Preventive use of a hepatoprotectant against anti-tuberculosis drug-induced liver injury: A randomized controlled trial
Abstract
Background and Aim
Hepatoprotectants are routinely prescribed in China to prevent anti-tuberculosis drug-induced liver injury (ATLI). However, their biological mechanisms have not yet been clearly demonstrated. This study aims to evaluate the preventive effects of Silybum marianum against drug-induced liver injury among tuberculosis patients and to provide clinical guidelines for tuberculosis management in China.
Methods
A randomized controlled trial was performed in Jiangsu, China. Tuberculosis patients were randomly allocated to the experimental group (anti-tuberculosis therapy plus S. marianum capsule) or the control group (anti-tuberculosis therapy plus vitamin C tablet). The primary outcomes were the occurrence of probable and possible ATLI, the peak aspartate aminotransferase/alanine aminotransferase ratio and the maximum altered alkaline phosphatase or gamma-glutamyl transferase.
Results
The final analysis comprised 183 cases in the experiment group and 187 cases in the control group. The risk of developing probable ATLI was not significantly different between the two groups. During the follow-up period, 43.72% of cases in the experiment group and 35.83% of cases in the control group were determined to have possible ATLI (relative risk = 1.23, 95% confidence interval: 0.94–1.54). When using a more strict definition of possible ATLI, the adjusted relative risk (95% confidence interval) was 1.76 (1.14–2.56). The risks of adverse drug reactions, prolonged treatment length, taking second-line tuberculosis drugs, and the clearance of tuberculosis bacteria were similar between the two groups.
Conclusions
No significant preventive effect of silymarin was found for either lowering the risk of liver injury or boosting the positive outcomes. Worse, we even found a potential risk of liver damage caused by the hepatoprotectant.
Introduction
With the 2015 deadline for the Millennium Development Goals approaching,1 the target of a 50% reduction in the prevalence of tuberculosis remains distant. China is experiencing the world's second largest tuberculosis epidemic, contributing to 12% of total global cases.2 To further exacerbate the situation, 5.7% of new cases are estimated to be multi-drug resistant. Among previously treated cases, this proportion has increased to 26%.2
The World Health Organization recommends directly observed therapy, short course to treat tuberculosis. This strategy emphasizes the use of the most effective anti-tuberculosis drugs, and this involves a standard cocktail regimen that includes isoniazid (H), rifampicin (R), pyrazinamide (Z), and ethambutol (E) for 6 to 8 months.3 As most anti-tuberculosis drugs are metabolized in the liver, treatment for tuberculosis can potentially lead to hepatic injury.4, 5 Anti-tuberculosis drug-induced liver injury (ATLI) or anti-tuberculosis drug-induced hepatotoxicity is a common adverse reaction encountered by patients during the treatment period.6, 7 ATLI and other drug-related adverse reactions contribute to the treatment interruption, a prolonged disease course, and unfavorable outcomes.8 Severe ATLI can lead to a fatality rate of 6–12% if the drug use continues after the onset of symptoms.9
To prevent ATLI, physicians in China routinely prescribe hepatoprotectants to tuberculosis patients. However, the biological mechanism and preventive effects of these hepatoprotectants have not yet been clearly demonstrated. Some studies have even found that these hepatoprotectants can cause liver damage.10 The overprescription of hepatoprotectants adds a further financial burden to patients, as these drugs are not included in the China government's “free TB service policy.”11, 12 The rationale behind the use of hepatoprotectants should receive much more attention.13
This study aims to evaluate the effects of the preventive use of the hepatoprotectant Silybum marianum against drug-induced liver injury among tuberculosis patients and to provide clinical guidelines and policy recommendation for tuberculosis management in China.
Methods
Design
A randomized controlled trial was performed to evaluate the preventive effect of the S. marianum capsule, a commonly used hepatoprotectant in China. The Institutional Review Board of Nanjing Medical University approved this project. Written informed consent was obtained from all participants. We registered this study through the Chinese Clinical Trial Register (http://www.chictr.org/cn/); the registration number was ChiCTR-TRC-12002076. We followed the guideline of the Consolidated Standards of Reporting Trials (CONSORT) statement (http://www.consort-statement.org/) to draft the manuscript.
Study subjects
The study was performed in the cities of Zhenjiang and Suzhou, China. Patients who initiated anti-tuberculosis therapy from May 1, 2012 onwards were enrolled in the trial. The case recruitment ended in December 2013. Patients recruited to this study were required to meet the following criteria: (i) older than 12 years; (ii) normal renal function; and (iii) no severe cardiovascular, cerebrovascular, renal, or thyroid disease. The exclusion criteria included the following: (i) patients with disorders directly affecting liver function (e.g. acute hepatitis, cirrhosis of the liver, encephalopathy, or cancer); (ii) patients taking concomitant hepatotoxic medications; and (iii) heavy alcohol intake. The estimated sample sizes were 199 cases in each group (two-sided significance level = 0.95; power = 0.8; percent of unexposed with outcome = 0.1; relative risk [RR] = 2; Fleiss method).
Randomization
Patients were randomly allocated to either group A or B according to their enrollment order. The classification of group A or B referred to the random number generated by the stata software (StataCorp, College Station, TX, USA). Group A was designated as the experiment group, and group B was designated as the control group. The seed for randomization was set at 20 000.
Intervention
Patients assigned to group A (experiment group) received the standard anti-tuberculosis therapy plus the S. marianum capsule (oral, 200 mg, twice a day). Patients assigned to group B (control group) received the standard anti-tuberculosis therapy plus a vitamin C tablet.
Baseline investigation
After enrollment, all patients were interviewed face to face using a questionnaire to collect their sociodemographic characteristics (age, sex, education, occupation, marital status, etc.), behaviors (tobacco smoking and alcohol intake), and clinical features (sputum smear microscopy tests, treatment history, time of symptom onset and diagnosis, history of hepatitis virus infection, and previous liver disease). Before initiating the anti-tuberculosis treatment, each patient was tested for alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBiL), gamma-glutamyl transferase (GGT), and alkaline phosphatase (ALP) using peripheral blood samples.
Follow-up
All patients were followed for at least 2 months (8 weeks) and, if available, until the end of the anti-tuberculosis treatment period (usually 6–8 months). Liver function biomarkers such as liver enzymes and bilirubin were documented. The patients' adherence to the treatment, changes in drug regimen, tuberculosis-related symptoms, and adverse drug reactions were recorded. The primary outcomes were the occurrence of probable and possible ATLI, the peak AST/ALT ratio, and the maximum altered ALP or GGT value. To better elucidate the occurrence of liver injury, we adopted two definitions of ATLI: probable ATLI and possible ATLI. Probable ATLI was based upon the criteria of Hy's law,14 which were defined as a serum ALT level or AST three times greater than the upper limit of normal (ULN) and a serum TBiL level two times greater than the ULN in asymptomatic patients or in those with obvious hepatitis symptoms such as anorexia, nausea or vomiting, or abdominal pain.15 The criteria for possible ATLI were less strict. As there are no standard criteria defining possible ATLI, we used two different definitions to estimate possible liver injury. Possible ATLI-A was defined as those with abnormal level of ALT, AST, or TBiL. Possible ATLI-B was defined as the serum level of ALT or AST greater than two times ULN and the serum level of TBiL greater than two times ULN. The ULNs of ALT, AST, and TBiL vary from 26 to 66 IU/L between studies and across the globe.16 In this study, we adopted the thresholds common among the Chinese population: 40 U/L for ALT (or AST) and 34.2 µmol/L for TBiL. We also applied the AST/ALT ratio as an alternative index to describe the drug-induced hepatotoxicity. We used GGT and ALP as additional biomarkers to evaluate each patient's liver function.17 Secondary outcome measures included the occurrence of adverse drug reactions, prolonged treatment duration, taking second-line drugs, and the clearance of tuberculosis bacteria from the sputum after 2 months of treatment.
Data analyses
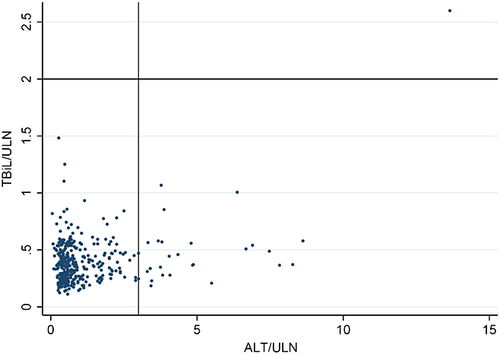
Data were recorded with EpiData 3.1 (The EpiData Association, Odense, Denmark) and analyzed using stata 10.0. The Student's t-test (for continuous variables with normal distribution), Wilcoxon rank-sum test (for continuous variables with skewed distribution), or chi-squared test (for categorical variables) was used to analyze the difference between the experiment and control groups. Changes in ALP and GGT between the two groups were compared using the general linear regression model. To graphically display the peak value of serum ALT and TBiL, we employed the “evaluation of drug-induced serious hepatoxicity” (eDISH) software to draw the plot.18, 19 Patients who experienced elevations in both ALT three times greater than the ULN and TBiL two times greater than the ULN appeared in the right upper quadrant of the eDISH plot. The effect of the S. marianum capsule on preventing ATLI and boosting positive clinical outcomes was estimated using the RR and 95% confidence interval (CI). Considering the potential confounders, we used multiple regression models and stratification analyses to adjust for their confounding effects.
Access to study data
All the authors had access to the study data and reviewed and approved the final manuscript.
Results
General characteristics
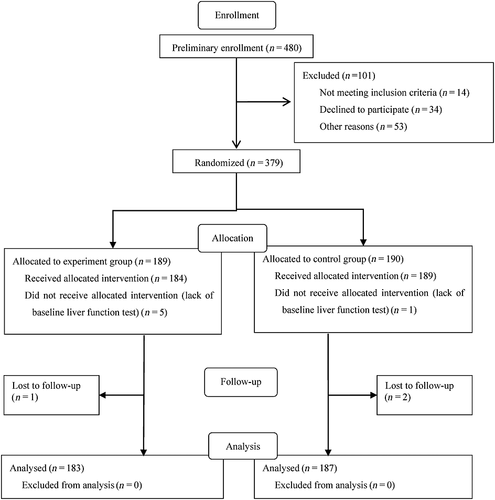
Patient recruitment was initiated on August 1, 2012 and ended on December 31, 2013. In total, 480 tuberculosis patients were assessed for eligibility for the study, and 379 patients were ultimately enrolled into the trial. Among them, 189 were randomly assigned to group A (experiment group) and 190 to group B (control group). Because of the lack of detailed baseline tests of liver function, six patients (five in the experiment group and one in the control group) were excluded after further evaluation. The final analysis contained 183 cases in the experiment group and 187 cases in the control group. The flow chart of the study is shown in Figure 1. There was no significant difference in the distribution of basic characteristics between the two groups at the baseline investigation except for age (Table 1). More than 73% patients were followed up for over 8 weeks. The median follow-up time was 60 days.
| Variables | Total, n (%) | Experiment group, n (%) | Control group, n (%) | χ2 | P |
|---|---|---|---|---|---|
| n = 370 | n = 183 | n = 187 | |||
| Age (years) | |||||
| <41 | 180 (48.65) | 72 (39.34) | 108 (57.75) | — | — |
| ≥41 | 190 (51.35) | 111 (60.66) | 79 (42.25) | 12.55 | <0.001 |
| Sex | |||||
| Men | 274 (74.05) | 129 (70.49) | 145 (77.54) | — | — |
| Women | 96 (25.95) | 54 (29.51) | 42 (22.46) | 2.39 | 0.122 |
| Previous tuberculosis history | |||||
| No | 309 (83.24) | 149 (81.42) | 160 (85.08) | — | — |
| Yes | 61 (16.76) | 34 (18.58) | 27 (14.92) | 1.15 | 0.283 |
| Sputum smear test | |||||
| Positive | 123 (33.42) | 63 (34.62) | 60 (32.26) | — | — |
| Negative | 247 (66.58) | 120 (65.38) | 127 (67.74) | 0.23 | 0.633 |
| Multi-drug resistant tuberculosis | |||||
| No | 360 (97.3) | 180 (98.36) | 180 (96.26) | — | — |
| Yes | 10 (2.7) | 3 (1.64) | 7 (3.74) | 1.56 | 0.212 |
| Chronic diseases | |||||
| No | 283 (76.49) | 137 (74.86) | 146 (78.07) | — | — |
| Yes | 87 (23.51) | 46 (25.14) | 41 (21.93) | 0.53 | 0.466 |
| Diabetes | |||||
| No | 336 (90.81) | 168 (91.80) | 168 (89.84) | — | — |
| Yes | 34 (9.19) | 15 (8.20) | 19 (10.16) | 0.43 | 0.513 |
| Hypertension | |||||
| No | 346 (93.51) | 169 (92.35) | 177 (94.65) | — | — |
| Yes | 24 (6.49) | 14 (7.65) | 10 (5.35) | 0.81 | 0.369 |
| Abnormal baseline liver function | |||||
| No | 320 (86.49) | 159 (86.89) | 161 (86.10) | 0.05 | 0.824 |
| Yes | 50 (13.51) | 24 (13.11) | 26 (13.90) | — | — |
Effect of the Silybum marianum capsule on preventing ATLI
Only one patient developed probable ATLI (Table 2). This patient appeared in the right upper quadrant of the eDISH plot in the Figure 2. The risk of developing probable ATLI was not significantly different between the experiment and control groups (P = 0.311).
| Variables | Experiment group | Control group | t | P | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (inter-quartile) | Mean (SD) | Median (inter-quartile) | |||
| ALT (U/L) | 21.56 (15.69) | 17 (13–26) | 22.01 (16.56) | 18 (13–27) | 0.26 | 0.792 |
| AST (U/L) | 27.34 (12.05) | 25 (20–32) | 26.42 (12.47) | 24 (19–31) | 0.72 | 0.470 |
| AST/ALT ratio | 2.13 (6.17) | 1.4 (1.1–2) | 1.56 (1.35) | 1.3 (1–1.7) | 1.25 | 0.214 |
| ALP (U/L) | 90.53 (36.95) | 85 (68–105) | 92.01 (31.85) | 87 (72–103) | 0.36 | 0.723 |
| GGT (U/L) | 27.95 (31.53) | 19 (14–27) | 31.31 (31.15) | 21 (15–34) | 0.89 | 0.373 |
| TBiL (µmol/L) | 12.10 ( 6.59) | 10.4 (7.8–14.75) | 11.12 (5.41) | 10 (8.1–13) | 1.38 | 0.168 |
- ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; TBiL, total bilirubin.
During the follow-up period, 147 cases were categorized as possible ATLI-A, including 80 cases in the experiment group and 67 cases in the control group. None of them had experienced an elevation in serum ALT level exceeding 10 times ULN. The risk of developing possible ATLI-A was slightly higher in the experiment group, but the difference was not significant (RR = 1.22, 95% CI: 0.95–1.57, P = 0.121). After adjusting for potential confounders such as age, sex, and baseline liver function test level, the risk was not significantly altered (adjusted RR = 1.23, 95% CI: 0.94–1.54) (Table 3). Stratification analysis also could not demonstrate the positive effect of taking the S. marianum capsule in preventing the occurrence of ATLI-A (Table 4).
| Variables | Experiment group | Control group | cRR (95% CI) | P | aRR (95% CI)† |
|---|---|---|---|---|---|
| Probable ATLI | |||||
| No | 182 (99.45) | 187 (100) | — | — | — |
| Yes | 1 (0.55) | 0 (0) | — | 0.311 | — |
| Possible ATLI-A | |||||
| No | 103 (56.28) | 120 (64.17) | 1 | — | 1 |
| Yes | 80 (43.72) | 67 (35.83) | 1.22 (0.95–1.57) | 0.121 | 1.23 (0.94–1.54) |
| Possible ATLI-B | |||||
| No | 137 (74.86) | 159 (85.03) | 1 | — | 1 |
| Yes | 46 (25.14) | 28 (14.97) | 1.68 (1.11–2.41) | 0.010 | 1.76 (1.14–2.56) |
| Peak AST/ALT ratio | |||||
| Normal | 63 (34.43) | 92 (49.20) | 1 | — | 1 |
| Abnormal | 120 (65.57) | 95 (50.80) | 1.29 (1.08–1.53) | 0.004 | 1.20 (0.98–1.39) |
| Occurrence of adverse reactions | |||||
| No | 114 (62.30) | 122 (65.24) | 1 | — | 1 |
| Yes | 69 (37.70) | 65 (34.76) | 1.09 (0.83–1.42) | 0.556 | 1.07 (0.79–1.37) |
| Prolonged treatment length | |||||
| No | 115 (63.89) | 125 (67.57) | 1 | — | 1 |
| Yes | 65 (36.11) | 60 (32.43) | 1.11 (0.84–1.48) | 0.459 | 1.02 (0.74–1.35) |
| Taking the second-line drugs | |||||
| No | 136 (74.32) | 144 (77.01) | 1 | — | 1 |
| Yes | 47 (25.68) | 43 (22.99) | 1.12 (0.78–1.60) | 0.547 | 1.02 (0.68–1.46) |
| Sputum smear test after 2 months treatment | |||||
| Negative | 176 (96.17) | 176 (94.12) | 1 | — | 1 |
| Positive | 7 (3.83) | 11 (5.88) | 0.65 (0.26–1.64) | 0.358 | 0.60 (0.23–1.54) |
- † Adjusted for age, sex, and baseline liver function.
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; ATLI, anti-tuberculosis drug-induced liver injury; CI, confidence interval; RR, relative risk.
| Variables | Possible ATLI-A, n (%) | χ2 | P | Possible ATLI-B, n (%) | χ2 | P | ||
|---|---|---|---|---|---|---|---|---|
| Experiment group | Control group | Experiment group | Control group | |||||
| Age (years) | ||||||||
| <41 | 30 (41.67) | 32 (29.63) | 2.77 | 0.096 | 15 (20.83) | 16 (14.81) | 1.10 | 0.295 |
| ≥41 | 50 (45.05) | 35 (44.30) | 0.01 | 0.919 | 31 (27.93) | 12 (15.19) | 4.28 | 0.040 |
| Sex | ||||||||
| Men | 59 (45.74) | 55 (37.93) | 1.71 | 0.191 | 36 (27.91) | 22 (15.17) | 6.63 | 0.010 |
| Women | 21 (38.89) | 12 (28.57) | 1.11 | 0.291 | 10 (18.52) | 6 (14.29) | 0.31 | 0.581 |
| Previous tuberculosis history | ||||||||
| No | 63 (42.28) | 60 (37.50) | 0.74 | 0.391 | 38 (25.50) | 25 (15.63) | 4.64 | 0.031 |
| Yes | 17 (50.00) | 7 (25.93) | 3.65 | 0.056 | 8 (23.53) | 3 (11.11) | 0.84‡ | 0.360‡ |
| Sputum smear test | ||||||||
| Positive | 25 (39.68) | 30 (50.00) | 1.32 | 0.25 | 15 (23.81) | 12 (20.00) | 0.26 | 0.610 |
| Negative | 55 (45.83) | 37 (29.13) | 7.36 | 0.007 | 31 (25.83) | 16 (12.60) | 7.01 | 0.008 |
| Multi-drug resistant tuberculosis | ||||||||
| No | 78 (43.33) | 64 (35.56) | 2.28 | 0.131 | 43 (24.43) | 25 (14.29) | 5.78 | 0.016 |
| Yes | 2 (66.67) | 3 (42.86) | — | 0.079† | 2 (50.00) | 1 (12.50) | 0.50‡ | 0.480‡ |
| Chronic diseases | ||||||||
| No | 59 (43.07) | 52 (35.62) | 1.65 | 0.200 | 32 (23.36) | 21 (14.38) | 3.74 | 0.053 |
| Yes | 21 (45.65) | 15 (36.59) | 0.73 | 0.391 | 14 (30.43) | 7 (17.07) | 2.11 | 0.146 |
| Abnormal liver function | ||||||||
| No | 61 (38.36) | 49 (30.43) | 2.23 | 0.135 | 33 (20.75) | 19 (11.80) | 4.71 | 0.030 |
| Yes | 19 (79.17) | 18 (69.23) | 0.64 | 0.424 | 13 (54.17) | 9 (34.62) | 1.94 | 0.164 |
- † Fisher's exact test.
- ‡ Yates' adjusted.
When using a stricter definition of possible ATLI, 74 cases were detected as possible ATLI-B, including 46 cases in the experiment group and 28 cases in the control group. The risk of developing possible ATLI-B was significantly higher in the experiment group (RR = 1.68, 95% CI: 1.11–2.41, P = 0.010; adjusted RR = 1.76, 95% CI: 1.14–2.56) (Table 3). Stratification analysis showed that the risk of taking the S. marianum capsule was more significant among older patients, men, new cases, sputum smear negative cases, and patients with normal baseline liver function, although the heterogeneity was not significant (Table 4).
We further compared the peak AST/ALT ratio between the two groups. In all, 215 patients experienced an AST/ALT ratio in excess of the ULN, including 120 cases in the experiment group and 95 cases in the control group. Patients in the experiment group had a significantly higher abnormal AST/ALT ratio (RR = 1.29, 95% CI: 1.08–1.53, P = 0.004). After adjusting for age, sex, and baseline liver function test level, the RR (95% CI) was 1.20 (0.98–1.39) (Table 3).
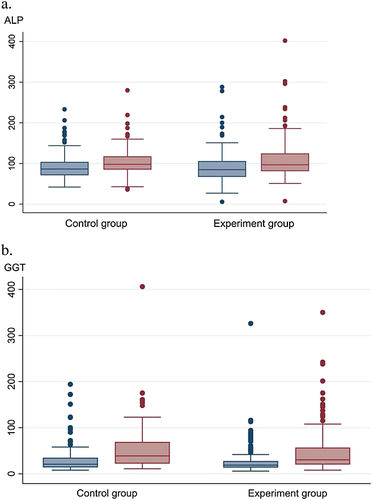
The maximum increased ALP was slightly higher in the experiment group (mean: 18.44, median: 13, inter-quartile: 3–30) than in the control group (mean: 11.08, median: 10.5, inter-quartile: 0–24.5) but without a difference that was significant (Z = −1.516, P = 0.130) (Fig. 3). The maximum altered GGT was also similar between the two groups (Z = 0.377, P = 0.707) (Fig. 3).
 , Baseline ALP;
, Baseline ALP;  , Maximum ALP. (b)
, Maximum ALP. (b)  , Baseline GGT;
, Baseline GGT;  , Maximum GGT.
, Maximum GGT.Effect of the Silybum marianum capsule in preventing adverse drug reactions
We inquired about the occurrence of adverse drug reactions such as rash, vomiting/nausea, allergy, blurry vision, leucopenia, arthralgia, hearing loss, and peripheral neuropathy. The proportion of these adverse drug reactions was 37.71% in the experiment group and 34.76% in the control group, and the difference was not significant between the two groups (P = 0.556) (Table 3).
Effect of the Silybum marianum capsule on treatment duration and outcomes
We collected data concerning the duration of anti-tuberculosis treatment and the use of second-line drugs. We did not find that taking hepatoprotectants could prevent patients from prolonging their treatment course (P = 0.459) or reduce the use of second-line drugs (P = 0.547). After adjusting for age, sex, and baseline liver function test level, the adjusted RR (95% CI) was 1.02 (0.74–1.35) and 1.02 (0.68–1.46), respectively (Table 3). We further compared the sputum bacteria conversion rate. After 2 months of treatment, there were 3.83% sputum smear positive cases in the experiment group and 5.88% in the control group (P = 0.358). The effect of the S. marianum capsule on the clearance of tuberculosis bacteria from the sputum was not statistically significant (Table 3).
Discussion
Physicians in China commonly prescribe the S. marianum hepatoprotectant capsule to prevent liver injury during anti-tuberculosis treatment episode. However, its protective effect could not be demonstrated in our study. Worse, we even found that hepatoprotectants could possibly cause liver damage. To our knowledge, this is the first randomized controlled trial to explore the protective effect of silymarin for lowering the risk of liver injury induced by anti-tuberculosis drugs. Our findings may serve as a wake-up call for the rational use of hepatoprotectants.
One of the most common forms of hepatitis that occurs among tuberculosis patients is ATLI.20 This condition has been emerging as a threat to tuberculosis control in China, although limited data are available at the population level.21 As ATLI can only be diagnosed without any other causes of liver injury, it is frequently underdiagnosed,22 although high rates of hepatotoxicity have been reported worldwide.23-25 In China, the reported incidence of ATLI varies from 2.6% to 7.5%.21, 26 In a population-based prospective study by Shang et al., 106 of 4304 tuberculosis patients receiving directly observed therapy, short course treatment developed ATLI, with a cumulative incidence rate of 2.55% (95% CI: 2.04–3.06%).21 This incidence further led to a 9.25-fold risk of unsuccessful treatment outcomes and a 2.11-fold risk of a prolonged intensive treatment phase.21
Silymarin, which is the main active compound in the S. marianum capsule, has been used as a hepatoprotectant since antiquity.27 However, the effectiveness and safety of these hepatoprotectants have long been under debate.10, 28, 29 Some clinical studies have concluded a positive effect, recommending routine prescription for tuberculosis patients.30 In contrast, however, other studies have reported an increased risk of developing liver injury when using hepatoprotectants in tuberculosis patients.19 Most previous studies, especially those conducted decades ago, have suffered from the shortcomings noted in other trials of herbal medications, including small sample size, lack of appropriate randomization, different periods of treatment, ill-defined patient population, lack of etiology or severity of disease, and ignoring potential confounders.31 To overcome these limitations and obtain better insight into the preventive effect of silymarin, we performed a randomized controlled trial in a Chinese population.
Based on our study results, no significant protective effect was observed in the prevention of tuberculosis patients from developing either possible or probable ATLI. Considering that ATLI may manifest clinically with symptoms of jaundice, fatigue, nausea, vomiting, and abdominal pain and these nonspecific symptoms can sometimes be an initial indictor of disease; we also explored the effect of preventing these adverse drug reactions, but the findings were not statistically significant. Until now, there has been no scientific evidence to support the hypothesis that hepatoprotectants can lower the risk of hepatotoxicity imposed by anti-tuberculosis drugs.26 Hepatoprotectants are not free under the current National Tuberculosis Control Program in China. Overprescription of these medications to patients, regardless of the risk, can increase patients' financial burden.11
There are several limitations to this study. First, most patients were followed for 2 months. Although ATLI usually develops within the first weeks after initiating the anti-tuberculosis treatment,32 we may have missed some delayed liver injury cases. Second, the study group exhibited an imbalance in age between the control and experiment groups. This imbalance might have resulted from physicians' exclusion of older patients from the experiment group. To avoid confounding by age and other factors, we used a multivariate logistic regression model together with the stratification analysis to overcome this issue. Third, although we used multiple biomarkers including ALT, AST, TBiL, GGT, and ALP to identify ATLI, we cannot guarantee the detection of liver injury at a very early stage.33 Fourth, this study was open labeled. Information bias was unavoidable because of the lack of blinding. Fifth, we used vitamin C as the control rather than a placebo. Previous reports have shown the potential effects of vitamin C in anti-hepatotoxicity,34 which may lower the preventive effect of silymarin in ATLI. Vitamin C is inexpensive and easy to access. If something like vitamin C has a similar liver protective effect, why use expensive hepatoprotectants that impose a heavy economic burden on patients?
In conclusion, our study showed no significant preventive effect of silymarin either in lowering the occurrence of liver injury or in boosting positive treatment outcomes for patients receiving anti-tuberculosis therapy. Worse, we even found that the hepatoprotectant may cause liver damage. The decision to use hepatoprotectants preventively against ATLI should be made cautiously by physicians.
Acknowledgements
The National Natural Science Foundation of China (81473027), Qing Lan Project (2014), Six Talent Peaks Project in Jiangsu Province (2014-YY-023), Philosophy and Social Science Research Fund of Jiangsu College (2014SJB164), Jiangsu Natural Science Foundation (BE2012694), Zhenjiang Key Lab for Drug Resistant Tuberculosis (SS2013018), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) supported this study. The funders had no role in the study design, data collection and analysis, decision to publish, or in the preparation of the manuscript.




