Effect of a concomitant elemental diet with maintenance anti-tumor necrosis factor-α antibody therapy in patients with Crohn's disease: A multicenter, prospective cohort study
Abstract
Background and Aim
The aim of this study was to clarify the additional effect of a concomitant elemental diet (ED) for patients with Crohn's disease on maintenance anti-tumor necrosis factor-α antibody (anti-TNF).
Methods
Crohn's disease patients who received anti-TNF induction therapy were enrolled. Patients who achieved clinical response (defined as delta Crohn's disease activity index [CDAI] > 70 and CDAI < 200) at 10–14 weeks after the start of infliximab or adalimumab were included. Eligible patients took a tolerability test of ED (900 kcal/day) for 3 days. Then, patients who preferred concomitant ED and whose ED tolerance was confirmed were allocated to the ED group and given Elental 900 kcal/day or more. Other patients were allocated to the non-ED group. The primary endpoint was the cumulative remission rate at 2 years after baseline. Clinical relapse was defined as CDAI > 200 and/or need for additional treatment. Adherence to the ED was confirmed at each visit.
Results
Seventy-two patients were included. Thirty-seven were allocated to the ED group, and 35 were allocated to the non-ED group. The cumulative remission rate at 2 years was not significantly different between the two groups (60.9% vs 56.7%, P = 0.98). Adherence to the ED in the ED group was relatively low, and only 11 patients were maintained on an ED of 900 kcal/day.
Conclusions
The addition of ED for Crohn's disease patients who responded to initial anti-TNF induction therapy was not found to improve outcomes. The efficacy of concomitant ED in other clinical settings, such as loss of response, needs to be clarified in the future (UMIN000009789).
Introduction
Crohn's disease (CD) is known to be a chronic inflammatory disease showing relapsing and disabling course due to the lack of curative therapy. However, the elucidation of its disease mechanisms has led to the discovery of effective therapeutics, such as anti-tumor necrosis factor-α antibody (anti-TNF) and anti-interleukin-12 and anti-interleukin-23 antibodies, which have become widely used.1-5 Anti-TNF, currently the primary approach for treating CD, is extremely effective in inducing and maintaining remission.1-4 However, it also has several problems, such as primary nonresponse, intolerance, and loss of response (LOR).6, 7 LOR has been reported to affect 25–61% of patients on anti-TNF maintenance therapy,6-9 and the main reason for this has been attributed to the decrease in its circulating concentration or the appearance of antidrug antibodies, and such cases are typically treated by dose escalation, such as doubled dosage, or shortened duration.10, 11 Risk factors for LOR include male sex, being a smoker, long-term illness, family history of inflammatory bowel disease, and previous use of anti-TNF.7 In addition, it has been demonstrated that the concomitant use of an immunomodulator (IMM), especially infliximab (IFX), increases the long-term effect,12 and IMM is added or its dose is increased in patients who develop LOR.13-15 An elemental diet (ED) is an extremely safe therapeutic approach that is effective for maintaining remission and preventing postoperative recurrence even when used alone.16, 17 Recently, the concomitant use of anti-TNF and an ED has been gaining attention as a therapeutic approach for CD, and many reports have demonstrated its efficacy for maintaining remission and continuing anti-TNF treatment.18-21 A meta-analysis that assessed four studies regarding concomitant use of anti-TNF and an ED concluded that there was a threefold effect in attaining clinical remission with concomitant IFX and ED compared with IFX without ED.22 However, only one study has prospectively analyzed the effects of concomitant ED.18 Therefore, there is no enough evidence to determine whether the concomitant use of anti-TNF and an ED is useful for maintaining remission in CD. The present prospective, multicenter, cohort study was designed to elucidate whether this combination brings additional long-term effects in patients who have achieved remission with anti-TNF.
Methods
Study subjects
Among CD patients who were newly receiving anti-TNF therapy, those with Crohn's disease activity index (CDAI) of at least 220 but less than 400 within 16 weeks prior to anti-TNF induction were eligible to enroll in the study. Patients who had previously used anti-TNF and those who had undergone surgery within 6 months prior to anti-TNF induction were excluded. During the 2-week period before anti-TNF induction, patients were given an explanation of the study objectives and the usefulness and side effects of anti-TNF and an ED. Patients were provisionally enrolled after giving written, informed consent. Provisionally enrolled patients underwent a tolerability evaluation by taking an ED of 900 kcal/day for at least 3 days during the 2-week period before anti-TNF induction. The ED was given orally as a general rule, but tube feeding by self-intubation was also allowed in patients who had previously received tube feeding and wished to receive an ED via this route. Subsequently, among patients with confirmed tolerability, those who wished to receive ED concomitantly during anti-TNF maintenance therapy were assigned to the ED group, and all other patients were assigned to the non-ED group. Anti-TNF treatment consisted of IFX at a dose of 5 mg/kg at 0, 2, and 6 weeks or adalimumab (ADA) with an initial dose of 160 mg, second dose of 80 mg, and a dose of 40 mg every other weeks thereafter. The baseline CDAI was calculated on the day of anti-TNF induction and was verified to be at least 220 but less than 400. At 10–14 weeks after anti-TNF-α induction, response to anti-TNF treatment was determined using the CDAI. A CDAI of < 200 with a decrease of at least 70 (delta CDAI > 70) was defined as a “clinical response,” and patients who did not meet this criterion and patients who discontinued anti-TNF therapy were defined as having “no response.” Only patients who were determined to have a “clinical response” were officially enrolled in the study.
Endpoints and assessments
Study design
This study was named the Additional Power of Elemental Diet on Maintenance Biologics Therapy in Crohn's Disease (ADORE) Study and was conducted as an investigator-initiated, multicenter, prospective, cohort study in Kyushu area, Japan. A total of 17 institutions that obtained ethics approval for this study participated. Provisional and official enrollment of patients was performed by the clinical study administrative office (Fukuoka University Chikushi Hospital), and the participating institutions communicated with the office via fax regarding enrollment. Responses were verified by the office, and officially enrolled patients were assigned a study enrollment number. Personal information was solely managed by each institution, and electronic data capture (EDC) was completed on a later date in a manner such that individuals could not be identified and was mailed to the office as password-protected electronic media after study completion for each patient. Adverse events were described in the EDC and were also reported as appropriate for each case. We registered this study to the University Hospital Medical Information Network of Japan (register no. UMIN000009789).
Endpoints
The primary endpoint of the present study was the rate of maintaining remission at 2 years after anti-TNF induction. Recurrence was defined as CDAI ≥ 200 or the need for additional treatment, which included additional medications such as IMM, dose escalation such as a dose increase and a shortened interval of anti-TNF, surgery, addition of the ED in the non-ED group, and hospitalization due to exacerbated CD. Shortened interval of IFX was defined as duration within 6 weeks or less. As for ADA, shortened interval was defined as 40 mg every week. The enrolled patients visited the participating institutions at least every 8 weeks for a checkup to determine CDAI, the C-reactive protein (CRP) level, and the presence/absence of recurrence. The EDC was submitted at the time when patients met the definition of recurrence or after 2 years when the 2-year study period was completed.
Secondary endpoints were analysis of adherence in the ED group, relapse risk factor analysis comparing those with and without a recurrence, and adverse events of anti-TNF and the ED. Adherence was determined through patient interviews conducted by physicians at each institution and through the prescribed amount of Elental (EA Pharma Co., Ltd., Tokyo, Japan). The amount of ED during the follow-up period was verified for each patient, and the amount that was maintained for at least two-thirds of the follow-up was designated as that patient's ED amount.
Statistical analysis
Sample size
The 1-year rate of maintaining remission after anti-TNF monotherapy from a retrospective study with an approximately 2-year follow-up period conducted in Kyushu region was approximately 50%,20 and this was similar to the outcomes from previous studies from Western countries, such as the ACCENT-1 study,1 CLASSIC II study,3 and CHARM study.4 In the earlier study from Kyushu, the hazard ratio of concomitant enteral nutrition therapy to IFX monotherapy for maintaining remission was 0.426. Based on these previous findings, the hazard ratio of the ED group to the non-ED group was set to be 0.426, and the rate of maintaining remission after 1 year was set to be 50% in the non-ED group. With these values, along with a follow-up duration of 2 years, two-tailed significance of 5%, and power of 80%, the necessary number of patients was calculated to be 37 in each group using the SAS 9.1 POWER procedure. Because this was not a randomized, controlled trial, provisional enrollment of 90 patients at participating institutions was planned with consideration for the imbalanced assignment to the ED group and the non-ED group and potential patient dropout at enrollment.
Statistical methods
The analysis for this study was conducted by Satt Co., Ltd. (Tokyo, Japan), a company independent from all participating facilities, the clinical study administrative office, and industry collaborators. With SAS ver. 9.4 software (SAS Institute, Cary, NC), the t-test and Wilcoxon rank-sum test were used to perform unpaired comparisons between two groups, and the chi-squared test or Fisher's exact test was used to compare frequencies. The cumulative rate of maintaining remission was analyzed with the Kaplan–Meier method and compared between two groups using the log-rank test. Cox regression analysis was used for multivariate analysis. P < 0.05 was considered significant for all analyses.
Results
Demographic and baseline characteristics
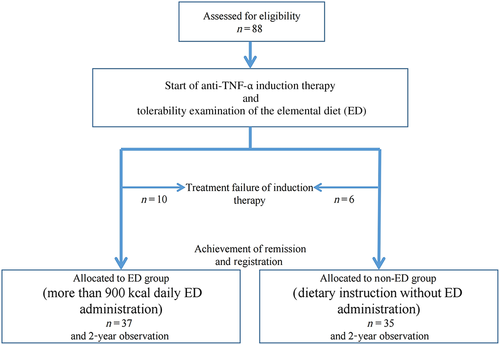
A total of 88 patients were provisionally enrolled in the study between July 2011 and March 2014 to assess patients' desire to receive an ED and their tolerability of the ED. Of these patients, a total of 16 patients with a poor response to anti-TNF (10 patients who wished to receive the ED and had good ED tolerability and six patients who either had poor ED tolerability or did not wish to receive the ED) were not officially enrolled. Ultimately, 72 patients who met the clinical response criteria were officially enrolled, and 37 patients were allocated to the ED group, while the remaining 35 patients were allocated to the non-ED group (Fig. 1). Patient characteristics are shown in Table 1. The number of patients who had received an ED in the past was greater in the ED group than in the non-ED group (P = 0.03), but none of the other patient characteristics were significantly different between the two groups. Seven patients (9.9%) in the ED group and two patients (5.7%) in the non-ED group concomitantly used IMMs.
| Demographic characteristics | ED group (n = 37) | Non-ED group (n = 35) | P value | |
|---|---|---|---|---|
| Age | Mean (±SD) | 31.6 (12.5) | 31.9 (12.4) | 0.86 (W) |
| Median (range) | 27.0 (18–59) | 28.0 (18–70 | ||
| Gender | Male | 28 (75.7%) | 24 (68.6%) | 0.60 (F) |
| Female | 9 (24.3%) | 11 (31.4%) | ||
| Induction therapy | Infliximab | 26 (70.3%) | 19 (54.3%) | 0.22 (F) |
| Adalimumab | 11 (29.7%) | 16 (45.7%) | ||
| Disease location | Ileum | 5 (13.5%) | 4 (11.4%) | 0.69 (F) |
| Ileum and colon | 25 (67.6%) | 21 (60.0%) | ||
| Colon | 7 (18.9%) | 10 (28.6%) | ||
| Disease periods (year) | Mean (±SD) | 6.5 (10.1) | 6.9 (8.0) | 0.40 (W) |
| CDAI | Mean (±SD) | 273.3 (50.1) | 268.3 (45.9)) | 0.85 (W) |
| Smoking status | No | 32 (86.5%) | 24 (68.6%) | 0.15 (F) |
| Past | 2 (5.4%) | 2 (5.7%) | ||
| Yes | 3 (8.1%) | 9 (25.7%) | ||
| Perianal lesion | No | 18 (48.6%) | 13 (37.1%) | 0.35 (F) |
| Yes | 19 (51.4%) | 22 (62.9%) | ||
| Fistula | No | 33 (89.2%) | 31 (88.6%) | 0.85 (F) |
| Internal fistula | 0 (0.0%) | 1 (2.9%) | ||
| Enterocutaneous | 4 (10.8%) | 3 (8.6%) | ||
| Intestinal stricture | No | 23 (62.2%) | 26 (74.3%) | 0.32 (F) |
| Yes | 14 (37.8%) | 9 (25.7%) | ||
| Previous enteral nutrition† (year) | 0 | 17 (45.9%) | 27 (77.1%) | 0.03 (F) |
| 1–3 | 8 (21.6%) | 3 (8.6%) | ||
| ≧4 | 12 (32.4%) | 5 (14.3%) | ||
| Dosage of enteral nutrition† (kcal/day) | Mean (±SD) | 1037.8 (296.6) | 171.1 (101.4) | < 0.001 (W) |
| Number of patients | n = 20 | n = 8 | ||
| Previous operation (times) | 0 | 26 (70.3%) | 25 (71.4%) | 0.80 (F) |
| 1–3 | 11 (29.7%) | 9 (25.7%) | ||
| ≧4 | 0 (0.0%) | 1 (2.9%) | ||
| Concurrent medications Azathioprine | No | 30 (81.1%) | 33 (94.3%) | 0.15 (F) |
| Yes | 7 (9.9%) | 2 (5.7%) | ||
- † ED and/or semi-digestion nutrition agent.
- CDAI, Crohn's disease activity index; ED, elemental diet; F, Fisher's exact test; TNF, tumor necrosis factor; W, Wilcoxon rank-sum test.
Cumulative remission rate
The cumulative remission rate 2 years after starting anti-TNF was not significantly different between the ED group (60.9%) and the non-ED group (56.7%) (P = 0.98) (Fig. 2). Clinical relapse occurred in 13 patients in the ED group, as indicated by CDAI > 200 in eight patients or additional treatment in five patients (surgery, n = 0; anti-TNF dose escalation, n = 2; and other additional treatment, n = 3). Similarly, clinical relapse occurred in 13 patients in the non-ED group as indicated by CDAI > 200 in six patients and additional treatment in seven patients (surgery, n = 0; anti-TNF dose escalation, n = 3; switching anti-TNF from ADA to IFX, n = 1; and other additional treatment, n = 3).
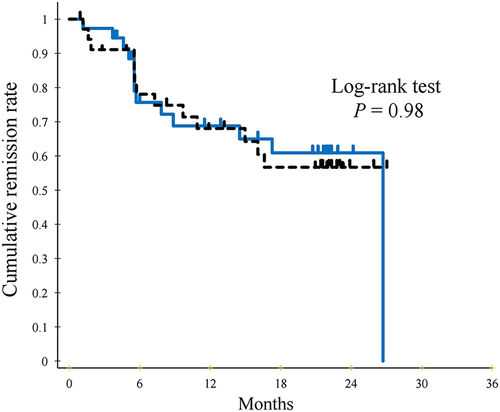
 ) elemental diet (ED) group and the (
) elemental diet (ED) group and the ( ) non-ED group determined using the Kaplan–Meier method. There is no significant difference in the cumulative remission rate between the two groups (log-rank test P = 0.98). [Color figure can be viewed at wileyonlinelibrary.com]
) non-ED group determined using the Kaplan–Meier method. There is no significant difference in the cumulative remission rate between the two groups (log-rank test P = 0.98). [Color figure can be viewed at wileyonlinelibrary.com]Crohn's disease activity index, C-reactive protein, and albumin at the time of study endpoint
Many factors on clinical efficacy were compared between two groups at the time of study endpoint as the additional investigation. CDAI of ED group was lower than that of non-ED group (100.1 ± 94.0 vs 124.1 ± 94.0); however, there was no significant difference (P = 0.21). Likewise, CRP level showed the same tendency (0.9 ± 1.8 vs 1.6 ± 2.7 mg/dL, P = 0.20). Although albumin was slightly higher in the ED group (3.9 ± 0.7 g/dL) compared with the non-ED group (3.8 ± 0.6 g/dL), there was no significant difference (P = 0.57).
Adherence to the elemental diet
The ED amounts of the subjects are shown in Table 2. In the ED group, only 11 patients (29.7%) were taking an ED of ≥ 900 kcal/day; four patients (10.8%) were on < 300 kcal/day; nine patients (24.3%) were on ≥ 300 kcal/day but < 600 kcal/day; and 13 patients (35.1%) were on ≥ 600 kcal/day but < 900 kcal/day, indicating poor ED adherence even in the ED group. Figure 3 shows the average dosage of enteral nutrition (kcal/day) administration in details. According to the real dosage that the subjects took in the observation periods, we alternatively performed an analysis stratifying the different amounts of ED. However, the analysis also showed no significance according to the real dosage of ED. For instance, when the cutoff was taken to be 600 kcal (in that case, 24 patients were classified as the ED group and 48 as the non-ED group), nine patients (41.7%) in the ED group and 17 patients (35.4%) in the non-ED group showed clinical relapse. There was no significant difference of cumulative remission rate by log-rank test (P = 0.69).
| Dosage of enteral nutrition (kcal/day)† | |||||
|---|---|---|---|---|---|
| <300 | ≦300 | ≦600 | ≦900 | Total | |
| ED group (n = 37) | 4 (10.8%) | 9 (24.3%) | 13 (35.1%) | 11 (29.7%) | 37 |
| Non-ED group (n = 35) | 34 (97.1%) | 1 (2.9%) | 0 (0.0%) | 0 (0.0%) | 35 |
- † Average intake of enteral nutrition during 2 years.
- ED, elemental diet.
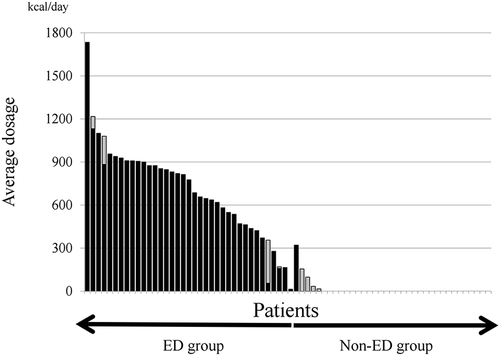
 )Black bar is elemental diet (ED), and (
)Black bar is elemental diet (ED), and ( )gray bar is other nutrients (e.g. polymeric formula).
)gray bar is other nutrients (e.g. polymeric formula).Risk factors for clinical relapse
Factor analysis of clinical relapse was conducted using variables such as patient characteristics, treatment history, disease state, and baseline test results. As described in the demographic and baseline characteristics part, there were no significant difference except ED experience between the ED group and the non-ED group. So we mainly selected the items, which were considered as important factors of long-term clinical course of CD, for multivariate analysis. Multivariate analysis showed that intestinal stenosis (hazard ratio [HR]: 4.99, 95% confidence interval [CI]: 1.64–15.18, P = 0.005), steroid therapy before anti-TNF induction (HR: 4.02, 95% CI: 1.08–14.89, P = 0.038), increased baseline leukocytes (> 8000/mm3) (HR: 13.83, 95% CI: 3.18–60.12, P = 0.0005), and high CRP level (> 5 mg/mL) (HR: 6.53, 95% CI: 1.75–24.41, P = 0.0053) were independent risk factors for clinical relapse (Table 3). Taking ED did not affect the clinical relapse (HR: 1.23, 95% CI: 0.46–3.27, P = 0.68).
| Predictors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (≧51) | 1.63 (0.56–4.74) | 0.37 | 1.84 (0.30–11.08) | 0.51 |
| Gender (female) | 1.58 (0.70–3.58) | 0.27 | 1.99 (0.50–7.96) | 0.33 |
| Taking ED | 0.99 (0.46–2.14) | 0.98 | 1.23 (0.46–3.27) | 0.68 |
| Intestinal stricture | 3.64 (1.58–8.39) | 0.002 | 4.99 (1.64–15.18) | 0.005 |
| Steroid therapy | 2.85 (1.11–7.27) | 0.029 | 4.02 (1.08–14.89) | 0.038 |
| WBC† (≧8000 mm3) | 3.90 (1.40–10.90) | 0.009 | 13.83 (3.18–60.12) | 0.0005 |
| Albumin† (≧3.5 g/dL) | 0.21 (0.07–0.61) | 0.004 | 0.44 (0.07–2.71) | 0.37 |
| CRP† (≧0.5 mg/dL) | 4.89 (2.13–11.27) | < 0.001 | 6.53 (1.75–24.41) | 0.0053 |
| CDAI† (≧100) | 2.07 (0.92–4.65) | 0.08 | 0.56 (0.14–2.20) | 0.41 |
| Surgical history | 0.33 (0.10–1.06) | 0.006 | 0.58 (0.12–2.69) | 0.48 |
- † At the time of registration.
- CDAI, Crohn's disease activity index; CI, confidence interval; CRP, C-reactive protein; ED, elemental diet; HR, hazard ratio; WBC, white blood cell.
Safety profile
Two patients on IFX developed an infusion reaction, and one of these patients discontinued the drug treatment. Three patients on ADA showed redness or a rash at the site of injection. In addition, progressive liver dysfunction and anemia were observed in five and two patients, respectively. Side effects of the ED consisted only of exacerbation of diarrhea in two patients, and none of the patients discontinued the ED because of side effects.
Discussion
Anti-tumor necrosis factor-α antibody has been reported to be an extremely effective drug for treating CD, with established efficacy, such as long-term steroid-free clinical remission and avoidance of hospitalization and surgery.1, 4, 23 On the other hand, LOR is one of the major challenges of anti-TNF treatment and is known to necessitate a dose increase of anti-TNF or additional treatment with drugs such as IMM.10 However, safety issues, including comorbid malignant diseases such as lymphoma, have been a concern with the concomitant use of IMM.24, 25 Furthermore, the long-term safety of dose escalation of anti-TNF has not been clarified. ED is known to be effective in CD not only through improving the nutritional status but also by minimizing food antigens, acting on mucosal cytokines, normalizing gut permeability, and ameliorating the gut flora.26-29 Compared with the drugs, ED therapy is extremely safe because of the nature, which consists of the intake of an amino-acid formulation. Based on these properties of an ED, it has been reported recently that the concomitant use of an ED with anti-TNF augments the therapeutic effects, resulting in the maintenance of remission without LOR and continuation of anti-TNF treatment.18-21 However, most of these were retrospective cohort studies, and only one report was a prospective study.18 This single-center, prospective, cohort study by Yamamoto et al. investigated 56 patients whose remission was induced by IFX, and they found no significant difference in the maintenance of remission during a 56-week follow-up between the ED group (78%) and the non-ED group (67%).18 As far as we know, the present prospective study was the largest cohort to validate the effects of concomitant ED use. The rate of remission maintenance 2 years after anti-TNF-α induction was not significantly different between the ED group (60.9%) and the non-ED group (56.7%). Risk factors for clinical relapse included intestinal stenosis, steroid therapy prior to anti-TNF induction, increased leukocytes, and high CRP levels at baseline, but did not include the absence of ED intake. There are several reasons why this study did not demonstrate the effectiveness of concomitantly taking an ED. First, the cumulative rate of maintaining remission with anti-TNF alone (non-ED group) was higher in this study compared with previous reports. In the actual clinical setting, better treatment effects than the clinical trial data are sometimes observed; thus, even though the number of enrolled patients was essentially as planned, results that were different from the sample size validation conducted at the study planning stage were attained. Moreover, this study included patients who received anti-TNF for the first time and subsequently achieved remission. Our previous retrospective study included all patients on anti-TNF-α maintenance therapy, and this may have led to divergent results between the two studies. Hisamatsu et al. investigated the effects of concomitant ED in LOR patients who required a dose increase of IFX (10 mg/kg).30 This prospective study concluded that IFX with an ED of 900–1200 kcal/day resulted in clear superiority of the subsequent clinical effect compared with IFX without an ED. Based on the overall results from the present study and the previous reports, it is suggested that the concomitant use of an ED is not necessary for patients who achieved remission after the initial induction of anti-TNF; however, the concomitant use of an ED is desirable in patients with LOR. These corroborate the results from a retrospective study by Sugita et al. who showed that the effects of a concomitant ED were greater in patients with LOR to ADA than in ADA-naïve patients.31 Second, validation of adherence, which was one of the secondary endpoints, found poor ED adherence even in the ED group that was composed of patients who wished to receive an ED. Only 11 patients in the ED group were taking the ED of ≥ 900 kcal/day, the designated study dose, and it is likely that the non-adherence to the ED was the chief factor affecting the results of this study. While this study did not analyze the reasons for non-adherence, it is necessary in due course to develop a strategy to improve ED adherence.
There are several limitations to this study. First, this was not a randomized, controlled study, and patients were assigned to the ED or non-ED group based on their own wishes. In fact, over 50% of the patients in the ED group had been given an ED previously, and this was significantly greater than the non-ED group, in which about 30% of the patients had been given an ED previously. It was speculated that the patients taking an ED previously might have selected keeping ED because they felt the potential risk of relapse. Moreover, the low number of patients who newly received an ED after anti-TNF-α induction may have also been associated with the absence of effect with taking an ED concomitantly. However, it is not feasible for every patient to take an ED, and this may be the ultimate limitation of a clinical study that investigates the effects of an ED. The non-adherence in the ED group from this study indirectly proves this point. Second, this study did not examine patients' quality of life (QOL). Improvement of QOL is an important treatment goal for CD, and analyses of how an ED affects patients' QOL are likely necessary in the future. Third, in the current study, intestinal stenosis, steroid therapy before anti-TNF induction, increased baseline leukocyte, and high CRP level were independent risk factors for clinical relapse. Steroid dependency, greater baseline leukocyte, and CRP level seem to be the predictive factors of LOR as high disease activity.7 Intestinal stenosis, which is the main reason for poor long-term outcome in CD patients, is also reasonable as a predictive factor.32 However, one of the most important factors supposed to be predictors for LOR or dose escalation is anti-TNF inhibitor's serum concentration and antibodies. It was desirable to assess the pharmacokinetics and antidrug antibody of anti-TNF inhibitors for evaluating long-term outcome; however, we could not check them because of the disapproval by Japanese medical insurance.
In conclusion, this investigation was the largest clinical study that prospectively examined whether concomitant ED is beneficial for maintaining remission during anti-TNF-α maintenance therapy. The results did not demonstrate the effectiveness of the concomitant use of an ED, and the added effect of an ED in patients who achieved remission with anti-TNF alone was minimal, indicating that concomitant therapy is not recommended for all patients. However, because non-adherence in the ED group may have affected the study results, further validation with better adherence is necessary to accumulate evidence for ED use. Furthermore, it is essential to revalidate the effects in different conditions, including patients with LOR.
Acknowledgments
The authors would like to thank the following participating institutions and physicians for their cooperation in this study. Osamu Zaha (Department of Gastroenterology, Nakagami Hospital), Kunihiko Aoyagi (Department of Gastroenterology, Fukuoka Red Cross hospital), Hiroyuki Kobayashi (Division of Gastroenterology, Fukuoka Sanno Hospital), Kazuoki Hizawa (Department of Gastroenterology, Kyushu Central Hospital of the Mutual Aid Association of Public School Teachers), Kazuya Makiyama (Gastroenterology Unit, Shunkaikai Medical Corporation Inoue Hospital), and Akira Hokama (Department of Infectious Diseases, Respiratory, and Digestive Medicine, Graduate School of Medicine, University of the Ryukyus). The authors would also like to thank Masakazu Washio (Department of Community Health and Clinical Epidemiology, St. Mary's College) for his advice on statistical analysis for this study.




