Use of a phage cocktail to reduce the numbers of seven Escherichia coli strains belonging to different STEC serogroups applied to fresh produce and seeds
Abstract
The aims of this research were to evaluate the effectiveness of a phage cocktail at reducing seven Shiga toxigenic Escherichia coli (STEC) serogroups on different food matrixes: mung bean sprouts (MBP), lettuce, and mung bean seeds (MBS) and to test the phage cocktail effectiveness to reduce E. coli O157 on Romaine and iceberg lettuce. To study the effect of the type of food matrix on the STEC phage cocktail effectiveness, a mixture of seven highly sensitive STEC strains designated as phage propagation strains (PPS) were used to adulterate Romaine lettuce, MBP, and MBS matrixes at a concentration of 105 logs CFU/g. A subsample of the treated MBS was germinated to assess STEC survival. Recovered STEC strains were confirmed using latex agglutination and PCR. To test the phage cocktail effectiveness to reduce E. coli O157:H7 on Romaine and iceberg lettuce, a mixture of four STEC strains (different than phage propagation strains, non-PPS) at both low (103 CFU/g) and high (105 CFU/g) concentrations were used to spike the samples in scaled up trials for the purpose of potential commercialization. Phage treatments including a combination of STEC phage cocktail and chlorinated water treatment were then applied to lettuce in a simulated scaled-up trial. STEC was assessed on the treated samples at different storage time and temperatures (0, 24, 48, and 72 hr at 2, 10 and 25°C). In the food matrix trial, the combination of STEC phage cocktail and chlorinated water-reduced PPS (p < 0.001) STEC on lettuce by 2.1 log10 CFU/g and on MBP by 2.2 log10 CFU/g. However, isolates from all 7 STEC serogroups remained viable after phage treatment in both lettuce and MBP; particularly those associated with serogroup O111, O121, O103, and O145, while only a few colonies of serogroup O26, O45, and O157 were detected. Lettuce adulterated with low levels of 4 non-PPS E. coli O157:H7 (103 CFU/g) achieved a reduction of 2.6–3.2 logs. While a reduction 1.7–2.3 logs was achieved by the phage cocktail when lettuce was inoculated with 105 CFU/g. Overall phage performance was more effective at 2 and 10°C and improved over storage time up to 72 hr. However, for MBS, the phage cocktail was not able to kill any of the STEC populations as all of them recovered during germination.
1 INTRODUCTION
Shiga toxigenic Escherichia coli (STEC) is widely recognized as major causes of foodborne illness (Luna-Gierke et al., 2014; Rangel et al., 2005). E. coli O157 was the first E. coli serogroup associated with Shiga-toxigenic-related foodborne outbreaks. However, more than 50 non-O157 STEC serogroups have been reported to be responsible for human illness. In North America, the most commonly reported non-O157 STEC are O26, O145, O103, O111, O121 and O145. These STEC serogroups, along with O157, are known as the “top seven” (Fratamico et al., 2017; Heymann, 2008; Luna-Gierke et al., 2014). From 2011 to 2021, the Centers for Disease Control and Prevention (CDC) indicated that pathogenic E. coli caused 34 outbreaks, 1,420 cases, and 12 deaths in the United States (CDC, 2022). From these recalls and outbreaks occurring in the last decade, 4 of them have been linked to Romaine lettuce, 5 of them have been linked to leafy greens, and 4 of them were linked with raw clover and alfalfa sprouted seeds. In January 2019, a foodborne outbreak linked to E. coli O157:H7 on Romaine lettuce was reported, causing illness in 167 individuals, of which 85 were hospitalized and 15 developed the hemolytic uremic syndrome. In April 2020, E. coli O103 in clover sprouts caused illness in 51 people, with 3 hospitalizations (CDC, 2020). This series of outbreaks have impacted the produce industry with implications for international trade as the Canadian Food Inspection Agency requested suppliers to certify that E. coli concentrations were below detectable levels or provide proof that the imported lettuce did not originate from counties in the Salinas Valley of California, USA (CFIA, 2020).
The consumption of fresh produce is steadily increasing as consumers seek to decrease meat consumption and increase their intake of dietary fiber (Tobler et al., 2011). As the demand for products has grown, foodborne illness outbreaks have been increasingly linked to contaminated fresh produce (Lynch et al., 2009). This is particularly concerning and difficult to prevent as leafy greens and sprouts are usually consumed raw. Chemical disinfectants suitable for fresh produce, such as chlorinated water, have been shown to be insufficient to eliminate STEC on fresh produce and sprouts (Boyacioglu et al., 2013).
Bacteriophages can control a variety of pathogenic bacteria associated with vegetable and meat products, with some strains approved for use both in Canada and the United States (Moye et al., 2018; Sharma, 2013). Lytic phages kill bacteria by commandeering their metabolic machinery to produce phage progeny that is released through the lysis of the host cell (Abedon, 2011a, 2011b). Phages are highly specific to unique surface lipopolysaccharides and proteins on the surface of host bacteria and are generally considered harmless to humans, animals, or plants (Sharma et al., 2008). Regarding STEC, there are commercial phage cocktails (EcoShield and Finalyse) approved for their use in food (Jassim & Limoges, 2017). EcoShield showed effectiveness in lettuce by reducing 2.22 log CFU/cm2 of E. coli O157:H7 when a phage cocktail was sprayed on lettuce (Ferguson et al., 2013). Spraying cattle with Finalyse is approved to reduce E. coli O157:H7 contamination before slaughter; the maximum reduction was 1.3 logs at 1 hr (Mies & Davis, n.d.). However, there are still gaps of information regarding phage effectiveness on food products, specifically addressing factors such as temperature, delivery method, contact time, and food type, information on the impact of these variables on phage effectiveness are urgently needed (Hong et al., 2016; Soffer et al., 2017; Zhang et al., 2019). For instance, fresh produce is processed and stored at low temperatures to maintain freshness and extend shelf-life. Low temperature inhibits bacterial growth but may also limit the effectiveness of phages as replication requires the host to be metabolically active (Obeso et al., 2008). However, there is evidence that phages can reduce host cell viability even without the release of phage progeny (Abedon, 2011a, 2011b). Some phages may also remain infective even within the cold chain, although the mechanisms whereby these phages cause host cell death is unclear (Huang et al., 2018). New natural and organic interventions to reduce foodborne pathogens are needed by the fresh produce industry, thus a new phage product capable to effectively reduce the risk of “top 7” STEC contamination will be a novel post-harvest intervention in fresh produce industries. Although phages have been shown to control E. coli O157:H7 on produce, there is limited information on the ability of phages to target other STEC serogroups and the effect of the food matrix, temperature, and storage on phage efficacy. The objectives of this research were to: (i) to test individual phage efficacy against STEC strains at various concentrations in an in vitro format; (ii) Evaluate the food matrix effect on a STEC phage cocktail effectiveness to reduce the “top 7” STEC using phage propagation strains (PPS) on Romaine lettuce, mung bean sprouts (MBP), and mung bean seeds (MBS) at 2, 10, and 25°C for 0, 24, 48, and 72 hr; (iii) Compare the effectiveness of a phage cocktail against STEC in combination with chlorinated water; (iv) and to test STEC phage cocktail effectiveness to reduce non-PPS O157:H7 on Romaine and iceberg lettuce at low (103 CFU/g) and high (105 CFU/g) inoculation levels.
2 MATERIALS AND METHODS
2.1 STEC strains and phage propagation
Seven Shiga toxigenic E. coli strains were used to propagate the phages, and they are referred to in this paper as phage propagation strain (PPS) (Table 1). These PPS strains were also used to test the effect of the food matrixes (lettuce, sprouts and seeds) on the phage cocktail's effectiveness in reducing STEC. It was hypothesized that by using PPS strains, which are highly susceptible to the phages, it could be possible to determine the food matrix effect on phage performance. On the other hand, to determine STEC phage cocktail efficacy on lettuce, four E. coli O157:H7 strains not used for phage propagation (non-PPS) (1931 from hamburger, 1934 from beef, 162-84 from humans and CO283 from cattle feces) were used. All STEC strains and phages were obtained from the culture collection of the University of Manitoba, Food & Human Nutritional Sciences, Winnipeg, Manitoba and the Alberta Agriculture and Agriculture and Agri-Food Canada in Lethbridge, AB, Canada. Bacterial cultures were maintained in Trypticase soy broth, TSB (BD Difco, Franklin Lakes, NJ) containing 15% glycerol and stored at −80°C. Each culture was thawed and streaked on to fresh TSB and MacConkey agar, MAC (Criterion Dehydrated Culture Media, Hardy Diagnostics, Santa Maria, CA) followed by incubation at 37°C for 24 hr. Bacterial suspensions were prepared by transferring one colony from each STEC serogroup into 10 ml TSB (BD Difco) and incubated overnight at 37°C to a density of 108 CFU/ml. The cultures were diluted as needed for phage propagation (104 CFU/ml), in an in vitro microplate assay (107 CFU/ml), food matrix trials, and scaled-up experiments (105 CFU/ml). The concentration of the mixture/individual bacteria culture was tested by reading OD at 600 nm wavelength and confirmed by enumeration on McConkey agar plates.
| Phage official designation | Phage type | Phage family | STEC host serotypes used for phage propagation | STEC strains used for phage propagation (PPS) | STEC target (s) serogroup (s) | Phage titer (PFU/ml) |
|---|---|---|---|---|---|---|
| vB_EcoM_AYO26A | T4 | Myoviridae | O26:H11 | EC19960464 | O26, O103, O145, and O157 | 7.55 × 108 |
| vB_EcoS_AXO45B | T5 | New taxonomy Tequintavirus | O45:H2 | EC19940040 | O45 | 5.7 × 109 |
| vB_EcoS_AXO103A | T5 | Siphoviridae | O103:H2 | EC20010670 | O26 and O103 | 2.3 × 109 |
| vB_EcoM_AXO111A | Vil | Myoviridae | O111:NM | EC20030053 | O26, O111, and O121, | 3.5 × 108 |
| vB_EcoM_AXO121A | rV5 | Myoviridae | O121:H19 | EC20040083 | O103, O121, and O157 | 1.7 × 1010 |
| AYO145A | O1 | Myoviridae | O145:NM | EC20020231 | O145 | 2.5 × 1010 |
| vB_EcoS_AKFV33 | T5 | Siphoviridae | O157:H7 | R508 | O157 | 1.6 × 1011 |
Phages used for this study were isolated from beef cattle feces from previous studies by Alberta Agriculture and Forestry in Lethbridge (Table 1) (Wang et al., 2015). A total of 37 lytic phages with activity against serotypes O26:H11, O45:H2, O103:H2, O111:NM, O121:H19, O145:NM, and O157:H7 were isolated. Phage genome size was measured by pulsed-field gel electrophoresis and ranged from 38 to 177 kb, and the phages were assigned to families based on morphology as visualized using transmission electron microscopy and genomic sequencing. Host range and lytic capability of the phages were tested using a microplate phage virulence assay at 2, 10, and 25°C. Phage candidates used in this study were selected based on their: (i) lytic activity, (ii) effectiveness over a broad temperature range, (iii) cross-infectivity against multiple strains of the targeted 7 STEC and the absence of unwanted genes (antimicrobial resistance and virulence). Phages were individually propagated as previously described (Niu et al., 2009) from phage master stock and the phage concentration for each working solution was quantified by soft agar overlay plaque assay (Kropinski et al., 2009). Propagated phages exhibiting titers ranging from 108 to 1010 PFU/ml were used in this study. The phage cocktail consisted of seven phages (Table 1) and was prepared by mixing 20 ml of each phage filtrate (108–1010 PFU/ml) in a sterile bottle prior to use. The microplate phage virulence assay, as described in the following sections, was then used to measure the multiplicity of infection (MOI) of the phage cocktail.
2.2 In vitro microplate phage virulence assay
The microplate phage virulence assay was used to determine the lytic activity of STEC phages (Niu et al., 2009). Seven phages were combined and tested as a cocktail against each E. coli PPS. Each phage was also tested individually against its PPS (Table 1). Briefly, 20 μl of each lytic phage candidate or phage cocktail were serially diluted (10−1–10−8) individually in a 96-well microplate (Thermo Fisher Scientific) containing 180 μl TSB supplemented with 10 mM MgSO4. (1%) (mTSB) per well. Wells were inoculated with 20 μl of 107 CFU/ml bacterial culture (OD600 = 1.1–1.2) of each STEC serogroup. Positive controls with STEC cultures and negative control with only mTSB and mTSB plus STEC phage were included. The microplate was incubated at 2, 10, and 25°C for 5 hr. After incubation, the microplate (25°C) was visually observed for clear wells, and then a microplate reader (BioTek Instruments, Winooski, VT) was used to measure the optical density (OD) at 630 nm of each well. The MOI for each phage-host assay was calculated based on three repetitions with six observations in total (MOI = initial phages concentration; PFU/ml) of the highest dilution showing lytic activity (highest dilution with clear wells)/initial number of bacteria host concentration (CFU/ml) (Zhang et al., 2019). The experiment was repeated three times, the data were collected from 12 observations for each STEC serogroup at three temperatures.
The microplate virulence assay was effective at showing the effectiveness of bacteriophages to reduce STEC based on the optical density reading at 25°C. However, STEC serogroups are mesophilic and are not able to grow or grow slowly at 2 and 10°C, thus clear wells were not conclusive. To confirm the STEC survival from microplates incubated at 2 and 10°C, spot plate technique was performed as previously described (Zhang et al., 2019). Briefly, 2 μl of sample from each well from the microplate was transferred to MacConkey agar. Plates were incubated at 37°C for 24 hr and colonies observed were considered as conformation of STEC survival. However, spot plate method only confirmed if STEC was completely killed by the phage at different concentrations; STEC reduction after phage treatment was investaged during in vitro trials in the following sections. Phages were not removed to test the effectiveness of remaining phages particles on STEC during storage.
2.3 Effectiveness of the STEC phage cocktail on different produce matrixes controlling STEC strains used for phage propagation (PPS)
Romaine lettuce heads and fresh MBP were purchased from a local grocery store and kept at 4°C before use. Outer leaves and the stems were removed from 3 cm the base of the head, and then lettuce leaves were peeled from the head. Whole seed sprouts with cotyledon, stem, and root were selected. On the other hand, all MBS (Mumm's Sprouting Seeds, Shellbrook, SK, Canada) used in this study were visually inspected to ensure they were not damaged.
The lettuce leaves, MBP and MBS were washed three times with tap water pre-chilled at 10°C and excess water was removed using a sterilized salad spinner. They were packaged in plastic bags and kept at 4°C, and MBS were kept at room temperature until needed.
Romaine lettuce, MBP, and MBS matrixes were spiked with a STEC bacterial mixture prepared with PPS strains. The PPS-STEC was delivered by immersion since it was found to be a more efficient method based on pre-testing data (data not shown).
In the food matrix experiment, 600 g lettuce, 300 g MBP, and 30 g of MBS were collected for each trial. Produce was then inoculated by immersion in a 105 CFU/ml of the STEC bacterial mixture for 30 min and excess liquid was removed using a sanitized salad spinner. Prior to treatment with chlorinated water or the phage cocktail, 10 g of leaves, MBP, and MBS that were only inoculated with the STEC mixture were collected and placed into a Whirl-Pak bag as a positive control. After inoculation, samples were then assigned in duplicate to one of three treatments: T1: STEC inoculated (adulterated samples) + chlorinated water wash. T2: STEC inoculated + phage cocktail. T3: STEC inoculated + chlorinated water + rinse + phage cocktail. Commercial liquid bleach containing sodium hypochlorite (Old Dutch®, Montreal, QC, Canada) was used to prepare chlorinated water at 150 (for lettuce and MBP) and 1,000 (for MBS) ppm. The concentration of the chlorinated water was measured using water quality test strips (HACH Co. ETS, Elkhart, IN). For treatments with chlorinated water (T1, T3), lettuce leave and MBP were washed with 10°C chlorinated water (150 ppm), followed by rinsing three times with chilled water to remove residual chlorine, a procedure that mimicked industry practice. MBS were immersed in 1,000 ppm chilled chlorinated water for 3 min and rinsed with chlorine-free water at room temperature. For treatments with phage cocktail (T2, T3), lettuce and MBP were immersed in the STEC phage cocktail (>108 PFU/ml) for 15 min while MBS were immersed in the phage cocktail for 1 hr at room temperature.
After treatment, 10 g of lettuce and MBP from each treatment was collected and placed in sterile bags (Seward Laboratory Systems, Inc., Bohemia, NY) and stored at 2, 10, and 25°C for 1, 24, 48, and 72 hr. MBS were dried in a biosafety cabinet at room temperature for a minimum of 1 hr and then stored at 25°C for 72 hr in a dark room. Half portion of the treated MBS were germinated to lab germinated (LG) sprouts by soaking in sterile water for 24 hr and then sprayed 2 times/day with sterilized distilled water for 3 days at 37°C. At the end of each storage period, the lettuce, MBP, MBS, and LG sprouts were placed in Whirl-Pak bags with 90 ml BPW and homogenized using a stomacher for 2 min (Intersciences, Inc., Markham, ON, Canada). For treatments with chlorinated water (T1, T3), 90 ml of D/E Neutralizing Broth (BD Difco) were substituted for BPW to neutralize the chlorine. For bacteria enumeration, 10-fold serial dilutions (10−1–10−6) were prepared for each sample type and plated on Rainbow and MacConkey agar plates. Plates were incubated at 37°C for 24 hr. Colonies from MacConkey plates were counted to determine the total reduction in STEC caused by treatments. To estimate the reductions and survivability for each STEC serogroup, colonies with different colors from Rainbow plates were counted. Five colonies from each color were randomly picked and tested with latex agglutination and confirmed by specific multiplex PCR for their serogroups (DebRoy et al., 2011). Immunomagnetic separation (IMS) was used to recover STEC strains below detectable levels following manufacturer recommendations (Dynabead; Dynal Wirral, UK). All experiments were performed as independent trials and replicated three times.
2.4 Targeting O157:H7 reduction on Romaine and iceberg lettuce using non-phage prorogation O157 strains (non-PPS) at high and low inoculation levels
Data obtained from preliminary experiments using PPS strains showed that the STEC phage cocktail was more effective at reducing E. coli O157:H7 strain on Romaine lettuce when compared with MBP and MBS. It was also challenging to differentiate STEC serogroups on Rainbow agar, and it required heavy work using serological test and PCR. Therefore, lettuce was chosen as the food matrix to further test the effectiveness of the STEC phage cocktail. For this purpose, a mixture of four strains of non-PPS E. coli O157:H7 at high (105 CFU/g) and low (103 CFU/g) concentrations was used. In this experiments we included Romaine and iceberg lettuce as they are the most popular lettuce types for consumption in North America, 62% of the lettuce production in the United States is the crisp head type (iceberg) (Mou, 2008). Romaine lettuce is often associated with outbreaks, so there is the question regarding potential differences in the two leafy greens concerning STEC predisposition for colonization. It is important to mention that the concentration of STEC contamination found in fresh produce industry production is likely to be lower than 5 logs, probably in the range of 2–3 logs CFU/g (Althaus et al., 2012). Thus, to test the capability of this phage cocktail at reducing STEC high and low STEC concentrations, 105 and 103 CFU/g of E. coli O157:H7 were chosen to be inoculated on lettuce samples. STEC phage cocktail and lettuce were prepared as described in the previous sections. Commercial liquid bleach containing sodium hypochlorite was used to prepare chlorinated water at 150 ppm (measured using water quality test strips). The phage cocktail was applied through immersion. Four treatments were applied to lettuce leaves samples: T1: STEC inoculated (adulterated samples) + chlorinated water wash. T2: STEC inoculated + phage cocktail. T3: STEC inoculated + chlorinated water + rinse + phage cocktail. T4: STEC inoculated + phage cocktails + chlorinated water + rinse. T4 is introducing a change to the order of the phage intervention, where the STEC phage cocktail was applied on lettuce first, before washing with chlorinated water and finalizing with a fresh cold-water rinse. T4 will show if the chlorinated water wash will interfere with the phage cocktail efficacy. Another reason for added T4, was to test a synergistic antimicrobial effect, if phages cause damage (holes) in the bacterial cell envelope, this could enhance the bactericidal effect of the chlorinated water. While T3, where the chlorinated water wash was applied first, followed by a fresh cold-water rinse and then the phage cocktail was applied, in this case T3 will likely allow the phage to remain in the packaged produce during storage and reduce STEC over time.
After treatment, Romaine and iceberg lettuce leaves were packaged in sterile bags and stored at 2°C for 1, 24, 48, and 72 hr. At the end of each storage period, conventional, and molecular microbiology techniques as previously described in food matrix trial were used for STEC enumeration, screening, and confirmation. All experiments were performed as independent trials and replicated three times.
Samples where E. coli O157 was not detected in agar plates were further analyzed for E. coli O157 survival via enriching 1 ml of liquid from the stomacher bag in 9 ml of trypticase soy broth (TSB) (BD Difco) for 24–48 hr at 37°C. Any growth observed in TSB was plated on MacConkey plate for E. coli O157 detection. Suspected colonies were confirmed using latex agglutination test for E. coli O157 serogroup and PCR if required. There was no further step taken to remove the background microflora as MacConkey is a selective and differential media for E. coli. In addition, there was no background microflora showing similar phenotype to E. coli on MacConkey plates.
2.5 Statistical analysis
All statistical analyses were conducted using the Statistical Analysis System (SAS), version 9.2. The MOIs from in vitro experiments were analyzed following the mixed linear procedure (for fixed and random effects). Data were analyzed using SAS with each produce type analyzed separately. The model used was a completely randomized design with factorial arrangement where the replications of treatments were assigned at random to independent experimental subjects. Treatments, storage time, temperature, and their interactions were considered as fixed effects.
Differences were considered significant where p < 0.05. Reduction in E. coli population was calculated as the difference between treated and untreated E. coli counts. Log reduction = Log N0/N (CFU/g), where N0 is the initial population and N is the population at a time interval where sample was collected.
3 RESULTS
3.1 In vitro microplate STEC phage virulence assay
For phage prorogation strains, an interaction (p = 0.003) between phage lytic activity and temperature indicated that lytic activity against STEC improved as temperature increased. At 25°C, both individual STEC phages and the phage cocktail were effective against targeted host(s), with at least a 5 log10 CFU/ml reduction in host concentrations. Five phages (AYO26A, phage O45, AXO103A, AXO121A, and AKFV33) were able to eliminate their targeted STEC host when they were individually tested at 25°C (Table 2). More details related to OD reading for microplate incubated at 25°C can be found in Figure S1. At 2 and 10°C, there was no detectable growth of STEC after 5 hr, and as a result OD was not a helpful indicator of phage effectiveness. Consequently, the spot plate technique was used at lower temperatures and showed that 4 STEC serogroups (O26, O103, O145, and O157) were eliminated when exposed to the phage cocktail or their individual targeted phages at 2 and 10°C (Table 2). Phages O45 and AXO121A failed to completely kill their PPS host at 2 and 10°C, whereas bacteria survivals from serogroup O111 were observed at any of the temperatures evaluated. Bacterium survivors detected through spot plate technique indicated phage 45, AXO121A, and AXO111A are less effective compared with other phages. They were effectively reducing their PPS host but could not eliminate them. It was found that the overall MOI was higher at 2 and 10°C (MOI = 5.5× 100 PFU/CFU) than at 25°C (MOI = 2× 10−2 PFU/CFU) (p = 0.001) for STEC O26:H11, O111:NM, O121:H19, O145:NM, and O157:H7 (Table 3). The higher MOI indicated that a larger number of phage particles (both as individual and/or phage cocktail) were needed to eliminate STEC (107 CFU/ml) at temperatures lower than 25°C.
| STEC serogroups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | O26 | O45 | O103 | O111 | O121 | O145 | O157 | |
| Individual. Phage | 2°C | N | D | N | D | D | N | N |
| 10°C | N | D | N | D | D | N | N | |
| 25°C | N | N | N | D | N | D | N | |
| Phage cocktail | 2°C | N | D | N | D | D | N | N |
| 10°C | N | D | N | D | D | N | N | |
| 25°C | N | N | N | D | D | D | N | |
- Note: One phage was used against its target serogroup at each temperature for treatment with individual phage. Phage cocktail was mixed with 20 μl of phage AKFV33, AYO26A, phage O45, AXO103A, AXO111A, AXO121A, and AYO145A at high titer (>108 PFU/ml).
- Abbreviations: D, STEC survival detected from spot plating; N, No STEC survival detected from spot plating.
| Temperature (°C) | |||
|---|---|---|---|
| STEC strains | 2 | 10 | 25 |
| O26:H11 | 3.4 × 10a | 3.4 × 101a | 2 × 10−2b |
| O45:H2 | 4 × 10−2a | 4 × 10−2a | 4 × 10−2b |
| O103:H2 | 8 × 10−2a | 8 × 10−2a | 9 × 10−2b |
| O111:NM | 2.9 × 100a | 2.9 × 100a | 2 × 10−2b |
| O121:H19 | 5 × 10−1a | 5 × 10−1a | 5 × 10−4b |
| O145:NM | 4 × 10−1a | 8 × 10−1a | 4 × 10−4b |
| O157:H7 | 8 × 10−1a | 7 × 10−1a | 4 × 10−4b |
- Note: Means in a row with different superscripts differ, p value = 0.01.
Regarding non-PPS strains, E. coli O157:H7 strains 1931, 161-84, 1934 and CO-238 (Table 4), had MOI >100 after being treated with phages O45, AXO103A, and AXO111A; those three phages were not effective against the four strains tested in this study. Phage AKFV33 was the most effective, as it had activity against all four tested O157 strains at all incubation temperatures. Phage AYO26A and AXO121A were effective against strains 161-84, 1934, and CO-283 at all tested temperatures (2, 10, 25, and 37°C), and effective in reducing E. coli O157 (1931) at 25 and 37°C (Table 4). Phage AYO145A was only effective at 25 and 37°C, which could help in conditions of temperature abuse. Overall, the MOI for AKFV33 ranged from 2 to 2 × 10−6. Phage AYO26A and AXO121A were more effective against O157:H7 strains 161–84, 1934, and CO-238 (MOI = 40–53, 4–50, respectively). Interestingly strain 1931 was insensitive to AYO26A at 2 and 10°C. In contrast, phage AYO145A exhibited lytic activity against all 4 E. coli O157:H7 strains only at 25 and 37°C. In general, the MOI was higher for phages at 2 and 10°C than at 25 and 37°C. Overall, the MOI of phage AKFV33 for all 4 non-PPS (MOI = 1 × 10−2 to 19) was higher than the MOI for the O157:H7 strain (R508) used for phage propagation (MOI = 4 × 10−4 to 8 × 10−1) at 2, 10 and 25°C, except for CO-238 at 10°C (Tables 3 and 4).
| Phage strain ID | 2°C | 10°C | 25°C | 37°C |
|---|---|---|---|---|
| 1931 | ||||
| AYO26A | R | R | 2 | 3 |
| O45 | R | R | R | R |
| AXO103A | R | R | R | R |
| AXO111A | R | R | R | R |
| AXO121A | R | R | 20 | 30 |
| AYO145A | R | R | 1 × 10−1 | 1 × 10−4 |
| AKFV33 | 21 | 19 | 1 × 10−2 | 2 × 10−6 |
| 161-84 | ||||
| AYO26A | 40 | 42 | 2 | 2 |
| O45 | R | R | R | R |
| AXO103A | R | R | R | R |
| AXO111A | R | R | R | R |
| AXO121A | 17 | 25 | 15 | 9 × 10−4 |
| AYO145A | R | R | 1 × 10−1 | 6 × 10−3 |
| AKFV33 | 3 | 2 | 2 × 10−2 | 2 × 10−6 |
| 1934 | ||||
| AYO26A | 44 | 47 | 2 | 9 |
| O45 | R | R | R | R |
| AXO103A | R | R | R | R |
| AXO111A | R | R | R | R |
| AXO121A | 4 | 14 | 12 | 2 × 10−4 |
| AYO145A | R | R | 2 × 10−1 | 2 × 10−5 |
| AKFV33 | 11 | 17 | 1 × 10−2 | 2 × 10−6 |
| CO-283 | ||||
| AYO26A | 45 | 53 | 5 × 10−1 | 5 |
| O45 | R | R | R | R |
| AXO103A | R | R | R | R |
| AXO111A | R | R | R | R |
| AXO121A | 26 | 50 | 1 | 6 × 10−4 |
| AYO145A | R | R | 1 × 10−1 | 4 × 10−6 |
| AKFV33 | 2 | 4 × 10−2 | 2 × 10−2 | 5 × 10−6 |
- Note: R indicates the STEC strain is resistant to phage, MOI >100.
3.2 Effectiveness of STEC of phage cocktail on produce matrixes using phage propagation (PPS) STEC strains
Use of the phage cocktail (T2) to reduce STEC on produce resulted in the highest reduction for MBP (1.71 log10 CFU/g), followed by MBS (1.69 log10 CFU/g), and lettuce (1.56 log10 CFU/g). However, STEC reduction decreased from 1.69 (MBS) to 0.61 (LG sprouts) log10 CFU/g, which indicates the phage cocktail was less effective in controlling STEC during germination (Figure 1).
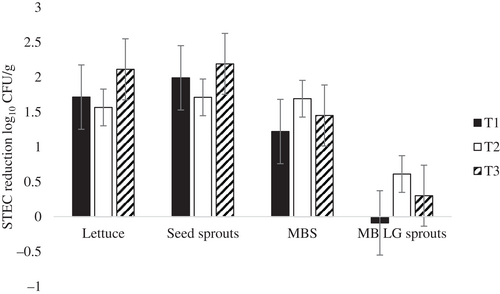
Significant differences were found between treatments for total STEC reduction (p < 0.0001) (Figure 1). The combination of chlorinated water with phage cocktail treatment (T3) achieved the highest overall reductions in STEC total counts on lettuce and sprouts with 2.1 and 2.2 log10 CFU/g reductions respectively. The application of only the STEC phage cocktail (T2) achieved 1.6 log10 CFU/g reductions on lettuce and 1.7 log10 CFU/g on MBP, which was not as effective at reducing STEC as T3. The STEC phage cocktail alone (T2) showed the highest reduction (1.7 log10 CFU/g) of STEC on MBS. In comparison, the reductions were lower in beans treated with 1,000 ppm chlorinated water (T1) (1.2 log10 CFU/g) and the combination of phage cocktail and chlorinated water (T3) (1.5 log10 CFU/g). In LG sprouts, regardless of the treatment, the reductions in total STEC numbers were less than 1 log10 CFU/g. Although the STEC phage cocktail was effective against all STEC serogroups on MBS either alone or when combined with 1,000 ppm chlorinated water wash, it did not prevent the regrowth of STEC during germination. After germination, none of the treatments was able to reduce more than 0.6 logs of STEC on lab germinate sprouts (Figure 1).
While 5 out of 7 STEC serogroups were eliminated in vitro (STEC O26, O45, O103, O121, and O157), isolates from all 7 STEC serogroups remained viable after phage treatment in food matrixes trials. There were no significant differences observed between treatments in lettuce (p = 0.82) (Figure 2a). In MBP, serogroups O111, and O121 were the dominating serogroups surviving from the treatments, while STEC O26, O45, O103, O145, and O157 were found in relatively lower numbers (<10 colonies) on plates, and treatment with phage cocktail was able to reduce 3.1–5.2 log10 CFU/g of those serogroups by itself or when combined with chlorinated water. The reduction (log10 CFU/g) of STEC after treatments was higher than the initial inoculation concentration, indicating the treatments effectively reduced the STEC number to low/undetectable levels, where the STEC number in positive control grew greater than inoculation concentrations during the storage period. The combination treatment was the most effective (p < 0.001), reducing 4/7 serogroups (O26, O45, O111, O145) in MBP (Figure 2b).
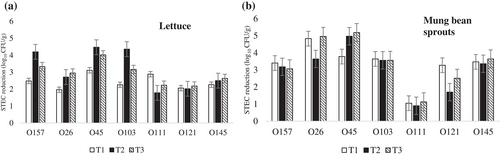
In MBS, there was a significant difference (p < 0.05) among treatments in their ability to reduce numbers of serogroups O157, O26, O111, O121, and O145 (Figure 3a), but no significant difference (p > 0.05) were found among treatments for control of O45 and O103. STEC phage cocktail alone (T2) was more effective against serogroups O157, O26, and O145 (≥ 3 log10 CFU/g), while less effective for control of O111 and O121. Chlorinated water alone (T1) was the most effective at reducing serogroup O121, with a 3.7 log10 CFU/g reduction. The highest reduction of serogroup O111 was 2.74 log10 CFU/g with STEC phage cocktail combined with chlorinated water treatment (T3).
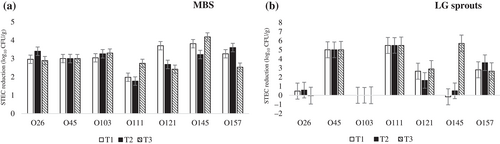
Serogroup O26, O103, O121, O145, and O157 isolates were the principal survivors in LG sprouts; particularly for serogroups O26 and O103, where there was low/no reduction either by the STEC phage cocktail, chlorinated water, or the treatment combination (Figure 3b). Serogroups O45 and O111 were below the countable range after all treatments, but they were detected through PCR/IMS, indicating that a 5 log10 CFU/g reduction was achieved.
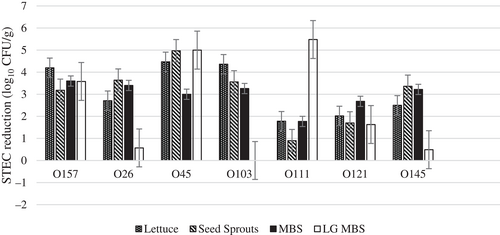
The phage cocktail was more effective at reducing STEC O157, O26, O45, O103, and O145 (2.5–5.0 log10 CFU/g) on lettuce and MBP than serogroups while poorly effective on O111 O121 (≤2 log10 CFU/g), which is the opposite to MBS and LG sprouts, where phages were most effective at reducing STEC O121 on MBS (3.4 log10 CFU/g), and O111 on LG sprouts (5.5 log10 CFU/g) (Figure 4).
3.3 Effectiveness of a phage cocktail treatment (T2) at controlling phage propagation STEC strains (PPS) on produce stored for different periods and temperatures
3.3.1 Lettuce
A significant temperature-by-storage time interaction (p < 0.0001) was found in lettuce. The phage cocktail was more effective at controlling total numbers of STEC at 2 and 10°C than 25°C (Table 5). The highest reduction achieved in STEC total numbers was 2.8 log10 CFU/g at 2°C after 72 hr. During storage, the reduction achieved by the STEC phage cocktail was increased at 2°C and remained relatively stable at 10°C. At 25°C, the phage cocktail reached a 2.5 log10 CFU/g reduction in total STEC within 1 hr, but the STEC population returned to levels that were similar to the control sample after 24 hr (Table 5). Phage cocktail was not able to control the growth of STEC population at 25°C overtime as they continued to grow after 1 hr.
| Lettuce | Sprouts | |||||
|---|---|---|---|---|---|---|
| Temperature | ||||||
| Time (hr) | 2°C | 10°C | 25°C | 2°C | 10°C | 25°C |
| 1 | 2.3a | 2.3a | 2.5a | 2.40a | 2.35a | 2.45a |
| 24 | 2.4a | 2.4a | 0.9b | 2.30a | 2.09a,b | 1.20b |
| 48 | 2.6a | 2.2a | 0.8b | 2.40a | 1.98b | 1.11b |
| 72 | 2.8a | 2.3a | 0.4b | 2.27a | 1.35b | 0.59b |
- Note: Reduction achieved by phage cocktail only (T2). Least square means lacking a common letter differ (p value <0.0001).
Regarding the individual serogroups, the phage cocktail exhibited high activity against all 7 serogroups at 2°C, causing reductions between 2.3 and 6.4 log10 CFU/g after 72 hr of storage (Figure 5a). At 10°C, the phage cocktail remained effective against isolates from serogroup O157, O26, O45, O103, and O145 with at least 2.1 log10 CFU/g reduction at the end of the 72 hr storage period, but had less effect on O111 and O121 as there were lower reductions in these serogroups (1.0 and 1.5 logs CUF/g respectively) (Figure 5b). At 25°C, STEC numbers increased after 1 hr in all serogroups except for STEC O103 where the phage cocktail controlled its numbers over 72 hr storage (Figure 5C).
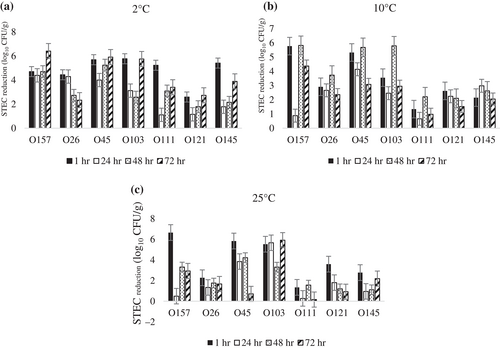
3.3.2 MBP
As expected, an interaction was found between treatment and temperature, as well as between temperature and time. At temperatures ≤10°C, the phage cocktail caused a similar reduction in STEC during storage and consistently controlled STEC (Table 5). The highest reduction of total STEC numbers, with the phage cocktail, was 2.4 log10 CFU/g both at 2 and 10°C within 1 hr. Similar to lettuce, the highest reduction at 25°C (2.45 log10 CFU/g) after phage cocktail treatment was at 1 hr, but it was only effective in the short term; reduction in STEC decreased as sprouts were stored longer (≤ 1 hr).
At 2°C, phage cocktail resulted in a > 2 log10 CFU/g reduction in serogroups O157, O103, O111, and O145 over 72 hr, and achieved a ≥ 5 log CFU/g reduction of E. coli O26 and O45 (Figure 6a). At 10°C, the phage cocktail remained efficacious against serogroups O157, O45, O103, O145, and O111 during storage, but it was less effective against O26 (3.8–2.13 log10 CFU/g reduction) and O121 (3.53–1.78 log10 CFU/g reduction) (Figure 6b). At 25°C, the phage cocktail caused a > 5 log10 CFU/g reduction in STEC O26, O45, 103, and O145 over 72 hr of storage (Figure 6C).
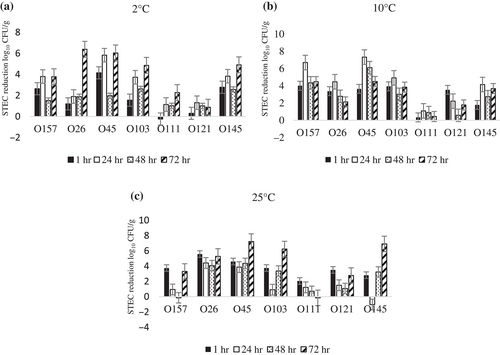
3.4 E. coli O157:H7 reduction on Romaine and iceberg lettuce using non-phage prorogation O157 strains (non-PPS) at high and low inoculation levels
There were significant differences found between all the tested treatments on both romaine and iceberg lettuce at high inoculation level (p < 0.001) (Table 6). Chlorinated water (T1) and phage cocktail combined with chlorinated water (T3, T4) had about 2 log10 CFU/g reductions, while phage cocktail alone (T2) achieved 1.7–1.8 log10 CFU/g reductions for both romaine and iceberg lettuce, respectively. Overall, the collected data on O157:H7 (105 CFU/g) adulterated lettuce indicated that the STEC phage cocktail combined with chlorinated water was the most effective treatment, which reduced E. coli O157:H7 by 2.3 logs on fresh Romaine lettuce, and 2.2 logs on iceberg lettuce. Phage cocktail alone was not an ideal treatment to control E. coli O157:H7 at high concentrations.
| Treatment | T1 | T2 | T3 | T4 | SEM | p-Value | |
|---|---|---|---|---|---|---|---|
| High inoculation | Reduction (log10 CFU/g) on iceberg | 1.95ab | 1.67a | 2.15bc | 2.09bc | 0.13 | <0.0001 |
| Reduction (log10 CFU/g) on Romaine | 2.07bc | 1.82a | 2.34c | 2.32bc | |||
| Low inoculation | Reduction (log10 CFU/g) on iceberg | 2.61a | 2.64a | 2.74a | 2.69a | 0.12 | <0.0001 |
| Reduction (log10 CFU/g) on Romaine | 3.22b | 3.23b | 3.11b | 3.23b |
- Note: a, b, c: Least square means without a common superscript letter indicate difference (P < 0.05).
- Abbreviations: T1, adulterated lettuce samples washed with chlorinated water (150 ppm) three times; T2, adulterated lettuce samples washed with STEC phage cocktail (108 PFU/ml); T3, adulterated lettuce samples washed with chlorinated water (150 ppm) three times before the STEC phage cocktail treatment; T4, adulterated lettuce samples washed with chlorinated water (150 ppm) three times after the STEC phage cocktail treatment.
Unlike the high inoculation level trial, all tested treatments showed similar E. coli O157:H7 reductions for all four treatments regardless of lettuce types at low inoculation level (103 CFU/g) (Table 6). The phage cocktail was effective at reducing the number of E. coli O157:H7, either alone or combined with chlorinated water with >2.6 and > 3.1 log10 CFU/g reductions on iceberg and Romaine lettuce, respectively. Interestingly, the reductions achieved were better than those observed in the high inoculation trials; only 44–46% of STEC was reduced in high inoculation trials, while 90–100% of STEC were reduced in low inoculation trials (percentage of STEC reduced = STEC reduction/STEC inoculated). In addition, no significant difference in phage efficacy was found regarding the order in which the phage was applied (before or after the chlorinated water wash) for both high and low inoculation levels (p > 0.05).
Significant differences (p < 0.0001) were found according to the type of lettuce at both high and low inoculation levels; interestingly, all the treatments were more effective at reducing E. coli O157:H7 on Romaine than on iceberg lettuce (Table 6). Regarding storage time, no time x treatment interaction was found at high inoculation levels (p = 0.9) (Figure S2A). At low inoculation levels (103 CFU/g), T1, T2, and T4 were more effective in romaine lettuce since E. coli O157 was only detected and enumerated at 1 hr or 48 hr. Treatment 3 showed itself to be the less effective treatment in iceberg lettuce since E. coli O157 was present at 1 hr, 24hr, and 48 hr. All the treatments effectively reduced O157 in Romaine and iceberg lettuce after 72 hr, as no E. coli O157:H7 was recovered (Figure S2B).
3.5 E. coli O157:H7 detection followed from low inoculation study
Samples, where E. coli O157 was not detected in agar plates, were further analyzed for E. coli O157 survival in the low inoculation study, whereas E. coli O157 was detected from all plates from the high inoculation study. After exposure to chlorinated water, recovery rates of E. coli O157:H7 were: 1 hr—100%, 24 hr—83.3%, after 48 hr—33.3%, and 72 hr—50% (Table 7). When only the phage cocktail was used, E. coli O157:H7 cells were recovered at 33.3% after 1 hr and were not isolated thereafter. The combination treatment with phage cocktail and chlorinated water (T3 and T4) was also more effective at keeping E. coli O157:H7 from recovering than using chlorinated water alone. It was found that only 1/36 samples (2.8%) had bacterial cells that were able to recover when treated with phage cocktail alone (T2) with storage time ≤ 24 hr and there were no bacterial cells recovered at 48 and 72 hr. Using chlorinated water treatments without phages (T1) allowed for 66.7% (16/24) E. coli O157:H7 recovery in romaine lettuce and 54.2% (13/24) bacterial cells recovery in iceberg lettuce (Table 7). Recovery percentage [% (n/N)] was calculated according to the number of positive sample (n) divided by the total sample size (N) at 1, 24, 48, and 72 hr. These findings suggest that the phage cocktail is causing more cell damage than chlorinated water. The phage cocktail was able to completely kill E. coli O157:H7, followed by a phage cocktail combined with chlorinated water treatments.
| Percent recovery without enrichment, % (n/N) | Percent recovery with enrichment,a % (n/N) | Total recovery rate % (n/N) | |||||
|---|---|---|---|---|---|---|---|
| Storage time (hr) | Treatment | Romaine | Iceberg | Romaine | Iceberg | Romaine | Iceberg |
| 1 | T1 | 33.33(2/6) | 16.67(1/6) | 100.00(4/4) | 80.00(4/5) | 100.00(6/6) | 83.33(5/6) |
| T2 | 33.33(2/6) | 0.00(0/6) | 0.00(0/4) | 0.00(0/6) | 33.33(2/6) | 0.00(0/6) | |
| T3 | 50.00(3/6) | 0.00(0/6) | 0.00(0/3) | 0.00(0/6) | 50.00(3/6) | 0.00(0/6) | |
| T4 | 0.00(0/6) | 16.67(1/6) | 33.33(2/6) | 0.00(0/5) | 33.33(2/6) | 16.67(1/6) | |
| 24 | T1 | 0.00(0/6) | 16.67(1/6) | 83.33(5/6) | 40.00(2/5) | 83.33(5/6) | 50.00(3/6) |
| T2 | 0.00(0/6) | 16.67(1/6) | 0.00(0/6) | 0.00(0/5) | 0.00(0/6) | 16.67(1/6) | |
| T3 | 16.67(1/6) | 0.00(0/6) | 0.00(0/5) | 0.00(0/6) | 16.67(1/6) | 0.00(0/6) | |
| T4 | 0.00(0/6) | 16.67(1/6) | 16.67(1/6) | 0.00(0/5) | 16.67(1/6) | 16.67(1/6) | |
| 48 | T1 | 0.00(0/6) | 16.67(1/6) | 33.33(2/6) | 40.00(2/5) | 33.33(2/6) | 50.00(3/6) |
| T2 | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | |
| T3 | 16.67(1/6) | 16.67(1/6) | 0.00(0/5) | 0.00(0/5) | 16.67(1/6) | 16.67(1/6) | |
| T4 | 33.33(2/6) | 0.00(0/6) | 0.00(0/4) | 0.00(0/6) | 33.33(2/6) | 0.00(0/6) | |
| 72 | T1 | 0.00(0/6) | 0.00(0/6) | 50.00(3/6) | 33.33(2/6) | 50.00(3/6) | 33.33(2/6) |
| T2 | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | |
| T3 | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | 0.00(0/6) | |
| T4 | 0.00(0/6) | 0.00(0/6) | 16.67(1/6) | 0.00(0/6) | 16.67(1/6) | 0.00(0/6) | |
- Note: Recovery percentage [% (n/N)] was calculated according to the number of positive sample (n) divided by the total sample size (N).
- a If the O157 was not recovered, 1 ml of the homogenized sample was transferred into 9 ml modified TSB for 24 hr enrichment at 37°C.
4 DISCUSSION
The lack of effective pathogen reduction interventions for fresh produce is a concern worldwide. Shiga-toxigenic E. coli (STEC) outbreaks linked to contaminated produce are rising in North America (Lynch et al., 2009). The use of lytic phages as a post-harvest intervention to eliminate STEC could be a safe and effective way to reduce STEC on leafy greens and other vegetables as a hurdle intervention. However, there are still information gaps regarding the efficacy of phage at controlling pathogens within produce-processing environments.
Our results showed that a higher MOI of phages was required to eliminate PPS STEC as well as for non-PPS E. coli O157:H7 strains as temperature decreased. Similar results were found when phage product (SalmoFresh) was applied to Salmonella (108 CFU/ml) in vitro under different temperatures (2, 10, 25°C); more phages (higher MOI) were required at lower temperatures for all five strains tested (p < 0.05) (Zhang et al., 2019). Result obtained in vitro shows the STEC phage cocktail has the potential to reduce STEC (both PPS and non-PPS strains) when applied at refrigeration temperatures; such results set the path for testing phages effectiveness on refrigerated produce.
Treatment of lettuce, MBP, and MBS adulterated with PPS STEC, with the phage cocktail reduced STEC to various degrees (1–5 log10 CFU/g) at storage temperatures of 2, 10, and 25°C at 1, 24, 48, and 72 hr. The phage cocktail was most effective at reducing STEC at lower temperatures (2 and 10°C) after 72 hr of storage, which is surprising, as the optimal phage activity has been reported at 30–45°C. Storage in colder temperatures is typically not preferred as it could reduce the activity of phage as they depend on the metabolic activity of the host to complete their lytic cycle (Boyer & McKinney, 2018). At low temperature, E. coli growth and metabolism is generally limited as membrane fluidity is decreased and membranes become rigid, which makes it more difficult for phage replication when bacteria growth is reduced to a minimal pace (Graumann & Marahiel, 1996; Parola et al., 1990). The findings of the present study are encouraging since the recommended shelf-life for fresh lettuce and sprouts at 4°C is 1 week (168 hr), and 3–5 days, respectively (Obeso et al., 2008), and the STEC phage cocktail was still effective at refrigeration temperatures during washing and storage. Some of the phages might be active at these lower temperatures; however, the mechanism in unknown. In addition, the STEC phage cocktail was effective against non-PPS STEC on lettuce stored at 2°C as well, especially at low inoculation levels (103 CFU/g). It is possible that those phages release lytic enzymes such as endolysins which are capable of degrading peptidoglycan to the bacterial cell wall, which also results in a rapid lysis of the bacterial cell at low temperatures (Hermoso et al., 2007; Shannon et al., 2020). Phage endolysins may have remained active during storage at low temperatures and could have contributed to the observed results found. The investigation of reasons for phage activity at lower temperatures is essential since most fresh produce is stored at ≤4°C prior to sale. More research is required to understand the mechanism(s) that the phages use to reduce targeted bacterial populations at refrigeration temperatures.
Overall, phages exhibited the potential to control all targeted O157:H7 non-PPS with MOI from 10−6 to 21 at all tested temperatures in vitro at 2, 10, 25 and 37°C. Similar results have been reported by others, where some phages were effective under a wide range of temperatures. For example, Hudson et al. reported that phage FAHEc1 effectively infected multiple E. coli O157 strains under temperatures ranging from 5°C (4 log10 reductions) to 37°C (>2.7 log10 reduction) (Hudson et al., 2013). Results from this current study show the phage cocktail can be used as an effective antibacterial method for reducing E. coli O157:H7 under different temperatures, including those conditions of temperature abuse.
Besides the impact of different temperatures, phage effectiveness may be influenced by different food matrixes. In this study, it was hypothesized that the use of PPS STEC would shed some light on the food matrix's role in phage efficacy. We observed that phage performance varied according to the food matrix, even with the same host. For example, E. coli O157:H7 showed the best reduction on romaine lettuce (4.2 logs), followed by MBS (3.6 logs), LG sprouts (3.6 logs), and MBP (3.2 logs). The structures of lettuce and sprouts influenced the effectiveness of the phage cocktail. The phage cocktail itself (T2) was more efficient at reducing STEC in MBP as compared to lettuce (p < 0.0001). These results could be due to the fact that the lettuce was cut and that some STEC cells entered the into internal plant tissues, restricting the contact of phage with host bacterial cells. STEC outbreaks have often been linked to shredded produce which promotes the internalization of foodborne pathogens within plant tissues (Beuchat, 1999). Previous research showed that phage treatment has lower efficacy on foods with an uneven surface than those with an even surface due to the limited diffusion and contact between bacteria and phage particles (Guenther et al., 2009). The leaf surface is less complicated than the interior structure of a leaf, where the bacteria internalized and are less accessible to phages, reducing their effectiveness at decontaminating produce (Solomon et al., 2003; Stine et al., 2005). This founding is supported by Snyder et al., who found in their research that immersion of the fresh produce in the phage treatment was more effective in removing the cells attached to the undamaged leaf as opposed to the cells which potentially internalized into the tissues through the cut surface (Snyder et al., 2016). More research is needed to understand specific mechanisms limiting phages’ ability to reach bacteria within the tissue.
Bacterial cells are often found residing in the crevices of the seed coat, as well as in the cotyledon, which can shield bacteria from sanitizers (Bang et al., 2011). Sprouting occurs as a result of exposure of the seed to higher temperatures and moisture, and these conditions, combined with nutrient release from the seed, create conditions that are ideal for the rapid growth of E. coli O157:H7 (FSAI, 2011; Stewart et al., 2001). In the present study, the phage cocktail was effective to some degree but is unlikely to eliminate STEC on MBS. During sprout germination, any pathogens that survive in the seeds can grow rapidly (FSAI, 2011), and this was not prevented by phage cocktail or chlorinated water. Perhaps phage-based interventions have most merit as part of a multi-hurdle approach but may lack efficacy if they are used as the sole intervention.
Regarding trials using STEC non-PPS, significant differences were found between lettuce type (p < 0.01), where non-PPS E. coli O157:H7 was reduced slightly more in Romaine than in iceberg lettuce at both high and low inoculation levels. These differences in reduction were small, about 0.6 logs; however, it is unclear what these differences in reduction could be attributed. E. coli O157:H7 outbreaks have been more related to Romaine than iceberg lettuce, perhaps due to the shape of Romaine lettuce head, where the leaves are more exposed to the environment than iceberg lettuce; increased exposure could lead to bacterial contamination in the field (Mou, 2008). From 2008 to 2018, there were 48 outbreaks related to leafy green products in Canada and United States, 37.5% of the outbreaks occurred in romaine lettuce, while only 14.6% were linked to iceberg lettuce (Coulombe et al., 2020). However, of all outbreaks associated with iceberg and Romaine lettuces, 28.6% (iceberg) and 11.1% (Romaine) were caused by shredded or ready-to-eat products. A potential reason is that iceberg lettuce leaves are more fragile than romaine lettuce leaves, and it is possible that during the experimental conditions, the iceberg lettuce leaves were structurally more damaged, increasing the risk that STEC becomes internalized within plant tissues. However, according to results from previous studies, no differences were found in E. coli O157:H7 attachment and survivability on Romaine and iceberg at 4°C and 15°C (Patel et al., 2011; Tian et al., 2012). More research is needed to understand potential differences in phage effectiveness in Romaine compared to iceberg lettuce.
Nevertheless, we found that the STEC phage cocktail was very effective at reducing STEC when in low inoculation levels (103 CFU/g) on both romaine and iceberg lettuce; thus, it seems that the lettuce matrix may not be the critical factor that inhibited the effectiveness of the phage cocktail. The concentration and strains of STEC inoculated on the fresh produce may play a more critical role on STEC reductions after treated by bacteriophages. The phage cocktail was more effective at reducing non-PPS E. coli O157:H7 when lettuce was adulterated with low O157 levels (103 CFU/g) than high levels (105 CFU/g). Similar results were shown elsewhere when the ability of a phage cocktail to reduce a mixture of E. coli O157:H7 strains was tested on baby romaine lettuce and baby spinach leaves (Viazis et al., 2011). The E. coli strains were spot inoculated onto the leaves at low (104), medium (105), and high concentrations (106 CFU/ml) followed by applied phage cocktail at a concentration of approximately 106 PFU/piece. Similar to our result, the efficacy of the phage cocktail also increased as the bacterial concentrations decreased in their study. A previous survey was conducted among produce from the United States and Mexico and found E. coli level of all E. coli serogroups, including both generic and pathogenic E. coli ranged in concentration from 1.0 to 4.0 log10 CFU/g (Bhagwat, 2003; Johnston et al., 2005; Johnston et al., 2006; Snyder et al., 2016). The concentration of E. coli O157:H7, which caused outbreaks in produce, can vary, but is generally believed to be less than our inoculated concentration (103 CFU/g) based on the result from a previous microbiological survey (Bohaychuk et al., 2009). Accordingly, we hypothesized that the phage cocktail will be more effective at eliminating E. coli O157:H7 at STEC concentrations lower than 103 CFU/g in fresh produce. There is a potential for this phage cocktail to be an effective post-harvest disinfection agent in real-world produce production.
It is important to mention that bacterial sensitivity to phages varies among different E. coli O157:H7 strains. When phage cocktail was used on romaine lettuce spiked at 105 CFU/g of inoculum, a more significant reduction was found in PPS (4.2 logs CFU/g) than non-PPS (1.7 logs CFU/g) E. coli O157:H7 strains. Furthermore, the MOIs for O157:H7 non-PPS were higher than for the PPS at all tested temperatures, indicating that more phage particles are needed to eliminate O157 with lower sensitivity to phage. In support of our study, Wang et al. (2015) reported phage lytic capabilities of 11 non-O157 STEC phages, phages AYO26A showed lytic capabilities in different levels to 4 STEC O26 strains tested. The MOI ranged from 6 × 10−6 to 1; and phage AXO121A showed lytic capabilities to four different STEC O121 strains with MOI from 10−3 to 10−5 (Wang et al., 2015).
When looking at the phage cocktail effectiveness in different STEC serogroups, it was found that not all serogroups had the same sensitivity to the phages included in the phage cocktail, even on the same food matrix. For example, phages were very effective for E. coli O157:H7 with the best reduction on romaine lettuce (4.2 logs), but for E. coli O26 only reduced it by 2.7 logs. It is important to mention that phages used in the present study were selected with a bias toward their effectiveness against O157, but with exception of phage AKFV33, only phages AYO26A, and AXO121A could lyse the 4 common STEC O157:H7 strains. Multiple phages used in the current study were effective against serogroup O26 (AKFV33, AYO26A, AXO103A, and AXO111A) and O103 (AYO26A, AXO103A, and AXO121A) (Wang et al., 2015), which may explain why the phage cocktail had the highest effectiveness against these serogroups. Bacteriophage are known to be able to infect only a narrow range of the bacterium, and the host range is determined by the specificity in the nature and structural peculiarities of bacteria cell surface receptors (Rakhuba et al., 2010). We hypothesized that when multiple phages are applied, some of them may be able to attach to receptors of multiple STEC serogroups, and this could increase phage efficiency. The combination of phages can target multiple STEC strains or cover multiple species that are associated with enteric diseases; a bacterium that is resistant to one phage type can be killed by another type (Chan & Abedon, 2012). Genetic similarities found in those more effective phages are very limited, phage AXO103A and AKFV33 are T5-like phages in Siphoviridae family. Phage AXO45B is a T5-like phage belongs to Myoviridae family. Phage AYO26A is a T4-like phage belongs to Myoviridae family. Phage AXO111A, AXO121A, and AYO145A are also in Myoviridae family but different in phage type (ViI, rV5, O1, respectively) (Wang et al., 2015). Pervious research shows the ability of phages to work in cocktails is enhanced if the phages are less closely related to each other, the diversities of the phage families of the phages chosen in the cocktail of the current study may promote the effectiveness of the phage cocktail (Yan D Niu et al., 2021). Although the phage mechanisms for STEC intervention was not analyzed in this research, we hypothesized that T4 and T5 phages may have more lytic activities than the other phage types involved in this study because phage AYO26A, AXO45B, AXO103A, and AKFV33 showed more effectiveness on their host serogroups. However, mechanisms involving underlying interactions between E. coli and anti-STEC phages are specific for each phage, new knowledge related to genetic factors of different phage types is needed and will be valuable to define criteria to select appropriate phage candidates as effective biocontrol agents. These questions remain unknown because the lack of information on phage genus, pharmacokinetic aspects, or the phage susceptibility type.
Our results obtained showed that the effectiveness of a phage cocktail to control PPS/non-PPS STEC on fresh produces improved when combined with chlorinated water, indicating the possibility of using this phage cocktail in the presence of such a sanitizer. The combination treatment of chlorinated water with phage cocktail achieved the highest reductions in both PPS and non-PPS STEC at high inoculation levels (105 CFU/g) in lettuce, as well as in MBP (PPS STEC). The use of wash water containing sodium or calcium hypochlorite is the primary method of preventing pathogen contamination during produce production. Chlorinated water has been proven effective at reducing pathogenic E. coli attached to surfaces, cut edges or stomata in produce (Sharma et al., 2009). Chlorine causes irreversible oxidation of sulphydryl groups of enzymatic sites in the cytoplasmic membrane, causing membrane damage (Estrela et al., 2002) which could make the cell envelope more unstable and susceptible to disinfectants. However, the combination treatments, including the phage cocktail were more effective than using chlorinated water alone in reducing STEC; likely because the potency of active chlorine can diminish rapidly when it comes in contact with seeds soaking in water due to the high levels of organic matter. High organic material can neutralize chlorine-based sanitizers (Taormina & Beuchat, 1999). A previous study reported using 50 μg/ml sodium hypochlorite (NaOCl) and a commercial phage cocktail known as Ecoshield treating lettuce leaves contaminated with E. coli O157:H7 that were stored at 4°C for 24 hr, the combination of NaOCl and Ecoshield achieved 1.54 log10 CFU/cm2 reduction in E. coli O157:H7, which was more effective than either NaOCl (1.38 log10 CFU/cm2) or EcoShield (1.32 log10 CFU/cm2) alone (Ferguson et al., 2013). The combination treatment resulted in a 1.54 log10 CFU/cm2 reduction in E. coli O157:H7, which was more effective than either NaOCl (1.38 log10 CFU/cm2) or EcoShield (1.32 log10 CFU/cm2) alone; (Ferguson et al., 2013). These previous findings align with the findings presented here and provide further evidence that chlorinated water and STEC phage cocktail can be used as hurdle technology by the fresh produce industry to reduce STEC populations. Future research should consider testing chlorine-resistant STEC strains to assess phage efficacy.
In order to answer the question from the industry regarding phage compatibility with chlorinated water interventions, different treatments were tested during the commercial simulation trials using lettuce adulterated with non-PPS E. coli O157:H7 strains. No significant differences were found regarding the order in which the phage was applied (before or after the chlorinated water wash). The residual chlorine remains on lettuce leaves did not reduce the lytic potential of the phages on E. coli O157:H7 or inactivate the phage cocktail. This is encouraging since it simplifies the use of phages by the food industry to use phages as a hurdle approach along with chlorine.
When examining STEC survival after exposure to the antimicrobial treatments, in trials testing the phages against non-PPS E. coli O157:H7 strains on lettuce, it was found that more E. coli O157:H7 cells recovered from chlorinated water treatment compared with phage cocktail treatment. The higher survival of E. coli O157:H7 with chlorinated water may cause by the organic substances from the damaged or cut surface of the leafy green, which will inactivate the oxidative effect of the free chlorine in chlorinated water (Marriott et al., 2018). Even though the interaction between the effectiveness of phage cocktail and organic substances was not tested in this study, there is no evidence that the phage cocktail is affected by leafy green organic substances compared with the chlorine disinfectants used in the food industry. The lower O157 recovery rate achieved by treatments with phages from this study indicated that the phage cocktail is a more practical intervention regarding limiting pathogens' survival and recovery compare with traditional chlorinated water treatment alone.
The phage cocktail remained effective against both PPS and non-PPS STEC strains that survived. This suggested that none of those isolates developed resistance to phage, and therefore, reasons for STEC survival remain unclear. Another theory is that under refrigeration, the STEC mutation rate and mechanism to overcome phage attacks should be lower since bacterial metabolism is reduced.
5 CONCLUSIONS
Overall, the present study demonstrates the effectiveness of using a phage cocktail to reduce STEC in different foods, including lettuce, MBP, and MBS. The reduction was affected by a time and temperature interaction; phages were effective at 2, 10, and 25°C in vitro, phage showed reductions of 5 log10 CFU/ml for the 7 phage-host STEC serotypes. At 2 and 10°C, phages were effective over 72 hr in both lettuce and MBP even when bacterial metabolism was limited by low temperatures.
The STEC phage cocktail was effective against isolates from 7 STEC serogroups, particularly on lettuce and MBP, mainly for serogroups O26, O45, and O157.
It was observed that phage performance varied according to the food matrix; the effectiveness of some phages was affected by the specific food matrix, even if the targeted bacteria was highly sensitive to the phage (in vitro). There was 84% of PPS E. coli O157:H7 reduced on romaine lettuce by phage cocktail, 72% reduced on MBS and LG sprouts, and only 64% reduced on MBP. Phage cocktail was not effective at fully eliminating pathogen contamination from the LG sprouts. In trials simulating commercial conditions, the STEC phage cocktail was able to reduce 2.6–3.0 logs of non-PPS E. coli O157:H7 on fresh Romaine and iceberg lettuce leaves at low inoculation levels (103 CFU/g). The findings of the present research could represent an important step towards understanding phage applications to enhance food safety on fresh-cut leafy produce. Our STEC phage cocktail was effective at reducing O157:H7 when present in low levels, in combination with chlorinated water and under refrigeration. Phages also showed maximal inactivation of non-PPS E. coli O157:H7 and the sustainable suppression of their regrowth. The promising results from this study provide more possibility of commercialization of this phage cocktail as a post-harvest disinfection treatment in fresh produce industries in the future.
AUTHOR CONTRIBUTIONS
Claudia Narváez-Bravo developed this project and obtained the funding. Tim McAllister provided the phages. Claudia Narváez-Bravo supervised the students and lab work. Yiran Ding executed the study and performed the experiment with the help from Yuchen Nan and Yang Qiu. Yiran Ding drafted the manuscript with the helps from Kim Stanford, Rick Holley, Tim McAllister, and Claudia Narváez-Bravo. Yiran Ding, Yuchen Nan, Yang Qiu, Dongyan Niu, Kim Stanford, Rick Holley, Tim McAllister, and Claudia Narváez-Bravo made a significant and direct contribution to this project and approved it for publication.
ACKNOWLEDGMENTS
The authors offer special thanks to the Ontario Ministry of Agriculture, Food and Rural Affairs and the Canadian Produce Marketing Association for funding the project.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




