Diagnosis, isolation and description of a novel amnoonvirus recovered from diseased fancy guppies, Poecilia reticulata
Abstract
The guppy, Poecilia reticulata, is one of the most common cultured ornamental fish species, and a popular pet fish highly desired by hobbyists worldwide due to its availability of many brilliantly coloured fish of many varieties. The susceptibility of guppies to diseases presents a remarkable concern for both breeders and hobbyists. In this study, we report the emergence of disease in fancy guppies caused by a previously uncharacterized virus in the USA. This virus was isolated from moribund guppies in two separate outbreaks in California and Alabama, from December 2021 to June 2023. The infected guppies presented with acute morbidity and mortality shortly after shipping, displaying nonspecific clinical signs and gross changes including lethargy, anorexia, swimming at the water surface, gill pallor, mild to moderate coelomic distension and occasional skin lesions including protruding scales, skin ulcers and hyperaemia. Histological changes in affected fish were mild and nonspecific; however, liver and testes from moribund fish were positive for Tilapia lake virus (TiLV), the single described member in the family Amnoonviridae, using immunohistochemistry and in situ hybridization, although the latter was weak. A virus was successfully recovered following tissue inoculation on epithelioma papulosum cyprini and snakehead fish cell lines. Whole genome sequencing and phylogenetic analyses revealed nucleotide and amino acid homologies from 78.3%–91.2%, and 78.2%–97.7%, respectively, when comparing the guppy virus genomes to TiLV isolates. Based on the criteria outlined herein, we propose the classification of this new virus, fancy tailed guppy virus (FTGV), as a member of the family Amnoonviridae, with the name Tilapinevirus poikilos (from the Greek ‘poikilos’, meaning of many colours; various sorts, akin to ‘poecilia’).
1 INTRODUCTION
Freshwater fish are among the most popular pets in North America, with more than 139 million fish owned (Bedford, 2018) in over 11 million households (Bedford, 2023). In 2018, the USA ornamental fish industry market value was estimated at approximately 45 million USD, with contributions from 263 farms (USDA, 2019). Guppies (Poecilia reticulata), native to South America, are highly prolific livebearers of the family Poeciliidae and one of the most popular pet fish species worldwide due to their variation in colour patterns and fin shapes. In recognition of their popularity, the International Fancy Guppy Association was established in 1965 to promote fancy guppies and their breeding. In addition to their popularity among ornamental fish hobbyists, guppies have served as model organisms in various fields of scientific research, such as aging (Imai et al., 2022) and spinal curvature (Gorman et al., 2007). Furthermore, they have also been used for mosquito control as they readily predate on mosquito larvae (Manna et al., 2008). However, it is important to acknowledge potential ecosystem risks associated with this practice, given guppies' adaptability to diverse environments, live-bearing reproduction and short gestation period (El-Sabaawi et al., 2016).
Most freshwater fish available to hobbyists, including guppies, are cultured primarily in South America, Asia and Europe (Hoseinifar et al., 2023; Tlusty, 2002). While guppies are easy to produce with a short reproductive cycle and a tolerance to diverse environments, they are susceptible to a range of fish pathogens. This susceptibility has led to large economic losses of unknown value within the industry and the use of various over-the-counter antibiotics and chemicals by hobbyists. Guppies have been documented to suffer from numerous parasitic infections (Kim et al., 2002; Ryan et al., 2004; Thilakaratne et al., 2003), including Tetrahymena spp. (Leibowitz et al., 2005) and Gyrodactylus spp. (King & Cable, 2007), as well as bacterial infections caused by Aeromonas spp. (Hossain et al., 2020; Lazado & Zilberg, 2018), Mycobacterium spp. (Pate et al., 2005; Sakai et al., 2005) and columnaris-causing bacteria (Declercq et al., 2013; de Sebastião et al., 2021). Nonetheless, only a few viruses have been isolated and reported from guppies (Hedrick & McDowell, 1995; Hegde et al., 2003). Due to the potential consequences posed by these infectious diseases, extensive research has been dedicated to developing effective control and prevention strategies in the ornamental fish trade (Motlagh et al., 2020; Schelkle et al., 2015; Sharon et al., 2014).
During two disease investigations among recently imported fancy-tailed guppies submitted for diagnosis (Case 1) or purchased online from a vendor in the USA (Case 2), a previously uncharacterized amnoonvirus was successfully isolated from moribund fish. This case report describes the diagnostic cases including histopathology, virus localization, virus isolation and phylogenetic analyses of the whole viral genomes. Based upon the criteria set forth herein, it is proposed that the virus, designated as fancy-tailed guppy virus (FTGV), as a new member of the family Amnoonviridae, with the name Tilapinevirus poikilos.
2 MATERIALS AND METHODS
2.1 Disease cases and diagnostics
Between 2021 and 2023, two cases involving guppies were investigated from distinct regions of the USA, the West Coast (Case 1; identified as Guppy-95-10-82) and the Southeast (Case 2; identified as CobraB-2), in response to increased morbidity and mortality. Guppies, aged less than 6months, showed varying degrees of morbidity and mortality following shipping and handling and were processed for microbiological and histopathological evaluation.
2.1.1 Case 1 (Guppy-95-10-82)
Thirty guppies, maintained at ~25°C in 1100 L tanks within an 11,350 L recirculating aquaculture system (RAS) operated by an ornamental fish wholesaler in California, were transported to the Aquatic Animal Health Laboratory, University of California, School of Veterinary Medicine, Davis, California, USA for examination. The fish had been recently imported from South Asia by the seller. Subsequent to a physical examination of fresh-dead and moribund fish, six guppies presenting clinical signs of disease were subjected to light microscopic examination of gill and fin clips, skin scrapes and internal organ squashes. Samples of brain tissue from three fish were inoculated on tryptic soy agar with 5% sheep blood and modified Thayer Martin agar (BD BBL[MTMII]) plates and incubated at 28°C for a duration of 2 weeks. Two distinct sets of pooled tissues from five fish each were collected and processed for virus isolation. External pooled tissues comprised the caudal fin and entire head (including gills) with both opercula removed. Internal pooled tissues included all major internal organs removed from the coelom. Pooled samples were diluted 1:25 (W:V) in MEM supplemented with 2 mM L-glutamine, 2% fetal bovine serum (FBS), penicillin (50 IU mL−1), streptomycin (50 μg mL−1) and 15 mM HEPES buffer (MEM-2 + HEPES) with a final pH of 7.4–7.8. Tissues were homogenized using a Stomacher 80 Lab Blender for 1 min and centrifuged at 1300 × g for 15 min. The supernatant was collected and diluted 1:1 with MEM supplemented with 4% fetal bovine serum, 15 mM HEPES buffer, amphotericin-B (25 μg mL−1) and gentamicin (1 mg mL−1) adjusted to pH 7.4–7.8. Tissue extracts (supernatant) were stored at 10°C overnight. The media from 12-well tissue culture dishes (3.5 cm2 surface area per well) containing epithelioma papulosum cyprini (EPC) (Fijan et al., 1983), fathead minnow (FHM) (Gravell & Malsberger, 1965), bluegill fry (BF-2) (Wolf et al., 1966), koi fin (KF-1) (Hedrick et al., 2000) and koi-goldfish fin (kgh fin) cell lines was removed, and replicate wells were inoculated with 0.2 mL of supernatant that was clarified by centrifugation at 1900 × g for 15 min. The kgh fin cell line was generated as in Gardell et al. (2014) from Hybrid goldfish (male goldfish Carassius auratus × female common carp Cyprinus carpio) at the University of California-Davis. After 1 h, 2 mL of media was added to each well, and the plates were incubated at 25°C for 2 weeks. A blind passage onto fresh cells was performed at 14 days. Media from a single well of EPC cell culture with cytopathic effect (CPE) was 0.2 μm filtered before inoculating onto fresh EPC cells to produce a virus stock (identified as Guppy-95-10-82/p2) that was cryopreserved for further investigation. Following identification as described below, the isolate Guppy-95-10-82 was propagated in striped snakehead (SSN-1) cells (Frerichs et al., 1991) incubated at 25°C.
2.1.2 Case 2 (CobraB-2)
In a separate case (CobraB-2), a young aquatic hobbyist in Alabama ordered six red cobra male guppies from an online vendor. Upon delivery, these fish were acclimated in a 20 L static quarantine tank, maintaining a water temperature of 26°C, with approximately 25% of the water replaced every other day. During acclimatization and initial quarantine, the hobbyist treated the fish with 200 ppm formalin for 1 h, Jungle Fungus Clear® fizz tabs (nitrofurazone and potassium dichromate), and oxytetracycline (20 mg L−1 for a 1 h static bath) as the fish were displaying clinical signs of disease. Four fresh-dead fish (guppy #1, 2, 3 and 4) were submitted to the USDA-ARS Aquatic Animal Health Research Unit in Auburn, Alabama, USA, for analysis. Given the antibiotic usage provided by the hobbyist and knowledge derived from Case 1, three guppies (#1, 2 and 3) were only processed for virological examination on SSN-1 cells. Two samples were collected from each fish, one from the viscera (liver, spleen, gastrointestinal organs and heart) and another from a pooled sample of head, gills and left pectoral fin. Tissue samples (<0.5 g) were placed into 1 mL filter-sterilized L-15 medium, supplemented with L-glutamine (0.3 g L−1), penicillin (100 IU mL−1), streptomycin (100 μg mL−1), amphotericin B (2.5 μg mL−1), 2% FBS and buffered with HEPES to achieve a pH of 7.4–7.6, followed by homogenization. After centrifugation at 4°C for 30 min at 2370 × g, 100 μL of clarified supernatant was removed and incubated either at 4°C for 48 h (guppy #1 samples) or 15°C for 2 h (samples from guppy #2 and #3). Following incubations, samples (100 μL of each clarified supernatant) were centrifuged at 4°C for 15 min at 2370 × g, and 25 μL from each of the six processed samples was inoculated in triplicate onto <48-h-old SSN-1 cells prepared in a 96-well plate. This culture was maintained at 25°C for 8 days, at which point, due to the poor condition of the prepared 96-well plate, all six samples were syringe filtered (0.22 μM) and inoculated onto fresh SSN-1 cells. Following passage onto fresh SSN-1 cells, samples were observed for a total of 28 days, with a blind passage onto fresh SSN-1 cells performed for cultures when no cytopathic effects were observed within 14 days. Cultures demonstrating cytopathic effects were subjected to 2–3 additional passages on SSN-1 cells to produce a sufficient volume of samples for virus cryopreservation and subsequent genomic analyses.
2.2 Histology
Three moribund guppies from Case 1 and six fresh-dead guppies (five from Case 1 and one from Case 2) were fixed in 10% neutral buffered formalin (NBF) for >24 h and processed routinely for histology. These specimens were sectioned at 4 μm and stained with haematoxylin and eosin (H&E) for light microscopic examination. A few selected tissue specimens from Case 1 were also stained by Gram, Giemsa and Ziehl–Neelsen staining methods.
2.3 In situ hybridization (ISH) and immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded (FFPE) guppy samples were processed for TiLV-specific RNA and protein detection using ISH and IHC respectively. The FFPE samples were sectioned at a thickness of 4 μm and placed on positively charged slides. Following deparaffinization with a sequence of graded alcohols and xylene, ISH and IHC were conducted as separate procedures, each with its own specific protocols. For ISH, the pretreatment processes were performed with slight modifications based on previously published protocols (Adamek et al., 2023; Yamkasem et al., 2021). Briefly, the sections were incubated in citrate buffer pH 6 at 96°C for 20 min, and then were prehybridized in a buffer containing 50% (v/v) formamide and 4× saline sodium citrate (SSC) buffer at 37°C for at least 15 min. The sections were then hybridized with the digoxigenin (DIG)-labelled TiLV probe covering 491 bp of segment 3 of TiLV strain VETKU-TV08 (Yamkasem et al., 2021) at 50°C overnight in a humidified chamber. After the hybridization step, the sections were washed with a series of SSC buffer. Nonspecific binding was blocked with 0.5% (w/v) bovine serum albumin (BSA), and a secondary antibody using anti-DIG-AP Fab fragments (Roche, Basel, Switzerland) (1:200 in 1× Blocking solution) was applied, along with Liquid permanent red (LPR) (Dako, Glostrup, Denmark). The slides were subsequently counterstained with haematoxylin, and red precipitates in conjunction with cellular morphology were considered as positive. Negative controls included slides incubated with a tilapia parvovirus probe (Piewbang et al., 2022) and liver sections obtained from TiLV-negative red tilapia (Piewbang et al., 2021). For IHC, procedures were carried out following a previous study (Piewbang et al., 2021). Briefly, after deparaffinization and modification of the antigen (unmasking) by autoclaving in citrate buffer pH 6 for 5 min, endogenous peroxidase activity was inhibited through incubation with 3% (v/v) hydrogen peroxide in absolute methanol. Nonspecific binding was blocked by incubating with 5% (w/v) skimmed milk at 37°C for 1 h, followed by incubation with rabbit anti-TiLV IgG antibodies (Piewbang et al., 2021) at 37°C for 1 h in a moist chamber. After washing with 1X phosphate-buffered saline (PBS), the sections were incubated with a goat anti rabbit Ig-specific Envision polymer K5007 (Dako, Germany) for 30 min at room temperature and visualized using 3,3′-diamenobenzidine (DAB). Mayer's haematoxylin was used for counterstaining, with brown precipitates within cellular morphology indicating positive results. Negative controls included slides incubated with normal rabbit IgG as the primary antibody. Positive controls consisted of liver sections obtained from TiLV-positive red tilapia (Piewbang et al., 2022) for both the ISH and IHC procedures.
2.4 Reverse transcription-PCR (RT-PCR)
Virus stocks from both Case 1 and 2 were submitted to the National Veterinary Services Laboratories (NVSL) in Ames, Iowa, USA for additional characterization. Total nucleic acid was extracted from the viral isolates, with and without Trizol-LS pretreatment, using the MagMAX-Ambion Kit 1836 (Life Technologies) on an automated MagMax Express magnetic particle processor (Applied Biosystems, Inc., Life Technologies, Foster City, California). The RNAs were evaluated by RT-PCR using primers and probes purported to be specific for TiLV targeting regions on segments 1, 3 or 10. These included two separate conventional RT-PCRs assays: (1) Dong FS (first stage) assay using primers ME1 and Nested-Ext-1, and (2) Dong SS (second stage) assay using ME1 and 7450/150R (Dong et al., 2017); two published Taqman RT-qPCR assays: (3) Megarani et al. (2022) assay; and (4) primers and probes published by Waiyamitra et al. (2018) adapted to a one tube reaction; and two unpublished real-time RT-PCR assays: (5) CEFAS (Center for Environment, Fisheries, and Aquaculture Science assay) (personal communication) and (6) Liu assay (personal communication). PCR amplicons generated by conventional RT-PCR were subjected to Sanger sequencing in both directions with the same primer used in the RT-PCR. The sequences obtained were assembled using Sequencer software followed by Basic Local Alignment Search Tool (BLAST) analysis.
2.5 Whole genome sequencing
Whole genome sequencing and assembly of the viral genome from Case 1 was performed at the Wildlife and Aquatic Disease Laboratory, University of Florida, Gainesville, Florida, USA. Total RNA was extracted from spent cell growth media from SSN-1 cells inoculated with guppy tissue homogenates from Case 1 using a RNeasy Mini kit (Qiagen) following the manufacturer's protocol. A cDNA library was generated using a NEBNext Ultra RNA Library Prep kit (New England Biolabs) and sequenced on an iSeq 100 sequencer (Illumina) using a i1 Reagent v2 (300-cycle) kit. Kraken v2.0 was used to remove cell culture host Channa striata sequences (SRA accession number SRX228596; Wood & Salzberg, 2014). The remaining paired-end reads underwent de novo assembly, which was performed using SPAdes v3.15.3 (Bankevich et al., 2012). The assembled contigs were then subjected to BLASTX searches against the National Center for Biotechnology Information (NCBI) non-redundant protein database using OmicsBox v1.2 (BioBam).
Whole genome sequencing and analysis of the Case 2 sample was performed at the NVSL. Previously extracted total RNA (Section 2.4) was subjected to sequence-independent single primer amplification (SISPA) to generate a cDNA with random primers (Chrzastek et al., 2017). Sequencing libraries were then generated using the Illumina DNA Prep kit followed by sequencing on the Illumina iSeq 100 sequencer using a i1 Reagent v2 (300-cycle) kit. Kraken 2.0 was used to remove cell culture host sequences and contamination, and the remaining reads were subjected to two different assemblies. De novo assemblies were conducted using SPAdes v.3.13.0 and the assembled contigs were subjected to BLASTN searches against the NCBI non-redundant nucleotide database to identify closest references for each segment. Reference-based assemblies were created using BWA (v.0.7.17) to align sequencing reads to each reference segments retrieved from NCBI GenBank, including BK063200.1, BK063201.1, BK063202.1, BK063203.1, BK063204.1, BK063205.1, BK063206.1, BK063207.1, BK063208.1 and BK063209.1).
2.6 Phylogenetic and genetic analyses
For the segment 1 (PB1) phylogenetic analysis, coding sequences for 26 TiLV isolates, two TiLV-like strains from guppies (Maracas-2015-1 and Maracas-2015-2; Li, 2023) and six representative members of the family Orthomyxoviridae (outgroup) were retrieved from the NCBI GenBank database and aligned with the viruses recovered from Cases 1 and 2 (Guppy-95-10-82 and CobraB-2 respectively) in this study. The alignment of nucleotide sequences guided by amino acid translations was performed within Geneious Prime v2022.2.2 using MAFFT. MEGA v11 (Tamura et al., 2021) was used to determine best-fit model and generate maximum likelihood phylogenetic tree with 1000 non-parametric bootstraps.
The BLASTX analyses revealed that the remaining segments (2–10) were only available for 23 TiLV isolates within the NCBI GenBank database and showed no sequence similarity to the representative members of the family Orthomyxoviridae. Hence, phylogenetic analyses for segments 2–10 were only performed based on the coding sequences of 23 TiLV isolates, two TiLV-like strains (Maracas-2015-1 and Maracas-2015-2; Li, 2023) and the guppy isolates (Guppy-95-10-82 and CobraB-2) from this study. Nucleotide sequence alignments and phylogenetic analyses were performed as described above. For the genetic analyses, nucleotide and amino acid sequences of all 10 segments of TiLV isolates, TiLV-like strains and guppy isolates in this study were compared using the Sequence Demarcation Tool v1.264 (Muhire et al., 2014) with the MAFFT 7 alignment option implemented.
3 RESULTS
3.1 Disease cases and diagnostics
3.1.1 Case 1
The wholesaler reported sustained mortality in the imported guppies for over 7 days post-shipping. Clinical signs and gross changes in affected fish included lethargy, anorexia, coelomic distention, pale gills, raised scales, hyperaemia and skin ulcers (Figure 1). Water quality parameters were found to be within acceptable ranges for guppies (ammonia 0 ppt, nitrite 0 ppt, nitrate 50 ppt, pH 8, dissolved oxygen 90%, temperature at approximately 25°C and salinity 2 g L−1). Before submitting for diagnostics, the guppies were treated with formalin, potassium permanganate, salt, malachite green, kanamycin, doxycycline, oxytetracycline, nitrofurazone, enrofloxacin, erythromycin, praziquantel and metronidazole by the wholesaler. After overnight shipping to the diagnostic laboratory, 13 out of 30 fish were found dead upon arrival. Among dead fish, eight exhibited cloudy eyes with moderate external autolysis, while the remaining five deceased fish had clear eyes and pink to red gills. Of the 17 fish that were still alive, one moribund fish was lethargic and swimming very close to the water surface with weakened caudal fin movements. Skin scrapes on six remaining fish revealed mild protozoan parasite infestation in three of them. No granulomas were observed in squash preparations of internal organs, except for one of the six examined fish, which presented a live motile metazoan (suspected nematode). Pseudomonas sp. and Aeromonas sp. were recovered in the inoculated agar plates from two out of the three and one out of the three evaluated fish respectively.

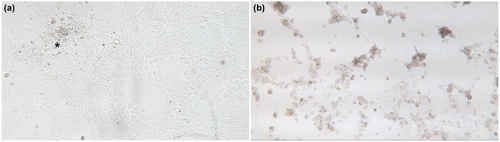
To investigate the possibility of a viral aetiology, pooled organ homogenates were used to inoculate five fish cell lines and observed over a period of 2 weeks. Cytopathic effect, characterized by vacuolation, was detected 4 days post-inoculation (dpi) in EPC cell lines, followed by cell rounding, lysis and lifting of the monolayer by 10 dpi (Figure 2). Similar CPE was observed in SSN-1 cells at 14 dpi (Figure 2). No CPE was appreciated in other tested cell lines. The virus isolate was subsequently designated as Guppy-95-10-82.
3.1.2 Case 2
The young hobbyst reported that approximately 3 days post-acclimation, guppies exhibited lethargy and flashing. Despite initial treatments, fish continued to display signs of disease, including loss of appetite, lethargy, hanging at the water surface, raised scales and skin lesions (Figure 1b).
At approximate 7 days post-inoculation onto fresh SSN-1 cells (following the abbreviated first passage detailed in methods), SSN-1 cells began exhibiting CPE in the form of moderate to severe cell lysis and vacuolated cells. These CPE were observed in two out of the six prepared samples, each from a separate guppy (guppy #2 and guppy #3). Following consistent observation of CPE, two virus isolates (one from each sample exhibiting CPE) were produced as described above and are designated herein as CobraB-2 and CobraB-3.
3.2 Histology
In Case 1, two out of the three submitted moribund fish exhibited microscopic renal pathology. Of particular note, one of the submitted fish showed evidence of intratubular organisms which were presumed to be myxozoans. The microscopic appearance of these organisms resembled that of Hoferellus sp., as described in other teleost species. In the liver, hepatocellular vacuolation was also observed in two of the three examined fish. Hepatocytes of captive-reared fish are often vacuolated due to the presence of intracellular lipid or glycogen (typically attributed to calorie-rich diets). In this case, one of the examined fish had appreciable hepatocellular vacuolation due to lipid, one with glycogen and the third fish did not express vacuolation. While non-specific, the lack of hepatocellular vacuolation can be associated with a variety of factors including stress, prolonged disease or malnutrition.
One of the five fresh dead guppies were also presented with intratubular organisms within the kidney. All other described renal changes were mild and non-specific. Tubular changes included dilation/ectasia (2/5), minimal to mild renal tubular epithelial degeneration and necrosis (3/5) and mild multifocal tubular mineralization or nephrocalcinosis (2/5). The sporadic degenerate/necrotic renal tubular epithelial cells were hypereosinophilic with pyknotic or karyorrhectic nuclei and were present within the lumen of the tubules. A subset of the ectatic tubules contained globules of eosinophilic proteinaceous material.
Karyorrhectic debris (2/5) and occasional small aggregates of macrophages with golden brown intracytoplasmic pigment (3/5) were noted within the renal interstitium. In four of the submitted fish, the hepatocytes throughout the liver contained clear intracytoplasmic vacuoles with sharply demarcated borders (consistent with lipid; Figure 3a,b). One of the five livers was not evaluated. In two of the submitted fish specimens, scattered myocytes were fragmented or moth-eaten with loss of cross-striations.
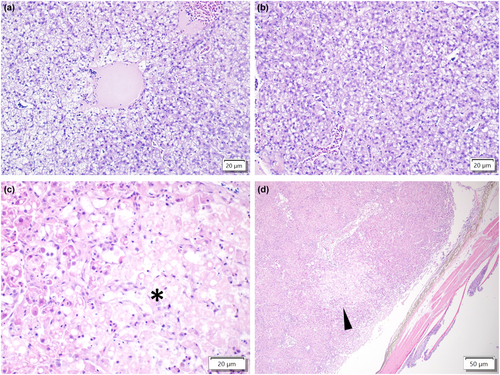
In Case 2, histology of a single fish was performed. Within the liver, there was a focal region of pallor characterized by hypoeosinophilia and loss of cellular detail consistent with acute hepatic necrosis (Figure 3c,d). Sporadic hepatocytes within the surrounding parenchyma had pyknotic or karyorrhectic nuclei. The remainder of the liver was largely unremarkable with rare binucleate hepatocytes, cells with mildly enlarged nuclei, low numbers of perivascular to interstitial lymphocytes, plasma cells, eosinophilic granulocytes and generalized hepatocellular vacuolation. These vacuoles were clear with sharply demarcated borders consistent with lipid. Few renal tubules contained basophilic mineralized material consistent with nephrocalcinosis. Small numbers of lymphocytes, plasma cells and histiocytes were noted within the epicardium of the heart. Endothelial cells lining the heart were often plump and reactive. Pigmented macrophage aggregates were noted in the spleen, kidney and heart.
3.3 In situ hybridization and immunohistochemistry
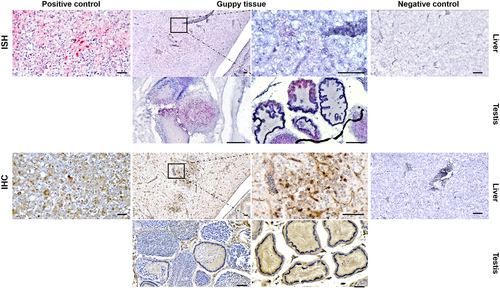
Weak DNA hybridizations using probes designed from segment 3 of TiLV strain VETKU-TV08 were observed localized in the cytoplasm of vacuolated hepatocytes. In contrast, specific staining with antibodies generated to TiLV in the IHC assay was abundant within the same cytoplasmic area that showed ISH positivity (Figure 4). Moreover, the positive antibody binding signals, indicating the presence of viral proteins, were easily observed in the germinative compartment, while the virus proteins were detected in both the germinative and interstitial compartments of the testis.
3.4 RT-PCR
The Guppy-95-10-82 and CobraB-2 RNA extracts from the growth media collected from infected cell cultures tested positive for some of the unpublished and published methods for the detection and quantification of TiLV (Table 1). The guppy viruses were positive for all the RT-PCR assays except for the unpublished assay of Liu and Waiyamitra et al. (2018).
| RT-PCR assay | Segment | Primer 1 | Primer 2 | Probe | Guppy-95-10-82 | CobraB-2 |
|---|---|---|---|---|---|---|
| Megarani et al. (2022) | 10 | TiLV-F | TiLV-R | TiLV-P | pos | pos |
| Waiyamitra et al. (2018) | 3 | TiLV-93F | TiLV-93R | TiLV-93Probe | neg | neg |
| Liu | 3 | TiLV-F1 | TiLV-R1 | TiLV-P1 | neg | neg |
| CEFAS | 1 | TiLV CEFAS qFor2 | TiLV CEFAS qRev | TiLV CEFAS qProbe | pos | pos |
| Dong FS (first stage) | 3 | ME1 | Nested ext-1(NE-1) | NA | posa | posa |
| Dong SS (second stage) | 3 | ME1 | 745/150R | NA | pos | pos |
3.5 Whole genome sequencing, phylogenetic and genetic analyses
The next-generation sequencing of Case 1 (Guppy-95-10-82) generated 10,319,134 reads, in which 88% of these reads were classified as the host genome and removed by Kraken v2.0. The de novo assembly of remaining paired-end reads (12%) by SPAdes 3.15.3 resulted in the complete coding sequences for all 10 segments.
Three libraries prepared from different cell passages of the CobraB-2 virus generated 124,184, 253,822 and 324,611 reads. Kraken v2.0 classified 73%, 59% and 65% of the reads as snakehead retrovirus, a known contaminant of both SSN-1 and E11 cells, and these reads were excluded from the analysis. The de novo and reference-based assemblies of the remaining reads (23%, 32% and 28%) yielded coding sequences for all 10 segments.
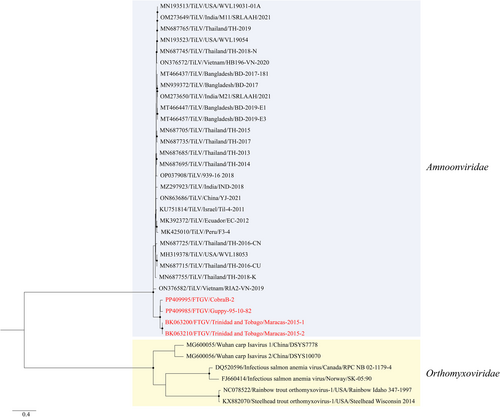
Phylogenetic analysis based on the coding sequence of segment 1 (PB1) showed that the Guppy-95-10-82 and CobraB-2 isolates of this study and the previously reported TiLV-like strains (Maracas-2015-1 and Maracas-2015-2) formed a well-supported basal branch within the family Amnoonviridae (Figure 5) with bootstrap value greater than 80%. Analyses based on segments 2, 3, 4, 6, 7, 8, 9 and 10 also showed the distinct and highly supported grouping of the guppy isolates (Guppy-95-10-82, CobraB-2, Maracas-2015-1 and Maracas-2015-2) separate from TiLV isolates (Figures S1–S9). Analysis of the segment 5 showed that the clade formed by guppy isolates were not supported with bootstrap value greater than 80%. The genetic analyses showed that all 10 segments of the guppy isolates shared 77.8%–92.2% and 77.9%–97.7% nucleotide and amino acid identities, respectively, to TiLV isolates (Table 2). On the other hand, the guppy isolates shared 92.7%–100% and 92.1%–100% nucleotide and amino acid identities, respectively, to each other. The most conserved segment between the guppy isolates was segment 9, presenting 95.7%–100% nucleotide identities between the isolates; yet, this segment only presented 88.6%–91.5% nucleotide identity between TiLV isolates and the guppy isolates (Table 2).
| Segment | Among FTGV isolates | FTGV versus TiLV | Among TiLV isolates | |||
|---|---|---|---|---|---|---|
| Nucleotide identity range (%) | Amino acid identity range (%) | Nucleotide identity range (%) | Amino acid identity range (%) | Nucleotide identity range (%) | Amino acid identity range (%) | |
| 1 | 93.1–100 | 96.9–100 | 84.2–86.2 | 94.8–97.7 | 90.4–99.9 | 97–1-100 |
| 2 | 93.6–99.9 | 97.6–99.6 | 83.5–85.3 | 95.2–96.9 | 92.4–99.9 | 97.2–100 |
| 3 | 94.0–99.8 | 97.4–100 | 85.2–87.1 | 93.1–95.5 | 91.0–99.8 | 96.4–100 |
| 4 | 93.0–100 | 94.1–100 | 84.6–86.5 | 87.9–91.0 | 92.2–100 | 96.0–100 |
| 5 | 91.5–100 | 92.1–100 | 80.6–83.2 | 83.0–86.5 | 90.4–100 | 94.7–100 |
| 6 | 92.7–99.9 | 94.6–100 | 77.8–80.9 | 77.9–81.4 | 88.7–100 | 92.1–100 |
| 7 | 93.7–100 | 95.4–100 | 80.3–83.8 | 88.1–90.3 | 92.2–99.8 | 95.9–100 |
| 8 | 95.6–100 | 98.3–100 | 89.7–92.2 | 96.0–97.7 | 95.4–99.6 | 97.7–100 |
| 9 | 95.7–100 | 93.1–100 | 88.6–91.5 | 79.3–84.5 | 94.6–100 | 93.1–100 |
| 10 | 93.9–100 | 93.8–100 | 88.3–91.8 | 85.8–93.8 | 93.9–100 | 91.2–100 |
4 DISCUSSION
Routine investigation of unusual mortality in guppies imported to the USA from an international source and in guppies ordered through an online vendor in the USA has revealed the presence of a new virus with a common name designated now as fancy-tailed guppy virus (FTGV).
Our results suggest the inclusion of FTGV into the family Amnoonviridae. This family is comprised of a single classified species, Tilapinevirus tilapiae (Koonin et al., 2023), but other unclassified members have been discovered (Li, 2023; Turnbull et al., 2020) through sequencing efforts, including two recent genomes discovered in transcriptomic data from guppies (Li, 2023). The Amnoonviridae have a negative sense genome with 10 segments. The phylogenetic analyses of all 10 segments clearly demonstrate that the FTGV isolates of this study, along with the previously published viral genomes from guppies (Li, 2023), formed a well-supported clade (bootstrap values >80%) distinct from TiLV (Figures 5 and S1–S9). Additionally, the nucleotide and amino acid identities between FTGV and TiLV are disparate from each other (Table 2). These phylogenetic and genetic analyses suggest inclusion of FTGV in the family Amnoonviridae with the closest relative being the only member in this family, TiLV. Since there are no criteria for species demarcation, we propose the classification of FTGV as a member of the family Amnoonviridae, with the scientific name Tilapinevirus poikilos (from the Greek ‘poikilos’, meaning of many colours; various sorts, akin to ‘poecilia’). Our demarcation of FTGV as a new virus species is based upon the apparent affinity of this virus for guppies and the aforementioned phylogenetic and genetic analyses.
The results of RT-PCR assays developed specifically for TiLV also exemplify the close yet distinct phylogenetic relationship between TiLV and FTGV. Six different assays were performed utilizing nucleic acid from the Guppy-95-10-82 and CobraB-2 isolates as the templates. Positive results were obtained with four of the assays, while the Liu (Hong) and Waiyamitra et al. (2018) assays generated negative results (Table 2). Examination of the genome sequences at the primer and probe binding sites for these assays fully explain the results. The sequences of the FTGV isolates contain numerous single nucleotide polymorphisms (up to five) at these regions of the Liu (Hong) and Waiyamitra et al. (2018) assays (Figure S10), which likely prevented suitable amplification. Future research should develop specific primers and probes for RT-PCR detection of FTGV.
Although River's postulates have not been fulfilled, the isolation of FTGV in both cases and lack of efficacy of antibiotic and other treatments strongly suggests its involvement in the mortality events observed. Given the close relationship of FTGV to TiLV, it is of interest to compare the clinical signs and pathology. The diseased guppies showed non-specific clinical signs including lethargy loss of appetite, coelomic distention, pale gills, raised scales and occasional lesions, much akin to those observed with TiLV (Bacharach et al., 2016; Surachetpong et al., 2017, 2020). Histological findings in the examined guppies from Cases 1 and 2 were generally mild and non-specific. While rare binucleated hepatocytes were appreciated in the liver of the guppy from Case 2, no syncytia or viral inclusions were appreciated in the examined tissues. Moreover, no lesions were observed in the brain of infected fish. In contrast, TiLV infections in tilapia have tropism to the liver and the brain. In the liver, they tend to cause hepatic lesions characterized by syncytial cell formation and hepatocellular degeneration and necrosis (Del-Pozo et al., 2017; Ferguson et al., 2014; Pierezan et al., 2019, 2020). In the brain, gliosis and perivascular cuffing are the most common pathology associated with TiLV infections (Eyngor et al., 2014; Jaemwimol et al., 2018; Pierezan et al., 2019, 2020; Tattiyapong et al., 2017).
FTGV was detected in the liver and gonad cells of guppies in both Case 1 and 2 using ISH and IHC assays designed for TiLV. The ISH reactions were weak due to poor binding of the probe that was designed using the sequence from segment 3 of the TiLV isolate KUVET-TV08. Analysis of the probe binding site in FTGV revealed several nucleotide differences likely accounting for poor probe binding (Figure S11). The IHC staining results were similar in localization, but stronger than the ISH. The positive reactions indicate cross-reactivity of the rabbit antibodies used and suggest antigenic similarity between FTGV and TiLV. It is not uncommon for polyclonal antibodies to cross-react with closely related viruses (Bollweg et al., 2018). In contrast, tilapia infected with TiLV typically exhibit positive IHC reactions in multiple organs, including the intestine, liver, gills and gonads (Piewbang et al., 2021, 2022; Pierezan et al., 2020). Both ISH and IHC techniques have proven useful in understanding the pathogenesis of TiLV, and such tools should be developed specifically for FTGV to increase our knowledge of its pathogenesis.
Our findings highlight the discovery of a new amnoonvirus, FTGV, associated with high morbidity and mortality in guppies. The research presented herein has built a foundation to increase our knowledge of its importance. Future research should focus on experimental infections in guppies and other fish species to fulfil River's postulates and to elucidate clinical disease signs and pathology, develop specific assays for identification and detection and perform surveillance or epidemiological studies to determine whether this virus may be emergent as defined by the World Organisation for Animal Health (WOAH).
AUTHOR CONTRIBUTIONS
Esteban Soto: Conceptualization; investigation; funding acquisition; writing – original draft; methodology; visualization; writing – review and editing; formal analysis; project administration; supervision; resources. Benjamin R. LaFrentz: Investigation; methodology; writing – original draft; writing – review and editing; formal analysis; conceptualization; visualization; supervision; project administration. Susan Yun: Investigation; writing – review and editing; formal analysis; methodology; visualization. Dorothea Megarani: Investigation; writing – review and editing. Eileen Henderson: Investigation; writing – review and editing; visualization. Chutchai Piewbang: Investigation; writing – review and editing. Amber E. Johnston: Investigation; writing – review and editing. Somporn Techangamsuwan: Investigation; writing – review and editing. Terry Fei Fan Ng: Investigation; writing – review and editing. Janet Warg: Investigation; visualization; methodology; formal analysis; supervision; resources; writing – original draft. Win Surachetpong: Investigation; writing – review and editing; visualization. Kuttichantran Subramaniam: Investigation; conceptualization; funding acquisition; writing – original draft; methodology; visualization; project administration; supervision; data curation; resources.
ACKNOWLEDGEMENTS
We acknowledge the University of California-School of Veterinary Medicine for financial support. The authors would also like to acknowledge Bryce LaFrentz for his enthusiasm and passion for all things fish.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




