 (Fig. S4).
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Total | |
|---|---|---|---|---|---|
| n = 5 | n = 7 | n = 6 | n = 7 | n = 25 | |
| CR70 | 1/5 (20) | 2/7 (29) | 4/6 (67) | 3/7 (43) | 10/25 (40) |
| CR100 | 1/5 (20) | 1/7 (14) | 4/6 (67) | 3/7 (43) | 9/25 (36) |
| Clinical remission | 0/5 (0) | 0/7 (0) | 3/6 (50) | 1/7 (14) | 4/25 (16) |
- Data were presented as n (%), CR70, CDAI decrease ≥ 70 from baseline; CR100, CDAI decrease ≥ 100 from baseline; and clinical remission, CDAI < 150. CR70, CR100, and clinical remission were evaluated at Week 12 for the patients who had a CDAI ≥ 220 at baseline. The doses of Cohorts 1–4 are presented in the notes of Table 1. Patients who discontinued the study drug prior to Week 12 were imputed as nonresponders (NRI).
Discussion
This phase 1 trial first demonstrated that repeated IV doses of E6011 were well-tolerated in patients with mild to moderate CD. We evaluated safety following the administration of 2, 5, 10, or 15 mg/kg IV doses of E6011 every 2 weeks for 12 weeks. Neither dose-dependent nor dose-limiting AEs occurred during the study. Overall, AEs were reported in 18 (64%) of 28 patients, and the most frequent AE was nasopharyngitis (18%) in the MAD phase. Serious AEs (CD and anemia) were observed in three patients (11%). These seemed to be related to the underlying disease; no exacerbation but progression of CD was observed in these patients. Acute infusion reaction-like signs were observed in some patients in Cohorts 1 and 3, but not in Cohort 4 receiving the highest dose. There were no clinically relevant changes in laboratory test results. Despite the very small number of patients who received E6011 in the Extension phase, E6011 seemed to be well-tolerated as long-term treatment. During this period, a serious AE of miscarriage was reported in the partner of a patient who had received E6011 at a dose of 2 mg/kg and was also taking azathioprine. Because the patient and the partner had a history of spontaneous abortion, other causes for this event were more likely. As an important safety concern, natalizumab, a monoclonal antibody against α4-integrin, is reportedly associated with a risk of PML, which is a rare but serious brain infection.30 Although E6011 has a distinctly different mode of action from that of natalizumab, E6011 may have an effect on the FKN–integrin interaction, which is speculated to play a role in strengthening leukocyte–integrin interactions.31 Therefore, the potential risk of PML cannot be ruled out in patients treated with E6011. Further evaluation focusing on this risk is needed in a larger number of patients with long-term follow-up.
Five (18%) of the 28 patients exhibited the presence of anti-E6011 antibodies during the course of this study. All patients received concomitant azathioprine. Further investigation is needed to assess the incidence of anti-E6011 antibodies in patients taking immunomodulators and in those not taking the drug. In addition, acute infusion reaction-like signs and symptoms, such as hot flush, headache, and fever were observed in three patients from Cohort 1 and one patient from Cohort 3. Among these four patients, only one patient (from Cohort 1) was positive for the anti-E6011 antibody. Accordingly, whether the presence of the anti-E6011 antibody contributed to the infusion reactions in these patients was not clear.
Many antibodies are known to be eliminated by two different processes: a nonlinear pathway known as target-mediated drug disposition model, particularly evident at low drug concentrations, and linear elimination, where the target is saturated with the drug.27 In the previous phase 1 trial of E6011 in healthy subjects, E6011 was shown to have a nonlinear PK profile after single-dose administration.24 The present results supported the previous PK data in CD patients after repeated administrations. Moreover, a simultaneous increase in the serum total FKN level was also confirmed in the patients treated with E6011, as observed in the healthy subjects in the single ascending dose study.24 These results reflect an increase in formation of the E6011 and sFKN complex in the peripheral circulation. Furthermore, a preliminary simulation suggested that E6011 may interact systemically with both mFKN and free sFKN after infusion.
Our preliminary results revealed that clinical response and remission were achieved in several patients with active CD after administration of E6011. Note that nearly all (96%) of the patients had a history of anti-TNFα therapy in the study, suggesting these patients to be refractory to anti-TNFα drugs. On the other hand, only three patients with mild CD (CDAI < 220) were included in this study, and there were no observed clear changes in CDAI in the small subpopulation; an increase in Cohort 1 (from 176 at baseline to 213 at discontinuation after 2 weeks), decrease in Cohort 2 (from 180 at baseline to 146 at Week 12), and almost no change in Cohort 3 (from 153 at baseline to 146 at Week 12). In an animal model, dextran sulfate sodium (DSS)-induced colitis and inflammatory cell infiltration were reportedly attenuated in CX3CR1-deficient mice as compared with wild-type mice.32, 33 Conversely, Medina-Contreras et al. reported that DSS-induced colitis was exacerbated in CX3CR1-knockout mice as compared with controls.34 Recently, Kuboi et al. demonstrated that mouse anti-FKN monoclonal antibody ameliorated colonic damage scores, along with a trend toward reduced leukocyte infiltration in the colon in oxazolone-induced and T-cell-transferred colitis.35 Despite the conflicting findings regarding the role of the FKN-CX3CR1 axis, no prior clinical trials of anti-FKN monoclonal antibody in patients with CD have been reported. The present study is the first, to our knowledge, to suggest that E6011 may improve clinical symptoms in CD patients. In addition, the study conducted on the DSS-induced colitis model has shown that anti-FKN monoclonal antibody reduced CX3CR1+ macrophages in the submucosa.35 In patients with active CD, sFKN and mFKN levels36 and FKN mRNA expression37 by endothelial cells in inflamed mucosa were elevated as compared with those in the normal mucosa. Both sFKN and mFKN were upregulated by mucosal microvascular endothelial cells upon stimulation with Th1 cytokines, such as TNFα and interferon-γ.36 Moreover, the numbers of CX3CR1+ T cells in the peripheral circulation were higher than those in normal subjects,36, 37 and the positive rate of peripheral CX3CR1+ CD4 cells correlated with disease activity.38 The percentage of CX3CR1+ cells in inflamed mucosa was also higher than that in normal mucosa. In addition, CX3CR1+ CD14+ CD16+ monocytes were increased in the peripheral circulation as well as inflamed tissues.38 Given that these leukocytes may contribute to the pathogenesis of CD, further investigation is warranted to examine the effects of E6011 on these changes in CD patients.
This study has limitations. First, this was an open-label study and lacked a control group. Therefore, placebo effects cannot be excluded based on the present data. Second, the sample size was very small, especially in the dose groups of the Extension phase. Third, the efficacy of E6011 was evaluated on the basis of clinical improvement without taking mucosal healing using colonoscopy into account.
In conclusion, E6011 was found to be safe and well-tolerated, and had an acceptable PK profile and immunogenicity in CD patients following repeated IV administrations. Furthermore, preliminary assessments for efficacy and PD simulation support the development of E6011 as a potential treatment for CD, although further large-scale randomized controlled trials are needed to confirm our preliminary findings.
Acknowledgments
The authors would like to thank the following principal investigators for their contributions to the study: Satoshi Tanida, Nagoya City University, Aichi, Japan; Kazuya Kitamura, Kanazawa University Hospital, Ishikawa, Japan; Toshiyuki Matsui, Fukuoka University Chikushi Hospital, Fukuoka, Japan; Makoto Arai, Chiba University, Chiba, Japan; Mikihiro Fujiya, Asahikawa Medical University, Hokkaido, Japan; Noriyuki Horiki, Mie University Hospital, Mie, Japan; Hiroko Nebiki, Osaka City General Hospital, Osaka, Japan; Fukunori Kinjo, Urasoe General Hospital, Okinawa, Japan; Takako Miyazaki, Hyogo College of Medicine, Hyogo, Japan; Takayuki Matsumoto, Iwate Medical University, Iwate, Japan; Motohiro Esaki, Kyushu University, Fukuoka, Japan; Keiichi Mitsuyama, Kurume University Hospital, Fukuoka, Japan; Masayuki Saruta, The Jikei University School of Medicine, Tokyo, Japan; Akio Ido, Kagoshima University, Kagoshima, Japan; Kiyonori Kobayashi, Kitasato University East Hospital, Kanagawa, Japan; Hiroshi Nakase, Kyoto University Hospital, Kyoto, Japan; Bunei Iizuka, Tokyo Women's Medical University, Tokyo, Japan; Ken Kawakami, Osaka Medical College, Osaka, Japan; Tetsuji Takayama, Tokushima University, Tokushima, Japan; Shinji Tanaka, Hiroshima University Hospital, Hiroshima, Japan; Shingo Kato, Saitama Medical University, Saitama, Japan; The authors also thank Mikiko Kawakatsu of Eisai Co., Ltd., for writing support which was funded by EA Pharma Co., Ltd.




