Marshall and Warren Lecture 2019: A paradigm shift in pathophysiological basis of irritable bowel syndrome and its implication on treatment
Abstract
Irritable bowel syndrome (IBS), a common functional gastrointestinal disorder (FGID), has often been considered rather inappropriately as psychogenic in the past. Though psychological issues are important comorbidities in a proportion of IBS patients, the evidences are far from enough to label this condition as psychogenic only. In the recent past, evidences are emerging that underscores the concept supporting pure psychogenic theory of IBS and suggest this disorder to be rather microorganic. Accordingly, a move of Rome IV Committee attempting to delete the term “functional” and designating these to be disorders of “gut-brain interaction” rather than that of “brain-gut interaction,” it emphasizes the importance of the gut over the brain in the pathogenesis. The introduction of the concept of multidimensional clinical profile in Rome IV requires attention to diagnostic category of FGID, overlap, severity, psychological issues, and physiological dysfunction or biomarkers; this attempts to recognize clinical variability and multidimensionality of pathophysiology and management of these disorders. The recognition of the biological factors in the pathogenesis of IBS is a significant paradigm shift in the recent time. This is somewhat similar to the progress in the pathogenesis of peptic ulcer disease from psychological factor to acid to Helicobacter pylori infection. It is expected that in the near future, therapeutic modalities targeting the different pathogenic mechanisms of different subtypes of IBS may bring revolution in management of the disorder.
Introduction
Irritable bowel syndrome (IBS), a common enigmatic functional gastrointestinal disorder (FGID), is characterized by abdominal pain, discomfort, bloating, altered stool forms, and frequency.1 IBS is diagnosed by symptom-based criteria such as Manning's criteria and different iteration of the Rome criteria.2, 3 The current iteration of Rome criteria (version IV) released in 2016 underscored the abdominal discomfort and bloating over pain, making the symptom-based diagnostic criteria for this condition somewhat insensitive.3, 4 However, the treatment of the condition largely depends on the subtyping of the condition such as constipation–or diarrhea–predominant syndrome and the underlying pathophysiology that has undergone substantial paradigm shift during the recent years.2, 3, 5, 6 In fact, earlier, it was thought that IBS is largely a functional condition. However, most started inappropriately interpret the term “functional” as idiopathic or cryptogenic; unfortunately, the patients suffering from this illness were labeled as neurotic, apprehensive, otherwise healthy individuals with an imaginary illness.3 The psychogenic origin of the condition is largely based on the uncontrolled or case–control studies that showed that the patients with IBS, particularly those with severe symptoms often have more psychological and psychiatric comorbidities and on the fact that these patients also respond to treatment with psychotropic agents though some studies did show IBS including postinfection IBS (PI-IBS) to develop more often among patients with anxiety and depression.7-9 However, IBS is a multifactorial condition, and the existing evidences are not enough to incriminate psychological comorbidity to be uniformly the cause for all patients with IBS. In fact, association does not prove causation, and patients with any chronic illness are expected to develop psychological comorbidities as compared with the healthy controls. Moreover, patients with IBS respond to much lower doses of the psychotropic drugs, and these drugs not only work in the big brain but also on the little brain present in the gut (the enteric nervous system).9 Hence, these evidences are far from enough to label IBS as psychogenic only. In fact, currently, the psychotropic agents are also classified as visceral neuromodulators when used in patients with FGIDs.9 A recent study showed that patients with IBS without psychological morbidities develop it during long-term follow-up in a frequency similar to development of FGIDs during long-term follow-up of patients with psychological conditions.10 It is important to note that in the Rome IV system, the term functional has been deleted quite appropriately, and these disorders have been labeled as “disorders of gut-brain interaction” rather than of “brain-gut interaction.”3, 11, 12 The Asian experts have designated the FGIDs as “microorganic disorders.” 11 This is somewhat analogous to understanding of the pathogenesis of peptic ulcer disease, which was initially thought to be a psychosomatic condition, then thought to be related to the diet and gastric acid before the discovery of the Helicobacter pylori by Warren and Marshall in 1983 that bags them the Nobel Prize in Physiology and Medicine in 2005.13, 14 Is the understanding of the pathogenesis of FGIDs in general and IBS in particular evolving through a similar path of psychogenic origin, to fermentable oligo-, di-, mono-saccharides and polyols (FODMAP)-related, to gut microbiota dysbiosis and immune interaction-related (Fig. 1)? In most diseases, treatment has evolved to be better once the pathogenesis has been explored. Would it happen similarly in IBS? This review aims to deliberate on some of these issues.
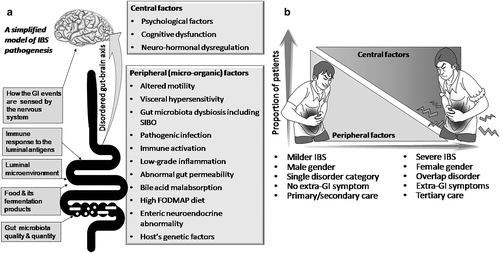
Factors implicated in the pathogenesis of irritable bowel syndrome
Irritable bowel syndrome is multifactorial in pathogenesis.1-3, 15, 16 These factors may be broadly classified into (i) peripheral factors17 (viz., altered gastrointestinal [GI] motility, GI inflammation and permeability alteration, altered luminal microenvironment including gut microbiota dysbiosis and small intestinal bacterial overgrowth [SIBO], host–microbe interaction, bile acid recirculation defect, pathogenic infection, dietary factors, and neurohumoral dysregulation including altered serotonergic transmission and visceral hypersensitivity) and (ii) central factors (psychological stress, cognitive dysfunction, abnormal emotional arousal system response, and sleep dysfunction). Genetic factors may underlie peripheral and central pathophysiological mechanisms.18 As listed above, a greater number of factors involved in the pathogenesis of IBS are peripheral rather than central in nature.
Altered gastrointestinal motility in irritable bowel syndrome
Whereas patients with constipation-predominant IBS (IBS-C) have slow gut, particularly colonic, transit, diarrhea-predominant IBS (IBS-D) patients have faster motility.1, 5, 19 Multiple studies showed that Bristol stool form is determined by colonic motility; slower colon transit is associated with harder stools.20, 21 Subsets of patients with IBS-C as well as functional constipation have slow gut motility with or without associated fecal evacuation disorder.5 Moreover, drugs that increase gut motility are known to improve chronic constipation in general and slow-transit constipation in particular,22 and the drugs that reduce gut motility often improve chronic diarrhea in these patients.23
Gut microbiota dysbiosis in irritable bowel syndrome
Recently, role of gut microbiota, which is the largest organ of the human body, has been investigated extensively for maintenance of human health and its alteration in several digestive and extra-digestive disorders.24 Gut microbiota dysbiosis includes increase in upper small bowel bacteria on quantitative culture (≥105 colony forming unit/mL of aspirate called SIBO; ≥103 but ≤105 colony forming unit/mL called low-grade SIBO) and qualitative change in microbiota (relative proportion of healthy and pathogenic microbes) by next-generation sequencing.25 Hydrogen breath tests (HBTs) such as glucose and lactulose HBTs are the other popular noninvasive, though not quite sensitive and specific tests for diagnosis of SIBO.25
Most studies found an association between SIBO diagnosed by quantitative upper gut aspirate culture, glucose and lactulose HBTs and IBS.26 In a recent meta-analysis, we found that the relative risk of SIBO among patients with IBS versus control using glucose HBT was 4.234 (95% confidence interval 3.015–5.947), and it was greater among patients with IBS-D than other subtypes of IBS.26
The above-mentioned studies do suggest an association between SIBO and IBS, but a mere association does not prove causation. A causative role of SIBO in IBS has been established by Koch's postulates.27 Moreover, three recent studies, one from India,28 one from China,29 and the most recent one from USA,30 showed that response of IBS patients with SIBO to treatment with antibiotics is far greater than when antibiotics are used without testing for SIBO. These studies suggest that in future, treatment of IBS patients may see a paradigm shift with a more personalized or individualized approach somewhat similar to several other fields of medicine.
Using real-time polymerase chain reaction and next-generation sequencing, several studies, mostly using fecal sample, showed gut microbiota dysbiosis among patients with IBS compared with healthy controls.24, 31 In a recent meta-analysis of 23 case–control studies on 1340 participants, patients with IBS had lower levels of good bacteria of genera Lactobacillus and Bifidobacterium and marginally higher levels of bad bugs of genera Enterobacter compared with the controls.32
The other sets of evidences on relationship between gut microbiota dysbiosis and IBS come from studies on patients with functional constipation, IBS-C, and functional dyspepsia (FD), which are often associated with IBS. Methanogenic microbes, particularly Methanobravibacter smithii, produce methane in the gut, which slows gut transit causing constipation.33 An association between high breath methane on lactulose HBT and constipation has been confirmed in meta-analysis of these studies.34 Moreover, three interventional studies, two from USA,35, 36 and one from India,37 showed that reduction in breath methane by treatment with either rifaximin alone or rifaximin and neomycin resulted in improvement in constipation and acceleration of colon transit. A recent randomized controlled trial from Hong Kong showed that gut microbiota manipulation with rifaximin resulted in improvement in symptoms in a subset of patients with FD, which is quite commonly associated with IBS.38, 39 The other methods of gut microbiota manipulation by probiotics showed mixed results, but these studies were quite heterogeneous.40
Immune activation and alteration in mucosal permeability in patients with irritable bowel syndrome
As early as 1962, Hiatt and colleague reported increased number of mast cells in the muscle layer of the gut in patients with “spastic colitis,” which was a name for the present day IBS.41 During the last decades, several studies showed that colonic biopsies in patients with IBS, particularly those with IBS-D, often show mononuclear inflammatory infiltrates including mast cells in the duodenum, jejunum, ileum, cecum, rectum, and the rest of the colon suggesting a low-grade inflammatory basis of a subset of these patients.42, 43 Mast cell is an important cell of innate immune response that on degranulation releases histamine, tryptase, and chymase. T lymphocytes are important in adaptive immune response to luminal antigen including microbes, and quite a few studies reported increased mucosal T cells in duodenum, jejunum, ileum, cecum, rectum, and recto-sigmoid region in patients with IBS as compared with controls though a few studies did refute this observation.43
A few studies showed increase in the pro-inflammatory cytokines such as interleukin (IL)-6 and IL-8 and decrease in the anti-inflammatory cytokines such as IL-10 in serum of IBS patients.43 An increase in fecal and jejunal fluid protease, fecal human beta-defensin-2,44 upper gut mucosal IL-1 alpha and beta, particularly in presence of SIBO, and rectal mucosal nitric oxide and serotonin, also suggest immune activation, particularly in patients with IBS-D.43 In an earlier study on 221 IBS patients and 273 healthy subjects, we found that IL-1 receptor antagonist (an anti-inflammatory protein) over-producer polymorphisms were uncommon and under-producers more common among IBS patients. Of 15/82 patients with IBS having SIBO on quantitative upper gut aspirate culture, mucosal IL-1 α and β (pro-inflammatory cytokine) levels were higher than those without SIBO, and higher IL-1 β levels were associated with loose stool and abdominal bloating.45 In a recent study on 47 IBS patients, colonic biopsy messenger ribonucleic acid (mRNA) levels of toll-like receptor (TLR)-4 and TLR-5, their protein expression, Il-6, C-X-C motif chemokine ligand-11 (CXCL-11), and C-X-C motif chemokine receptor (CXCR-3), which are pattern recognition receptors or pro-inflammatory cytokine or chemokine, were up-regulated in patients than controls46; in contrast, anti-inflammatory cytokine mRNA of IL-10 was down-regulated among IBS patients. Interestingly, number of gene copies of the good bugs such as Lactobacillus and Bifidobacteria showed correlation with anti-inflammatory cytokine IL-10.46 Weekly stool frequency had positive correlation with mRNA levels of TLR-4 and CXCR-3 but inversely correlated with IL-10.46 Most of the evidences mentioned above suggest that patients with IBS, particularly those with IBS-D, have immune activation in the gut suggesting the condition to be a low-grade inflammatory state.
Abnormal intestinal permeability in IBS, particularly those with IBS-D and PI-IBS has been shown in some studies.43 Most of these studies used recovery of orally administered sugars or radioisotopes in the urine. Though some studies could not find altered gut permeability in IBS, most studies did find abnormal small intestinal and colonic permeability, particularly among patients with IBS-D and PI-IBS.43
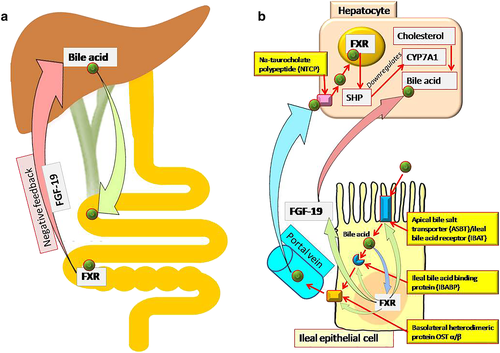
Bile acid recirculation defect in irritable bowel syndrome
Bile acid malabsorption is known to be associated with chronic diarrhea and IBS-D. Decrease in feedback inhibition of bile acid synthesis in liver because of down-regulation of the ileal fibroblast growth factor 19 (Fig. 2) is associated with over-production of bile acid and its delivery to the colon.47, 48 Patients with IBS-D are more likely to have bile acid malabsorption than those with IBS-C.47, 48 In a meta-analysis of 18 studies including 1223 patients,49 429 patients studied in five reports had severe bile acid malabsorption (7 day SeHCAT retention < 5% of baseline), 1073 studied in 17 reports had moderate malabsorption (SeHCAT < 10%), and 618 patients studied in seven reports had mild (SeHCAT < 15%) malabsorption. Response to treatment with bile acid binding agent (cholestyramine) showed a dose–response relationship as per degree of bile acid malabsorption (96% in patients with < 5% retention, 80% with < 10% retention, and 70% with < 15% retention).49
Postinfection irritable bowel syndrome
Following acute infectious gastroenteritis, 7% to 9% subjects continue to experience GI dysfunction fulfilling the Rome criteria for IBS or FD or both though they did not have these disorders earlier.50, 51 These conditions are called PI-IBS and postinfection FD. In a meta-analysis of 45 studies including 21 421 subjects with acute gastroenteritis, a pooled prevalence at 12 months following the episode was 10.1% (95% confidence interval, 7.2–14.1).17 Mechanism of development of PI-IBS includes alteration in gut microbiota, immune activation, low-grade inflammation, alteration in mucosal permeability, neuro-hormonal dysfunction, and alteration in GI motility.15, 52 Further evidence of organic basis of PI-IBS comes from the suggestion that postinfection bowel dysfunction is a spectrum disorder in which the milder end of the spectrum is PI-IBS, and more severe end is postinfection malabsorption syndrome, popularly known as tropical sprue.52 In fact, in a recent study, 9% of patients fulfilling Rome criteria for PI-IBS have been found to have tropical sprue on further investigation.53 The link between acute gastroenteritis, PI-IBS, and tropical sprue is one of the best evidences for organic basis of a subset of patients with IBS.52
Visceral hypersensitivity in irritable bowel syndrome
Visceral hypersensitivity is a major determinant of occurrence and severity of symptoms, phenotype of FGID, and consultation behavior.54 Visceral hypersensitivity is defined as an enhanced perception of mechanical triggers applied to the bowel, which seems as pain and discomfort. Allodynia refers to the elevated nociceptive sensation in response to normal stimuli. Frequency of visceral hypersensitivity among patients with IBS has been reported to vary widely between 33% and 90%.54 Visceral hypersensitivity may be at the local level in the gut wall, or at the level of posterior column of the spinal cord, subcortical thalamic level, at the cerebral cortex, and because of reduced descending inhibition of pain sensation or because of inappropriate exaggerated activation of emotional arousal system by visceral sensory stimuli.54
Visceral hypersensitivity may determine occurrence of symptoms and of FGID phenotype. For example, it has been found that patients with IBS-C often have visceral hypersensitivity as compared with the patients with functional constipation suggesting that the pain symptom might result from visceral hypersensitivity. In an earlier study from our group, of 124 patients with IBS and 53 healthy controls, frequency (by HBT, 82% and 77%, respectively) and degree (by peak breath hydrogen levels) of lactose malabsorption were comparably high; however, patients with IBS reported symptoms more often following lactose ingestion than the healthy subjects (55% vs 32%, respectively) that might be related to the presence of visceral hypersensitivity among the patients.55 This has been further confirmed in a similar Chinese study using a visceral sensitivity assessment machine (barostat) that showed degree of visceral hypersensitivity determined symptom development following lactose ingestion among IBS patients with this disaccharide malabsorption.56 The mediators of visceral sensation include serotonin, cannabinoid, tachykinin, histamine 1, tyrosine kinase, protease activated receptors, voltage-gated sodium and calcium channels, acid-sensing ion channel, and transient receptor potential vanilloid-1 receptor.54 Some of these have been found to be the therapeutic targets for treatment of patients with IBS.54
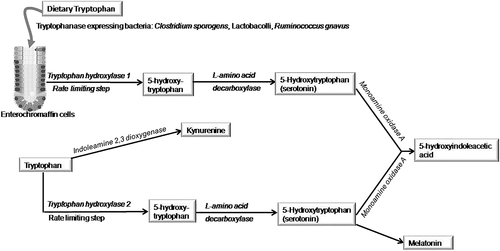
Serotonin is an important chemical that promotes gut motility, secretion, and sensation. 95% of body serotonin is in the gut, and only 5% is in the brain.57 Enteroendocrine cells present in the intestinal crypts contain most of the body's serotonin; it is also found in enteric neurons and platelets. Serotonin released from enteroendocrine cells acts as a mucosal signaling molecule and that released from enteric nerves in the myenteric and submucosal plexus as a neurotransmitter. Serotonin is synthesized from dietary tryptophan in the gut by tryptophan hydroxylase 1 and in the brain by tryptophan hydroxylase 2 (Fig. 3). Monoamino oxidase A converts serotonin both in the brain as well as gut to 5-hydrosyindoleacetic acid. Serotonin synthesized in the gut cannot enter into brain because of blood brain barrier. Serotonin in the brain also gets converted into melatonin, which is the normal sleep-inducing chemical. Because indoleamine 2,3-dioxygenase can shunt tryptophan in the brain to Kynurenine, it may lead to less serotonin production and its conversion to melatonin leading to sleep disorder.57-59 Some microbes such as Clostridium sporogens, Lactobacolli, and Ruminococcus gnavus express tryptophanase and hence, help in serotonin biosynthesis.60 As excess serotonergic function is associated with diarrhea and its reduction is associated with constipation, modulation of serotonin by its antagonists and agonists are useful in treatment of IBS-D and IBS-C (Table 1).3
| • Categorical Rome diagnosis (symptom-based criteria) |
| • Additional information that subclassifies the diagnosis leading to more specific treatment (e.g. diarrhea-predominant IBS, constipation-predominant IBS, pain-predominant IBS, postinfection IBS, overlap disorders) |
| • The personal impact of the disorder on the patient (severity) |
| • Physiological abnormalities or biomarkers |
| • Psychological influences of the disorder |
- IBS, irritable bowel syndrome.
Dietary factors in irritable bowel syndrome
Dietary intolerance contributing to the symptoms of IBS has been recognized for long time. In the past, several groups tried elimination diets of different kind to treat patients with IBS. Malabsorption of individual components of the diet, such as lactose and fructose, has been shown in several61 studies, and elimination of these components yielded variable results in treating these patients.62 Inconsistent response to elimination of individual components of foods might result from the fact that malabsorption of multiple elements might contribute to symptoms in a patient, and other mechanisms such as exaggerated immune response, visceral hypersensitivity, and noncarbohydrate components of the diet may be involved in the pathogenesis.57 Recently, Gibson and colleagues from Australia showed that elimination of all the high FODMAP foods consisting of a group of poorly absorbed short-chain carbohydrates including fructose and lactose, fructans, galacto-oligosaccharides, and polyols or sugar alcohols might be helpful to treat IBS.63 Till date, at least nine randomized controlled trials showed efficacy of low FODMAP diet (eliminating high FODMAP foods) in treatment of patients with IBS.64 The high FODMAP food components consisting of the above-mentioned carbohydrates generate IBS symptoms by two broad mechanisms: (i) Osmotic activity and poor absorption of fluid lead to its accumulation leading to distension; (ii) FODMAPs are rapidly fermented by the colonic microbiota, leading to colonic distention from gas production. Therefore, abdominal distension, bloating, flatulence, pain, and loose motion may develop.63
Multidimensional clinical profile of irritable bowel syndrome
Multidimensional clinical profile (MDCP), proposed in the Rome IV algorithm, is an important advancement in the diagnosis and management of FGIDs including IBS (Table 1).65 This is step toward individualization of patients based on their diagnostic category, overlap, severity, psychological issues, and physiological dysfunction or biomarkers so that each factor contributing to the symptoms can be treated, and patients with disorders of different severity are not managed in the same way. Each of these has important clinical implication. For example, overlap disorder, though common, has been ignored currently in spite of the fact that such overlaps influence the severity, treatment strategy, and outcome of illness.66, 67 It is expected that the concept of MDCP would lead to greater recognition of the pathophysiological abnormalities including the peripheral issues contributing to the symptoms of these patients in future.
Pathophysiology-directed treatment for irritable bowel syndrome
Each of the above-mentioned pathophysiological mechanisms, both peripheral and central, can be targeted by pharmacological and non-pharmacological agents to treat different subtypes of IBS and abdominal pain in this disorder (Tables 2,3).3, 68, 69 In fact, as can be seen from Tables 2 and 3, most of these agents used in the treatment of IBS target peripheral rather than central mechanisms of the disease. The psychopharmacological agents used in the treatment of IBS such as tricyclic antidepressants, serotonin reuptake inhibitors, and serotonin nor-epinephrine reuptake inhibitors, believed to target the central mechanisms, also work peripherally, and hence, these have been renamed as visceral neuromodulators when used in the treatment of FGIDs.9 In fact, these agents have prominent anticholinergic and serotonin modulatory activities at the level of enteric nervous system, work at a dose much lower than that used in the psychiatric disorders, and are known to work even in absence of psychological comorbidity in patients with FGIDs.9 Whereas some of drugs shown in the Tables 2 and 3 are established therapeutic agents for treatment of IBS, others are under development. The putative therapeutic target of most drugs useful in the treatment of IBS also underscores the conventionally believed hypothesis that FGIDs including IBS are largely psychological in origin.
| IBS subtypes | Outline of pathogenic mechanisms | Pharmacological agents targeting it | Peripheral vs central |
|---|---|---|---|
| IBS-C | Slow motility |
Serotonin agonists i. Cisapride (withdrawn) ii. Tegaserod (withdrawn) iii. Prucalopride iv. Plicanatide Osmotic agents (promotility by gut distension) i. Osmotic laxatives including fibers ii. Lubiprostone (CCl2 agonist) iii. Linaclotide (guanylate cyclate agonist) |
Peripheral |
| IBS-C | Reduced bile acid in lumen | Ileal bile acid transporter inhibitor: Elobixibat | Peripheral |
| IBS-C | Abnormal gut microbiota (particularly methanogens) |
Antibiotics i. Neomycin ii. Rifaximin Probiotics Fecal transplantation |
Peripheral |
| IBS-D | Increased serotonin activity |
Serotonin receptor blockers i. Alosetron ii. Cilansetron iii. Ramosetron iv. Ondasetron |
Peripheral |
| IBS-D | Increased gut motility |
Anti-motility agents i. Loperamide ii. Diphenoxylate iii. Asimadoline iv. Eloxadoline v. Anticholinergics including tricylic antidepressants (TCA) |
Peripheral (TCAs have central effect as well) |
| IBS-D | Increased bile acid in gut lumen |
Bile acid binding agents i. Cholestyramine ii. Cholestipol iii. Cholesevelam |
Peripheral |
| IBS-D | Gut microbiota dysbiosis |
Antibiotics i. Neomycin ii. Rifaximin iii. Norfloxacin Probiotics Fecal transplantation |
Largely peripheral though microbiota influences brain function |
| IBS, non-C | Food fermentation products | Low FODMAP diet | Peripheral |
| IBS-D | Low-grade inflammation | Anti-inflammatory (mesalamine [effective in postinfection IBS], mast cell stabilizer, not useful) | Peripheral |
| IBS-D | Bile acid malabsorption | Obeticholic acid (farnesoid X receptor agonist) | Peripheral |
- FODMAP, fermentable oligo-, di-, mono-saccharide and poyol; IBS, irritable bowel syndrome.
| Name of the drug | Peripheral vs central |
|---|---|
| Alverine | Peripheral |
| Cimetropium bromide | Peripheral |
| Dicycloverine/Dicyclomine | Peripheral |
| Drotaverine | Peripheral |
| Fenoverine | Peripheral |
| Mebeverine | Peripheral |
| Otilonium | Peripheral |
| Phloroglucinol | Peripheral |
| Pinaverium bromide | Peripheral |
| Rociverine | Peripheral |
| Scopolamine/Hyoscine | Peripheral |
| Tiropramide | Peripheral |
| Trimebutine | Peripheral |
| Tricyclic antidepressants | Central + peripheral |
| Serotonin reuptake inhibitor | Central + peripheral |
| Serotonin norepineprine reuptake inhibitor | Central + peripheral |
Some of the therapeutic modalities may work for both subtypes of IBS (IBS-C and IBS-D); these mostly include modulators of gut microbiota such as antibiotics, probiotics, and fecal transplantation.70 However, these may work through different mechanisms in different subtypes of IBS patients. For example, three studies showed that antibiotics are more often effective in patients with IBS-D with SIBO than those without SIBO.28-30 In patients with IBS-C, rifaximin with or without neomycin works primarily by targeting methanogens, which, by producing methane, slow colon transit. In fact, a recent randomized controlled trial showed that rifaximin led to improvement in patients with slow-transit constipation by reducing breath methane and increase in colon transit.37 The limited available evidence from the other methods of gut microbiota manipulation may also suggest different mode of action of this form of therapy in different subtypes of IBS. Of the three randomized controlled trials on fecal transplantation in IBS published till data, the study from Denmark showed lack of improvement in IBS symptoms despite a change in fecal microbiota71 though the two studies from Norway showed significant relief in IBS symptoms during short-term follow-up following the therapy. One of the two Norwegian studies showed that donor's fecal microbiota and the fecal microbiota doses were the key to the success.72, 73 The Denmark study might have limitation, as it included patients with IBS-C as well and used oral route of fecal transplantation with a potential risk of development of SIBO exacerbating IBS symptom, though one of the two Norwegian studies also had these limitations yet gave a positive result.73 More adequately powered long-term follow-up studies on fecal transplantation on well-characterized patients with different subtypes of IBS are needed. Till then, fecal transplantation is only in the experimental domain in management of IBS.
Conclusions and future directions
Though psychological issues are important comorbid conditions in a subset of patients with FGID including IBS, current evidences are far from supporting generalizing these disorders to be psychogenic only. In fact, in the recent years, more and more evidences are emerging to suggest the concept that a good proportion of patients with FGIDs including IBS have microorganic basis (mentioned earlier). In fact, experts in Rome IV suggested deleting the term “functional” from these disorders as this term is often misinterpreted leading to labeling these patients as neurotic, apprehensive, otherwise healthy individuals with an imaginary illness.3, 11, 12 Rome IV suggested that these are disorders of “gut-brain interaction” rather than that of “brain-gut interaction” recognizing the importance of the gut-related factors over the psychogenic issues.3, 11, 12 This is a major paradigm shift in understanding the pathogenesis of IBS, which is akin to the evolution in pathogenesis of peptic ulcer disease from psychogenic factor to acid to Helicobacter pylori infection.13 Targeting each of the mechanisms of the pathogenesis of different subtypes of IBS is expected to bring revolution in management of IBS in the coming years.




