N-myc and STAT interactor correlates with severity and prognosis in acute-on-chronic liver failure of hepatitis B virus
Abstract
Background and Aim
Hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF) is characterized by acute deterioration of chronic liver disease with excessive inflammation. N-myc and STAT interactor (NMI), an inflammation-mediated protein, involves in various inflammatory-related diseases, but the role of NMI in development and prognosis in HBV-ACLF remains to be elucidated.
Methods
Serum NMI from healthy controls (HCs, n = 20), chronic hepatitis B (CHB, n = 50) patients, and HBV-ACLF patients (n = 50) was determined using ELISA. NMI from peripheral blood mononuclear cells and liver was confirmed using quantitative real-time polymerase chain reaction, Western blot, and immunofluorescence.
Results
Serum NMI was increased 1.9-fold or 2.2-fold from HBV-ACLF patients compared with that from HCs (P < 0.01) or CHB patients (P < 0.01). Consistently, NMI from peripheral blood mononuclear cells was upregulated significantly from HBV-ACLF patients compared with that from HCs and CHB patients at mRNA and protein levels. Hepatic NMI from HBV-ACLF patients was 2.8-fold higher than that from HCs. Serum NMI was correlated with Model for End-stage Liver Disease, Chronic Liver Failure Consortium ACLF score, and ACLF grades. In contrast, serum NMI was significantly decreased in HBV-ACLF ameliorated patients during follow-up, whereas serum NMI was sustained at high levels in non-ameliorated patients. Elevated serum NMI (≥ 198.5 pg/mL) was correlated with poor survival rate of HBV-ACLF patients. Using receiver operating characteristics curves, it was suggested that serum NMI was a potential biomarker in predicting 3-month mortality of HBV-ACLF patients.
Conclusions
Our study highlights the potential role of NMI in assessing the development and prognosis of HBV-ACLF.
Introduction
Acute-on-chronic liver failure (ACLF) is a syndrome characterized as a severe deterioration of chronic liver disease accompanied with multi-organ dysfunction.1 Estimated 80% of ACLF patients infected with hepatitis B virus (HBV) as the underlying etiological factor in China.2 There is no effective strategy for ACLF patients at late stages apart from liver transplantation. However, ACLF patients identified at early stage have a potential for reversibility after receiving appropriate management.3 Therefore, novel biomarkers are urgently explored for early prognosis to identify the patients at early stage with HBV-related ACLF (HBV-ACLF).
N-myc and STAT interactor (NMI), as an inflammation-mediated protein, is first identified as a binding protein partner of N-myc and c-Myc oncogenes, and other transcription factors.4 NMI is typically localized to the cytoplasm and ubiquitously expressed, especially in the liver.5 NMI plays regulatory roles in immune cells, including T cells, natural killer cells, and macrophages.4, 6 NMI enhances immune function via STAT-mediated transcription in response to inflammatory stimulation.4 It is reported that macrophages-derived NMI promotes inflammation in different inflammation models.6 NMI is elevated rapidly in acetaminophen-induced liver injury.6 NMI is also involved in antiviral response against viral infections.7 Recently, it is demonstrated that NMI correlates with the severity of inflammation in sepsis patients.6 The severity of ACLF is also correlated with inflammation, which is contributing to prognosis of ACLF.8 However, the precise role of NMI in development and prognosis of HBV-ACLF remains to be explored.
In the current study, it was investigated that the potential role of NMI on the development of HBV-ACLF. Our data may provide useful information in the management of such devastating HBV-ACLF, particularly in identifying promising biomarker for prognosis of HBV-ACLF.
Methods
Human subjects
A total of 120 subjects were identified between September 2015 and September 2018 in Ruijin Hospital, Shanghai Jiaotong University School of Medicine, including HBV-ACLF patients (n = 50), chronic hepatitis B (CHB, n = 50) patients, and healthy controls (HCs, n = 20). Clinical data were presented in Table 1. Twenty age-matched and gender-matched healthy people for routine health check in our hospital were selected as HCs. All of the CHB patients had positive hepatitis B surface antigen for more than 6 months. HBV-ACLF patients, according to a recent Asia–Pacific consensus recommendation,9 had a history of CHB with total bilirubin (TBil) ≥ 5 mg/dL and prothrombin activity < 40%, complicated within 4 weeks by clinical ascites and/or encephalopathy. Exclusive criteria were the following: co-infection with human immunodeficiency virus or other hepatitis virus; metabolic or autoimmune liver diseases; alcohol-induced or drug-induced liver diseases; pregnancy; and hepatocellular carcinoma. The follow-up of HBV-ACLF patients started from the enrollment and lasted for at least 3 months. The first 3 days after enrollment was set as baseline. The ending point was referred to the end of 3-month follow-up survivors, death, or liver transplantation during follow-up. There were 26 ameliorated patients (survivors) and 24 non-ameliorated patients (including 18 deaths and six liver transplantations) among all HBV-ACLF patients at the end of follow-up.
| Characteristics | HCs | CHB | HBV-ACLF | P value | HBV-ACLF (n = 50) | ||
|---|---|---|---|---|---|---|---|
| Ameliorated | Non-ameliorated | P value | |||||
| Case | 20 | 50 | 50 | — | 26 | 24 | — |
| Male (n, %) | 17 (85.0) | 38 (76.0) | 46 (92.0) | NS | 24 (92.3) | 22 (91.7) | NS |
| Age (years) | 52.0 (33.3, 61.0) | 46.0 (32.8, 55.0) | 48.5 (40.8, 59.0) | NS | 47.5 (40.8, 54.8) | 53.5 (39.5, 61.0) | NS |
| ALT (U/L) | 22 (19.3, 25.3) | 103 (37.0, 501.3) | 105 (54.3, 496.3) | < 0.01 | 82.5 (27.8, 443.3) | 162.5 (59.3, 716.5) | NS |
| AST (U/L) | 23 (20.0, 28.5) | 87.5 (43.0, 239.8) | 144 (87.5, 288.3) | < 0.001 | 142 (58.8, 254.5) | 144 (112.3, 444.3) | NS |
| TBil (μmol/L) | 14.4 (12.0, 16.3) | 27.4 (16.6, 47.9) | 413.2 (278.3, 485.5) | < 0.001 | 365.6 (272.2, 421.9) | 477.2 (385.9, 592.2) | < 0.01 |
| PT (s) | ND | 12.4 (11.2, 13.9) | 22.9 (19.2, 28.0) | < 0.001 | 20.4 (18.3, 25.2) | 26.4 (21.6, 30.7) | < 0.01 |
| INR | ND | 1.1 (0.9, 1.2) | 1.9 (1.7, 2.3) | < 0.001 | 1.8 (1.5, 2.2) | 2.3 (1.8, 2.4) | < 0.01 |
| Albumin (g/L) | 44.0 (44.0, 46.8) | 37.0 (32.0, 39.0) | 27.0 (25.0, 30.0) | < 0.001 | 27.5 (24.0, 30.3) | 27.0 (25.3, 30.0) | NS |
| Creatinine (μmol/L) | 86.0 (75.8, 91.5) | 71.5 (63.8, 82.3) | 81.5 (69.8, 92.3) | < 0.01 | 77.0 (69.8, 93.8) | 84.5 (69.0, 89.5) | NS |
| Log10 (HBV DNA [IU/mL]) | ND | 5.0 (2.7, 7.3) | 3.7 (2.7, 5.8) | < 0.05 | 2.7 (2.4, 6.1) | 3.7 (2.7, 5.7) | NS |
| HBeAg positive | ND | 27 (54.0) | 19 (38.0) | NS | 9 (34.62) | 10 (41.67) | NS |
| HBsAg positive | 0 | 50 (100.0) | 50 (100.0) | < 0.001 | 26 (100.0) | 24 (100.0) | NS |
| MELD | ND | 7.0 (3.8, 10.3) | 25.0 (22.0, 27.3) | < 0.001 | 23.0 (21.8, 25.0) | 26.0 (23.0, 29.0) | < 0.05 |
| CLIF-C OFs | ND | ND | 9.0 (8.0, 10.0) | NS | 8.0 (8.0, 9.0) | 9.5 (8.0, 10.0) | < 0.01 |
| CLIF-C ACLFs | ND | ND | 40.7 (35.8, 45.5) | NS | 37.9 (35.0, 43.3) | 43.2 (40.0, 49.4) | < 0.01 |
| NMI (pg/mL) | 97.4 (55.0, 123.7) | 81.1 (40.2, 182.8) | 182.4 (113.3, 315.2) | < 0.001 | 128.1 (84.9, 193.6) | 306.9 (150.1, 494.9) | < 0.001 |
- Quantitative variables were expressed as the median (interquartile range). Categorical variables were expressed as numbers (percentage). ALT, alanine aminotransferase; AST, aspartate aminotransferase; CHB, chronic hepatitis B; CLIF-C ACLFs, Chronic Liver Failure Consortium ACLF score; CLIF-C OFs, Chronic Liver Failure Consortium Organ Failure score; HBeAg, hepatitis B envelope antigen; HBsAg, hepatitis B surface antigen; HBV-ACLF, hepatitis B virus-related acute-on-chronic liver failure; HCs, healthy controls; INR, international normalized ratio; MELD, Model for End-stage Liver Disease; ND, not determined; NMI, N-myc and STAT interactor; NS, no significance; PT, prothrombin time; TBil, total bilirubin.
This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki and approved by the Human Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Informed consent in writing was obtained from each subject prior to their inclusion in the study.
The clinic prognostic parameters
Diagnostic criteria of ACLF grades were described as follows10: ACLF grade 1 was indicated by appearance of kidney failure (serum creatinine ≥ 2 mg/dL) or other single organ/system failure (liver failure: serum bilirubin ≥ 12 mg/dL; coagulation: international normalized ratio ≥ 2.5 or platelet count ≤ 20 × 109/L; hepatic encephalopathy [HE]: III–IV HE based on West Haven criteria; circulation: received vasoconstrictors to maintain arterial pressure; and respiration: PaO2/FiO2 ≤ 200 or SpO2/FiO2 ≤ 214) associated with a serum creatinine level of 1.5–1.9 mg/dL and/or HE grades 1 and 2. ACLF patients with 2 or ≥ 3 organ failures defined as ACLF grade 2 or grade 3, respectively.
Chronic Liver Failure Consortium ACLF score (CLIF-C ACLFs)11 and Model for End-stage Liver Disease (MELD)12 were calculated as follows: CLIF − C ACLFs = 10 × [0.33 × CLIF − OFs + 0.04 × Age + 0.63 × ln (WBC count) − 2]. MELD = 3.8 × ln (bilirubin [mg/dL]) + 11.2 × ln (international normalized ratio) + 9.6 × ln (creatinine [mg/dL]) + 6.4 × (etiology : 0 if cholestatic or alcoholic, 1 otherwise).
Processing of blood and liver tissue samples
Peripheral blood mononuclear cells (PBMCs) obtained from the patients using EDTA-treated blood were separated with Ficoll density gradient. Serum was obtained from every participant for determining NMI level. Liver tissues were obtained from HBV-ACLF patients (n = 6), CHB patients (n = 5), and HC subjects (n = 5). The HC subjects were collected from the liver tissues adjacent of hepatic hemangioma during surgical liver resection, which were ready for disposing and part of the tissues were ready for pathological diagnosis (n = 5).
ELISA
Serum NMI (CUSABIO, China) was assessed, using ELISA kits according to the manufacturer's instructions.
Western blot
Cells and tissues were lysed with RIPA containing protease and phosphatase inhibitors (Roche), and proteins were separated by sodium dodecylsulfate–polyacrylamide gel electrophoresis after denaturation, as described.13 Immunoblot analysis was performed by initial transfer of proteins onto polyvinylidenefluoride filters, using a Mini Trans-Blot (Bio-Rad) followed by a blocking step with 5% non-fat milk in TBST for 2 h at room temperature and incubated with primary antibodies overnight at 4°C. The primary antibodies involved were antihuman NMI (Abcam, ab183724) and anti-glyceraldehyde phosphate dehydrogenase (GAPDH) (Cell Signaling Technology, D16H11). The blots were then incubated with a secondary antibody conjugated with horseradish peroxidase for 1 h at room temperature following three time washing. Signals were detected by a FluorChem E system (Alpha Innotech Corp). Immunoblot band densitometry was quantified using ImageJ software (National Institutes of Health).
Quantitative real-time polymerase chain reaction
Total RNA was prepared using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions, and RNA was reverse transcribed for cDNA using PrimeScript RT Master Mix (Takara Bio, Shiga, Japan). Quantitative real-time polymerase chain reaction (PCR) was performed using FastStart Universal SYBR Green Master (Rox) (Sigma-Aldrich, St. Louis, MO, USA), and signal was collected by VII7 real-time PCR system (Applied Biosystems, Foster City, CA, USA). GAPDH was used as an internal control for normalization, and the relative expression level of the related gene was calculated by the comparative (2−△△Ct) method as described.14
-
NMI: Forward, 5′-TCCGGGAGTGCAGTCATCACG-3′;
Reverse, 5′-TCTCCACCTCCATTCTTTGCCCG-3′;
-
GAPDH: Forward, 5′-GGAAGATGGTGATGGGATT-3′;
Reverse, 5′-GGATTTGGTCGTATTGGG-3′.
Immunofluorescence
To explore the relationship between NMI and Kupffer cells, double immunofluorescent labeling was applied to determine co-localization of NMI with CD68 in these human liver tissue, as described.15 Briefly, the liver sections were incubated with blocking solution at room temperature for 1 h, followed by an overnight incubation at 4°C with the mixture of anti-NMI antibody (1:100; Abcam) and anti-CD68 antibody (1:200; Abcam). Subsequently, Alexa Fluor 488-conjugated secondary antibody (1:200; Abcam) and Alexa Fluor 555-conjugated secondary antibody (1:200; Invitrogen) were incubated for 1 h at room temperature in the dark, followed by a 5-min incubation of DAPI at room temperature in the dark.
Statistical analysis
All results were analyzed, using the SPSS version 23.0 software (SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed as the median (range), and categorical variables were presented as number (percentage). The differences between two groups were evaluated by Student's t-test, Mann–Whitney U-test, or χ2 test, as appropriate. The differences among three or more groups were analyzed using one-way anova test, Kruskal–Wallis test, or χ2 test, as appropriate. The correlation between two variables was assessed using Spearman's rank correlation test. The contributing factors to deterioration in HBV-ACLF patients were investigated using multivariate logistic regression analysis. Survival analysis was determined by the Kaplan–Meier method and compared with the log–rank test. Predictive accuracy of any parameter was assessed by receiver operating characteristics curves. A two-sided P < 0.05 was considered statistically significant.
Results
Serum and hepatic N-myc and STAT interactor in hepatitis B virus-related acute-on-chronic liver failure patients
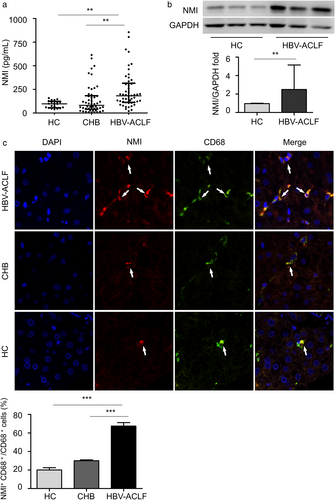
Excessive inflammation response is a major mechanism of ACLF. Inflammatory mediators are strongly associated with the severity of ACLF.16 It was investigated whether NMI was involved in the development of HBV-ACLF. Using ELISA, serum NMI from HBV-ACLF patients was 1.9-fold or 2.2-fold higher than that from HCs (P < 0.01) or CHB patients (P < 0.01) at baseline. There was no significant difference of serum NMI level between HCs and CHB patients (Fig. 1a). Then it was further investigated if hepatic NMI was elevated from HBV-ACLF patients, compared to that from HCs. Using Western blot, hepatic NMI was 2.8-fold higher in HBV-ACLF patients compared with that in HCs (P < 0.01) (Fig. 1b). Furthermore, double immunofluorescence identified that intrahepatic NMI+CD68+ cells (Kupffer cells) were increased in HBV-ACLF patients compared with those in CHB patients (P < 0.001) or HCs (P < 0.001) (Fig. 1c). Such data suggest that upregulated intrahepatic NMI is produced from Kupffer cells that may contribute to the progression of ACLF. These results suggest that NMI might participate in the progression of HBV-ACLF.
N-myc and STAT interactor from peripheral blood mononuclear cells in hepatitis B virus-related acute-on-chronic liver failure patients
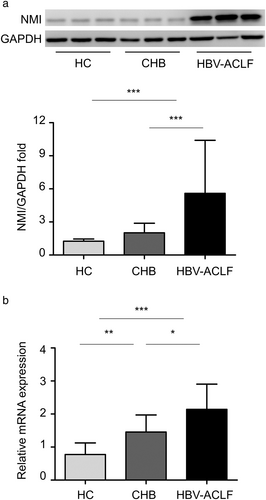
Peripheral blood mononuclear cells, including monocytes and lymphocytes, could reveal the immunity in various inflammatory diseases.17 Using Western blot, it was detected that NMI in PBMCs from HBV-ACLF patients was 4.3-fold or 2.8-fold higher than that from HCs (P < 0.001) or from CHB patients (P < 0.001) (Fig. 2a). The protein levels were confirmed using quantitative real-time PCR, showing that NMI mRNA in PBMCs from HBV-ACLF patients was increased 2.8-fold or 1.5-fold compared with that from HCs (P < 0.001) or CHB patients (P < 0.05). The expression of NMI in PBMCs was higher in CHB patients compared with that in HCs (P < 0.01) (Fig. 2b). These results indicate that upregulated NMI in PBMCs might be involved in the development of immune dysfunction in HBV-ACLF.
Correlation between serum N-myc and STAT interactor and clinical prognostic scores in hepatitis B virus-related acute-on-chronic liver failure patients
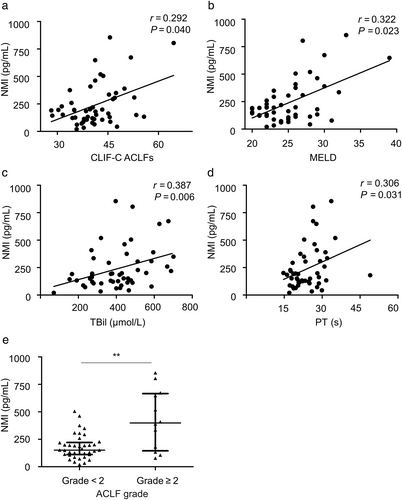
Model for End-stage Liver Disease is the most common predictor for prognosis evaluating of ACLF.18 CLIF-C ACLFs, considering multi-organ failure and inflammation level, is a newly developed prognostic parameter for ACLF with improved predictive accuracy.11 It was investigated the correlation between NMI and prognostic scores of HBV-ACLF. At baseline, NMI positively correlated with CLIF-C ACLFs (P < 0.05) (Fig. 3a) and MELD (P < 0.05) (Fig. 3b). NMI was also positively correlated with TBil (P < 0.01) (Fig. 3c) and prothrombin time (P < 0.05) (Fig. 3d). It is well known that ACLF grades base on organ failure level, which is a simplified prognostic parameter for ACLF classification.10 It has been reported that grade 2 is a significant cut-off point for ACLF grades in determining prognosis of ACLF patients.19 Thus, serum NMI was compared between HBV-ACLF patients grade < 2 and grade ≥ 2, observing that there was 2.6-fold higher in these patients grade ≥ 2 than these grade < 2 at baseline (P < 0.01) (Fig.3e).
Association between serum N-myc and STAT interactor and prognosis in hepatitis B virus-related acute-on-chronic liver failure patients
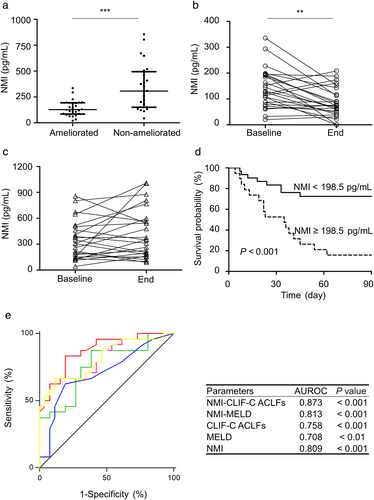
Because there was a positive correlation between NMI and the clinical prognostic scores, the prognostic value of serum NMI for HBV-ACLF patients was further investigated. Serum NMI of HBV-ACLF non-ameliorated patients was 2.4-fold higher than that of HBV-ACLF ameliorated patients at baseline (P < 0.001) (Fig.4a). Serum NMI was 42% reduced from the HBV-ACLF ameliorated patients at the ending point, compared with that from the baseline (P < 0.01) (Fig. 4b), whereas there was no significant difference of serum NMI from HBV-ACLF non-ameliorated patients at baseline and the ending point (P > 0.05) (Fig. 4c). Furthermore, NMI, CLIF-C ACLFs, and TBil were identified as contributing factors to deterioration in HBV-ACLF patients (Table 2).
 , NMI-CLIF-C ACLFs;
, NMI-CLIF-C ACLFs;  , NMI-MELD;
, NMI-MELD;  , CLIF-C ACLFs;
, CLIF-C ACLFs;  , MELD;
, MELD;  , NMI;
, NMI;  , reference line. **P < 0.01; ***P < 0.001. [Color figure can be viewed at wileyonlinelibrary.com]
, reference line. **P < 0.01; ***P < 0.001. [Color figure can be viewed at wileyonlinelibrary.com]| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| P value | OR | 95% CI | P value | OR | 95% CI | |
| Gender (male) | NS | 0.917 | 0.119–7.075 | — | — | — |
| Age (years) | NS | 1.018 | 0.968–1.071 | — | — | — |
| ALT (U/L) | NS | 1.001 | 0.999–1.003 | — | — | — |
| AST (U/L) | NS | 1.002 | 0.999–1.005 | — | — | — |
| TBil (μmol/L) | < 0.01 | 1.008 | 1.003–1.014 | < 0.05 | 1.007 | 1.000–1.014 |
| PT (s) | < 0.05 | 1.178 | 1.040–1.335 | — | — | — |
| INR | < 0.05 | 6.363 | 1.452–27.878 | — | — | — |
| Albumin (g/L) | NS | 1.015 | 0.903–1.140 | — | — | — |
| Creatinine (μmol/L) | NS | 0.997 | 0.981–1.013 | — | — | — |
| Log10 (HBV DNA [IU/mL]) | NS | 1.013 | 0.752–1.363 | — | — | — |
| HBeAg positive | NS | 1.349 | 0.429–4.240 | — | — | — |
| MELD | < 0.05 | 1.239 | 1.032–1.489 | — | — | — |
| CLIF-C OFs | < 0.01 | 2.218 | 1.236–3.981 | — | — | — |
| CLIF-C ACLFs | < 0.01 | 1.190 | 1.056–1.340 | < 0.05 | 1.163 | 1.008–1.343 |
| NMI (pg/mL) | < 0.01 | 1.011 | 1.004–1.018 | < 0.05 | 1.009 | 1.002–1.017 |
- 95% CI, 95% confidence interval; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CLIF-C ACLFs, Chronic Liver Failure Consortium Acute-on-Chronic Liver Failure score; CLIF-C OFs, Chronic Liver Failure Consortium Organ Failure score; HBeAg, hepatitis B envelope antigen; HBV, hepatitis B virus; INR, international normalized ratio; MELD, Model for End-stage Liver Disease; NMI, N-myc and STAT interactor; NS, no significance; OR, odds ratio; PT, prothrombin time; TBil, total bilirubin.
An optimal cut-off value of 198.5 pg/mL for serum NMI was identified to discriminate ameliorated patients and non-ameliorated patients at the end of 3-month follow-up, with sensitivity, specificity, positive predictive value, or negative predictive value as 66.67%, 88.46%, 84.21%, or 74.19%, respectively. In addition, the median survival time for HBV-ACLF patients with serum NMI ≥ 198.5 pg/mL was significantly shorter than those with serum NMI < 198.5 pg/mL (35 vs > 90 days, P < 0.001) (Fig. 4d). The area under the receiver operating characteristics curve (AUROC) of serum NMI was 0.809 (P < 0.001). Interestingly, no significant difference of AUROC value was observed between serum NMI and CLIF-C ACLFs or NMI and MELD score. However, a combination of serum NMI and CLIF-C ACLFs (NMI–CLIF-C ACLFs) showed significantly higher AUROC value than CLIF-C ACLFs (P < 0.05) or MELD (P < 0.05) (Fig. 4e). These data suggest that serum NMI may be good predictor of prognosis for patients with HBV-ACLF.
Discussion
The underlying mechanism of HBV-ACLF is not fully elucidated. It is suggested that excessive inflammation induced by local immune dysfunction is considered as the predominant factor and correlated with poor prognosis in HBV-ACLF patients.8 It is suggested that inflammatory mediators may be used as potential biomarkers for predicting prognosis in HBV-ACLF patients.16 In the present study, we demonstrated that NMI was involved in the development of HBV-ACLF and may become a prognostic biomarker for HBV-ACLF patients.
It has been demonstrated that NMI is involved in various inflammatory diseases. Pesciotta et al. report that upregulation of NMI correlated with inflammatory signature in the patients with congenital erythrocyte aplasia.20 Serum NMI is also found to be correlated to the severity of inflammation and mortality of patients with sepsis.6 HBV-ACLF syndrome has some similarities to sepsis,21 suggesting that NMI may also involve in the progression of HBV-ACLF. This is consistent with our study, showing that serum and hepatic NMI were significantly increased in HBV-ACLF patients. Moreover, serum NMI in HBV-ACLF non-ameliorated patients was significantly higher than that in HBV-ACLF ameliorated patients, suggesting that NMI may reflect the severity of HBV-ACLF and participate in the development of liver failure.
Peripheral blood mononuclear cells play an important role in immune dysfunction in the liver as infiltrating immune cells and reveal immunity in various inflammatory diseases.22 Recently, many genes related to immune regulation in PBMCs have also been identified and may be served as signatures of HBV-ACLF progression, of which expression profile could be altered by pathological process of HBV-ACLF.23 Brozzi et al report that intracellular NMI could be upregulated in response to inflammatory process in autoimmune disease.24 Our study also showed that NMI was significantly increased in PBMCs from HBV-ACLF patients, suggesting that upregulated NMI in PBMCs may be related to excessive inflammation response in the development of HBV-ACLF. Furthermore, intracellular NMI inhibits immune response to reduce inflammatory cytokines in cancer.25 Therefore, our observation showed that NMI in PBMCs from HBV-ACLF was increased, suggesting that NMI could also serve as an intracellular immune regulator in the development of HBV-ACLF in addition to act as a circulating inflammatory mediator. We acknowledge that there is certain discrepancy of NMI between serum and PBMCs from HCs and CHB. Apart from the point that both CHB and HCs were used as control for HBV-ACLF, serum NMI is secreted already in the systemic circulation, whereas PBMC NMI is still within the cellular structure prior to secretion. There may be timing issue and/or immune responses issue in these two distinguish conditions. The precise underlying mechanism will be determined in our future experiment.
Recently, some reports show that NMI could be used as an effective biomarker for prognosis evaluating in cancer patients.26 However, the prognostic value of NMI in HBV-ACLF patients remains unknown. As an important mediator involves in development of tumor, elevated NMI is also significantly correlated with poor prognosis in patients with glioblastoma.27 Although the mechanism of NMI in tumorigenesis is different from ACLF, our study is supporting the findings that NMI was strongly correlated with the commonly used prognostic scores for ACLF. Therefore, the relationship between the elevation of NMI level and the changes of prognostic scores indicates the potential usefulness of NMI in reflecting the prognosis of HBV-ACLF.
In addition to the established prognostic scores that based on conventional clinical parameters, biomarkers related to inflammation and immune for early prediction of HBV-ACLF are also being explored.28 Zhang et al. report that frequency of Th17 cells predicts 3-month prognosis equivalent to MELD and CLIF-C ACLF scores in HBV-ACLF.29 Similarly, our current study showed that serum NMI may have prognostic value equivalent to MELD and CLIF-C ACLFs in predicting 3-month mortality in HBV-ACLF patients. Furthermore, our data also showed that higher AUROC of serum NMI was observed than that of frequency of Th17 cells in predicting 3-month mortality for patients with HBV-ACLF. A combination of serum NMI and CLIF-C ACLFs (NMI–CLIF-C ACLFs) provided better prediction of short-term prognosis than that of MELD or CLIF-C ACLFs. The differences in prognostic value between NMI and other biomarkers may be related to their different functions in HBV-ACLF development and also related to differences in stages of liver diseases. Although mechanism of NMI in HBV-ACLF progression has not been well characterized, our study provided some evidence that increased NMI at baseline may predict poor prognosis in HBV-ACLF patients. Nevertheless, further validation for the prognostic value of NMI in a larger cohort of patients should be performed in the future.
In summary, the present study suggests that NMI is involved in the development of HBV-ACLF. Elevated NMI reversely correlates with disease severity in HBV-ACLF patients. Serum NMI shows a satisfactory prognostic accuracy in predicting the short-term mortality in HBV-ACLF patients. NMI not only may serve as a prognostic biomarker of HBV-ACLF but also might be a potential treatment target for clinic application.




