A stable isotope analysis of the dietary patterns of the aquatic apex predator, the African tigerfish (Hydrocynus vittatus)
Abstract
Stable isotope analyses, specifically δ13C and δ15N, are useful tools increasingly used to understand ecosystem function, food web structures, and consumer diets. Although the iconic tigerfish Hydrocynus vittatus is regarded as an apex predator in southern African freshwater systems, little information is available regarding their feeding behavior and how this may change with growth or differ between ecosystems, with most information stemming from stomach content analyses (SCA). The aim of the present study was to address this lack of information through a baseline study of the diet of large and small tigerfish in various lentic and lotic ecosystems in South Africa using stable isotope methods. Fish and various food web components and food sources were collected from two river and two lake ecosystems in South Africa. The δ13C and δ15N values for all samples were determined and multivariate analyses and Bayesian analytical techniques applied to determine the feeding ecology of H. vittatus and how this may differ with size and habitat type. Analyses revealed a substantial difference in the type and abundance of food sources contributing to the diet of H. vittatus between ecosystems, most prominently between the lotic systems, where less dietary specialization was observed, and lentic systems where more specialization was observed. Furthermore, there was a distinct difference in diet between small and large tigerfish, especially in the lotic system, indicating an ontogenetic diet shift as tigerfish grow and further supporting previous SCA studies. This is the first study of its kind on the African continent for H. vittatus and the findings illustrate the value of stable isotope analysis in providing in-depth information into the feeding ecology of consumers and how this may differ between size classes and habitat types.
1 INTRODUCTION
Stable isotopes analysis (SIA) of carbon and nitrogen stable isotope ratios can provide valuable and unique insights into trophic linkages of natural ecosystems and has increasingly been used in ecosystem research, especially ecology-based food web studies (de Necker et al., 2020; Keough et al., 1996; Peterson & Fry, 1987). Ratios of carbon isotopes (δ13C) are useful in indicating energy flow between primary producers and consumers as well as links between consumers, whilst nitrogen isotope ratios (δ15N) are indicative of the positions that organisms occupy within the food web hierarchy (Adams & Sterner, 2000; Keough et al., 1996; Kraemer et al., 2012). Food web studies form the basis of ecosystem function research since interactions such as those of predator–prey may impact vital ecosystem processes, including nutrient cycling (Bunn & Boon, 1993; Thompson et al., 2012; Wood et al., 2016).
The dominant carbon pathways within food webs are particularly identifiable through SIA. SIA can uncover many important feeding links between consumers and their primary food sources (Jepsen & Winemiller, 2002; Phillips & Eldridge, 2006) as well as effectively describing the ecological niche an organism occupies (Bearhop et al., 2004; Desta et al., 2006; Kraemer et al., 2012; Thompson et al., 2012). Isotopic approaches are advantageous as they provide researchers with temporally integrated information on the dietary habits of organisms. This is because stable isotopes accurately reflect the foods that are assimilated by the consumer (Davis et al., 2012; Jepsen & Winemiller, 2002).
Trophic assemblages in tropical and subtropical river systems are typified by increased taxonomic diversity and a wide array of foraging modes, including omnivory, herbivory, and piscivory (Jepsen & Winemiller, 2002; Pusey et al., 2010; Rayner et al., 2010). Omnivory occurs in large predatory fishes frequently occupying similar trophic positions to their smaller bodied counterparts, or because of reduced preferential food sources, which in turn may suggest that many freshwater fishes in fact feed across multiple trophic levels (de Necker et al., 2022; Jensen et al., 2012; Jepsen & Winemiller, 2002; Layman et al., 2005). The determination of ontogenetic dietary shifts in fishes using SIA is increasing, and the use of δ15N as an indicator of trophic position and size-related dietary shifts is commonplace (Davis et al., 2012; Galván et al., 2010; Herlevi et al., 2018). Most dietary shifts in fishes have been demonstrated from simple food webs where pronounced isotopic shifts were anticipated (Jensen et al., 2012; Post, 2002, 2003), such as lentic systems (de Necker et al., 2020, 2022; Thompson et al., 2012). However, river food webs have a more complex trophic structure with a more diverse array of production sources. This increased diversity makes isotopic evidence of dietary shifts in lotic ecosystems potentially difficult to document (Davis et al., 2012).
Tigerfish (Hydrocynus vittatus Castelnau 1861) are an ideal case study species to assess the practicality of SIA as a means of determining ontogenetic dietary shifts in subtropical freshwater fishes, as they are presumed to undergo several ontogenetic dietary shifts. A handful of studies using traditional stomach content analysis (SCA) have found evidence of these dietary shifts (Dalu et al., 2012; Gaigher, 1970; Marshall, 2011; Mhlanga, 2003; Winemiller & Kelso-Winemiller, 1994). It is postulated that H. vittatus fry feed on small invertebrates or zooplankton, progressively taking larger plankton and insects and, finally, fish (Kenmuir, 1975; Marshall, 2011; Skelton, 2001), as indicated by Marshall (2011). Due to the different life stages, H. vittatus potentially feeds across different carbon sources and several different trophic levels throughout their lifetime. The major limitation of SCA studies involving H. vittatus is that screened stomachs are often empty (up to 90% of captured individuals) and when stomach contents were found, they have been digested to such a degree that they are unidentifiable (Dalu et al., 2012). As such, SIA approaches may prove particularly useful in disentangling H. vittatus diet and ontogenetic shifts.
H. vittatus are both ecologically and economically important as they are one of Africa's primary predatory freshwater fish (Skelton, 2001; Winemiller & Kelso-Winemiller, 1994). These predators are considered the most sought-after freshwater gamefish in Africa and support a major fishery (Gerber et al., 2017; Smit et al., 2009). Additionally, H. vittatus are relatively long lived, up to 20 years (Gerber et al., 2009), and thus can exert significant top-down pressures or control within the systems that they inhabit. Consequently, the scant stable isotope-based evidence available regarding the feeding ecology of this important species serves as motivation that further research is needed to understand H. vittatus's ecological role in African ecosystems.
The main aim of this study was to use stable δ15N and δ13C isotopes to elucidate the feeding ecology of South African H. vittatus populations across lotic and lentic ecosystems. This is the first such study for H. vittatus from South Africa and the first for H. vittatus in lotic ecosystems. Throughout the African continent and H. vittatus's distribution, this study is second only to the study by Marufu et al. (2017). The specific objectives of this study were (1) to determine the trophic position of H. vittatus across four different aquatic ecosystems, (2) to determine and compare the dietary habits of large and small H. vittatus from lotic and lentic ecosystems, and (3) to use SIA to confirm ontogenetic dietary shifts in the species as previously indicated by SCA.
2 MATERIALS AND METHODS
2.1 Ethics statement
The care and use of animals complied with South African animal welfare laws, guidelines, and policies as approved by the University of Johannesburg Faculty Ethics Committee, South African National Parks, and Ezemvelo KZN wildlife (OP 674/2012, OP 5139/2012, OP 526/2014, and OP 839/2014).
2.2 Study area and sampling
Tigerfish and various food web elements were collected synchronously from four aquatic systems in South Africa during the period April 2010 to June 2011 (Figure 1). The selected aquatic ecosystems consisted of two lotic (Luvuvhu and Olifants rivers) and two lentic (Lakes Jozini and Nyamithi) systems along South Africa's eastern boundary with Mozambique. The sampled sections of the lotic systems fall within the conservation area of the Kruger National Park (KNP). Sites in the KNP were chosen as part of a larger project (Smit et al., 2013) where food webs were assessed using SIA. The Luvuvhu River was sampled twice, once during the high-flow period and once during the low-flow period. The two lentic systems form part of the Phongolo River system and its floodplain (PRF). Lake Jozini is dammed by the Pongolapoort Dam and Lake Nyamithi, located in Ndumo Game Reserve, is the largest conserved floodplain lake of the PRF. The lentic systems were also part of another larger study (Smit et al., 2016) during which food webs were assessed using SIA.
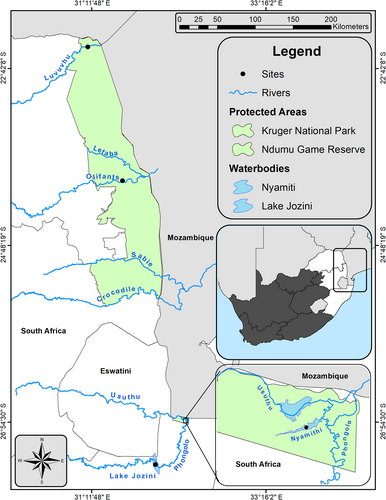
2.3 Field collections
2.3.1 Primary producers
In each of the systems as many elements forming the base of the food web (primary producers) as possible were sampled. Replicates of particulate organic matter (POM) were collected from a 25-cm2 area of sediment at each site. A single composite biofilm sample was taken from each site, consisting of four replicates of biofilm collected from rocks using a toothbrush. A single plankton sample was collected from each site, using a 64-μm plankton net. The biofilm and plankton samples were individually centrifuged at 1500g for 5 min. and the pellets of each sample were placed in individual tubes. Leaf litter from the surrounding riparian vegetation and hippo dung/grass litter present in the water were also collected. All primary producer samples were frozen at −12°C in the field and transported back to the laboratory for further analysis.
2.3.2 Fish and macroinvertebrate sampling
Due to the similarity in fish and macroinvertebrate assemblages of the selected systems, similar fish species and invertebrate taxa were targeted at each site. A minimum of four to a maximum of 10 individuals/replicates of each fish species and invertebrate taxa were collected. Various sampling techniques, including rod and line (targeting H. vittatus and Clarias gariepinus [Burchell, 1822]), cast netting, electrofishing, and seine netting, were applied to ensure a holistic composition of fishes was sampled. The H. vittatus individuals were separated into two groups, namely large H. vittatus (>200 mm standard length [SL]) and small H. vittatus (<140 mm SL); no tigerfish were sampled between these two size categories. Captured fishes were identified to species level (Skelton, 2001) and the necessary number of individuals euthanised by severing the spinal cord. Approximately 5 g of axial muscle tissue (skinned and boneless) was removed from the left side of larger specimens (>50 mm), whereas small individuals (≤50 mm) were sampled as whole specimens, with fins, scales, the head, and organs removed prior to freezing. Representative macroinvertebrate samples were collected from all available habitat biotopes using a 500-μm mesh size sampling sweep net. The aquatic macroinvertebrate identification guide by Gerber and Gabriel (2002) was used to identify the different representatives of the target invertebrate families. Individuals of the same families were picked out and placed in tubes to make up a minimum of four replicates (~5 g) of each family.
The sampled muscle tissue and macroinvertebrates were immediately frozen in liquid nitrogen (−196°C). Samples were removed from the liquid nitrogen on arrival at the analytical facilities and stored at −80°C until further processing. Samples were dried in an oven for 72 h at 50°C and ground into a fine powder using a sterile pestle and mortar. The 10% HCL was used to clean equipment between samples i.e. the grinding phase not between steps as such. The POM was sieved using a 0.5-mm sieve and the smaller particles were used for analysis. Approximately 1 g of the ground fish tissue and macroinvertebrate samples was transferred into test-tubes with screw caps and pre-treated with a 2:1 chloroform:methanol (v:v) solution for 12 h to remove lipids. Lipid extraction was carried out following Van der Merwe et al. (2022) since this method is more accurate than applying lipid correction factors. During this period, samples were kept in the dark at 4°C. Following lipid extraction, samples were centrifuged (1500×g) for 2 min, the supernatant was discarded, and the pellet was oven dried (24 h at 50°C). Each dried sample was accurately weighed (consumers ±0.5 mg, primary producers ±5 mg, POM ±100 mg) and encapsulated in ultrapure tin capsules (D1008, 8 × 5 mm; Elemental Microanalysis) prior to analysis.
2.4 Isotopic analysis
2.5 Data analyses
2.5.1 Trophic position
2.5.2 Trophic group variability
A discriminant function analysis (DFA) was performed for each of the sampled rivers using SPSS version 18 (PASW Statistics, IBM). The DFA was used to determine whether nitrogen and carbon isotope ratios and total C:N ratios could be used to accurately reclassify individual H. vittatus samples into their respective trophic guilds. It was also used to assess whether small and large H. vittatus from the different systems could be differentiated from other components and other fish species found in these systems. A posteriori tests using Fisher's function coefficients were used to examine the probability of membership of individual samples to each of the abovementioned groups. The reclassification success from the DFA was used to estimate feeding variability between trophic groups, but more specifically the feeding differences between H. vittatus (large and small) compared to other fish species.
2.5.3 Diet composition
To ascertain the potential food sources and their contributions to the diet of small and large H. vittatus the sources were pooled into biologically meaningful groups and the different analyses follow those of de Necker et al. (2020, 2022). The pooling of multiple sources improves the discriminatory power of Bayesian mixing models and is a recommended method for the improved discrimination between sources and in providing a better constrained solution to such models (Manfrin et al., 2018; Phillips et al., 2005, 2014; Stock et al., 2018). Sources were initially separated into three overarching groups based on their role in the food web: primary producers—various plants, detritus, POM, biofilms; invertebrates—all invertebrates and zooplankton; fish—all fish species from all trophic guilds. The results from the initial analyses (described in detail below) steered the further division of the biological groupings and which groupings were used in the secondary analyses. The secondary analyses were done to provide detailed insights to potential food sources and their respective contributions. For primary producers the groups were further subdivided into C3 and C4 plants, biofilms, detritus, and POM. Invertebrates were subdivided into their respective Orders, such as Hemiptera, Decapoda, Coleoptera, and Plecoptera, and fish were divided into family levels, i.e. Mochokidae, Clariidae, Alestidae, Cyprinidae, Mormyridae, and Cichlidae. Fish from the Cichlidae were further divided into the four species (Oreochromis mossambicus (Peters, 1852): detritivorous, Coptodon rendalli (Boulenger, 1897): omnivorous, Pseudocrenilabrus philander (Weber, 1897): predatory, and Tilapia sparmanii Smith, 1840: invertivorous) sampled across the systems as each of these species differs in their feeding and some were extremely dominant within some of the systems. The initial groupings specified which group small and large H. vittatus are feeding on, whereas the second, more in-depth, analyses elucidated which components of the food web they are specifically feeding on.
A mixing polygon was created for each system prior to each of the different diet composition analyses to ascertain whether the consumers were situated within the convex mixing polygon of the potential food sources (Lemmens et al., 2017, Phillips et al., 2014, Smith et al., 2013, Valladares et al., 2017). The sp and splancs packages for the R environment (Smith et al., 2013; R Core Team 2017) were used to simulate the mixing polygon region of each model. No H. vittatus individuals were located outside the 95% mixing polygon region, therefore all consumers could be included in the final model. The diet composition of H. vittatus in the various systems was assessed using the hierarchical Bayesian stable isotope mixing model MixSIAR (Stock & Semmens, 2016). By using Bayesian techniques paired with the isotopic ratios of food sources and consumers these mixing models provide the likely percentage contribution of each dietary source to the overall diet of the consumer in question (Boecklen et al., 2011; de Necker et al., 2020, 2022; Manfrin et al., 2018; Marchese et al., 2014; Parnell et al., 2010; Stock et al., 2018).
Several mixing models were investigated using MixSIAR: two each for the large H. vittatus in the Olifants River, Lake Jozini and Lake Nyamithi, respectively, two each for the small H. vittatus in Lake Nyamithi and the Luvuvhu River, respectively, and two each for a seasonal comparison in the large H. vittatus diet in the Luvuvhu River. Each mixing model was run using raw stable isotope data by survey from all collected H. vittatus as individual consumers and the potential prey groups as the food source groups. Fractionation correction factors of 1.0‰ ± 0.25‰ for δ13C (Akamatsu et al., 2004; DeNiro & Epstein, 1978) and 3.37‰ ± 1.3‰ for δ15N (Taylor et al., 2017) were applied to the consumer isotopic ratios. Consumer isotopic ratios were included as fixed effects into the models and ‘Process only’ error terms used. Mixing models were performed for each system, consumer size class, and, where applicable, per season using the three-chain Markov Chain Monte Carlo (MCMC) parameters. The model was only accepted on convergence of all three chains, where the convergence was based on the Gelman–Rubin (if <1.05) and Geweke diagnostic tests (if <5% of values were outside ±1.96) (Stock & Semmens, 2016). The MCMC chain length for the models was calculated using 50,000 iterations with a burn-in phase of 25,000 iterations and thinned by 25 iterations, producing the most likely dietary proportions with mean, mode, levels of uncertainty, and 95% credibility intervals.
3 RESULTS
Large H. vittatus were captured from all systems and both surveys to the Luvuvhu River. Small H. vittatus were captured in Lake Nyamithi and the Luvuvhu River during the high-flow survey. A total of 110 H. vittatus individuals were analyzed for their respective δ13C and δ15N values from the two lotic and two lentic systems. Their respective biometric and isotopic data as well as trophic position are presented in Table 1. Twenty-one of these were small tigerfish (<140 mm) and 89 were larger individuals (>200 mm). A further 544 food web components were analyzed for their respective δ13C and δ15N values from the various selected systems and formed part of the formal data analyses. These samples consisted of various primary producers (n = 135), invertebrates (n = 109), and fish species (n = 300). Primary producers included leaf litter, grass litter/hippo dung, POM, sediment, plankton, and biofilm. Invertebrates from 25 different taxa were collected and similar fish species from the selected systems comprising 23 species were collected. These samples represent all available habitats and different feeding modes in the selected systems.
| System | n | Standard length (mm) | δ13C (‰) | δ15N (‰) | C:N | Trophic position |
|---|---|---|---|---|---|---|
| Large Hydrocynus vittatus | ||||||
| Luvuvhu River (HF) | 19 | 340 ± 22 (221–509) | −20.16 ± 0.57 (−21.06–19.14) | 15.20 ± 0.59 (14.45–16.49) | 3.32 ± 0.15 (3.14–3.77) | 3.93 ± 0.17 (3.70–4.31) |
| Luvuvhu River (LF) | 16 | 290 ± 22 (205–430) | −19.94 ± 1.13 (−22.49–−18.22) | 15.23 ± 1.01 (13.29–16.93) | 3.23 ± 0.22 (3.07–3.77) | 3.78 ± 0.35 (3.05–4.33) |
| Olifants River | 16 | 314 ± 15 (233–456) | −21.45 ± 0.76 (−22.39–−19.66) | 18.33 ± 0.54 (17.27–18.93) | 3.40 ± 0.10 (3.22–3.65) | 4.28 ± 0.16 (3.97–4.46) |
| Lake Jozini | 18 | 443 ± 110 (260–614) | −18.81 ± 0.61 (−19.80–−17.80) | 16.72 ± 0.57 (15.85–18.06) | 3.16 ± 0.05 (3.09–3.24) | 4.01 ± 0.17 (3.75–4.41) |
| Lake Nyamithi | 20 | 322 ± 52 (263–435) | −22.21 ± 0.55 (−23.27–−21.26) | 14.12 ± 0.72 (12.64–15.24) | 3.40 ± 0.18 (3.10–3.80) | 3.34 ± 0.21 (2.90–3.67) |
| Small Hydrocynus vittatus | ||||||
| Luvuvhu River (HF) | 13 | 90 ± 11 (69–108) | −21.51 ± 1.16 (−23.43–−19.24) | 12.39 ± 0.32 (11.86–13.13) | 3.12 ± 0.03 (3.07–3.17) | 3.14 ± 0.10 (2.99–3.36) |
| Lake Nyamithi | 8 | 116 ± 16 (108–133) | −23 ± −0.52 (−23.74–−22.19) | 14.75 ± 0.23 (14.44–15.20) | 3.12 ± 0.02 (3.08–3.17) | 3.52 ± 0.07 (3.43–3.66) |
- Abbreviations: HF, high flow; LF, low flow.
3.1 Trophic position and trophic group assessments
The different trophic levels are clearly demonstrated in the biplots of δ13C and δ15N values indicated in Figure 2, with primary producers (sediments, plant matter, plankton, and biofilms) forming the base of the food webs. Macroinvertebrates and fishes formed second and third “tiers”, respectively, and the larger H. vittatus positioned at the very top of the food chains as apex predators. The small tigerfish showed some variability in their isotopic signatures, where the individuals from the Luvuvhu River fell between the other fish species in the food web. However, the individuals from Lake Nyamithi occupied similar positions to their larger counterparts. The food chains in the Olifants River and Lake Jozini were longer than those from the Luvuvhu River and Lake Nyamithi.
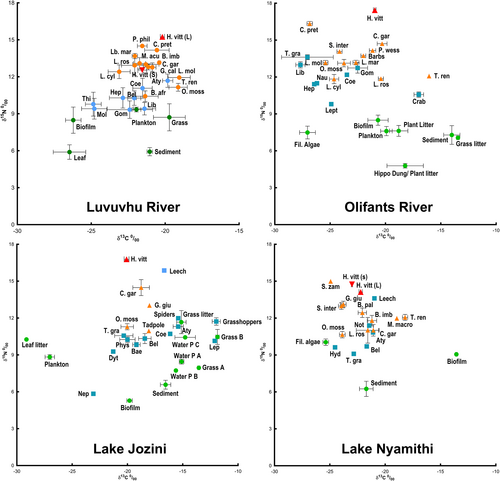
 , large Hydrocynus vittatus;
, large Hydrocynus vittatus;  , small Hydrocynus vittatus;
, small Hydrocynus vittatus;  , other fishes,
, other fishes,  , macroinvertebrates;
, macroinvertebrates;  , primary producers. The full identities of the abbreviated sample names are presented in Table S1.
, primary producers. The full identities of the abbreviated sample names are presented in Table S1.The isotope data were able to reclassify between 64% and 89% of the food web components into their respective predefined trophic categories depending on the system (Table 2). Large H. vittatus from the Luvuvhu River HF survey, Olifants River, and Lake Jozini were grouped separately from all other elements within their respective food webs. However, those from the Luvuvhu River LF survey and Lake Nyamithi showed more variability and some individuals were classified along with the other fish species. The small H. vittatus indicated substantial differences across systems where, as expected, those from the Luvuvhu River HF survey were classified separately but with overlap with the other fish species in the system, whereas those from Lake Nyamithi were almost entirely classified as large H. vittatus.
| System | Trophic grouping | n | Predicted trophic groupings reclassification success | Error | ||||
|---|---|---|---|---|---|---|---|---|
| P | I | F | H. vitt (S) | H. vitt (L) | ||||
| Luvuvhu River (HF) | P | 40 | 92.5 | 7.5 | 0.0 | 0.0 | 0.0 | 0.08 |
| I | 35 | 2.9 | 60 | 20 | 17.1 | 0.0 | 0.4 | |
| F | 67 | 0.0 | 13.4 | 47.8 | 25.4 | 13.4 | 0.51 | |
| H. vitt (S) | 13 | 0.0 | 0.0 | 15.4 | 76.9 | 7.7 | 0.23 | |
| H. vitt (L) | 18 | 0.0 | 0.0 | 0.0 | 0.0 | 100 | 0 | |
| Total error | 0.32 | |||||||
| Luvuvhu River (LF) | P | 30 | 73.3 | 26.7 | 0.0 | 0.0 | 0.27 | |
| I | 26 | 3.8 | 80.8 | 11.5 | 3.8 | 0.19 | ||
| F | 62 | 0.0 | 19.4 | 50 | 30.6 | 0.50 | ||
| H. vitt (S) | ||||||||
| H. vitt (L) | 17 | 0.0 | 0.0 | 23.5 | 76.5 | 0.23 | ||
| Total error | 0.36 | |||||||
| Olifants River | P | 73 | 90.4 | 2.7 | 6.8 | 0.0 | 0.10 | |
| I | 17 | 11.8 | 0.0 | 88.2 | 0.0 | 1.00 | ||
| F | 106 | 0.0 | 0.0 | 98.1 | 1.9 | 0.02 | ||
| H. vitt (S) | ||||||||
| H. vitt (L) | 47 | 0.0 | 0.0 | 2.1 | 97.9 | 0.02 | ||
| Total error | 0.11 | |||||||
| Lake Jozini | P | 34 | 94.1 | 5.9 | 0.0 | 0.0 | 0.06 | |
| I | 36 | 8.3 | 80.6 | 0.0 | 11.1 | 0.19 | ||
| F | 11 | 9.1 | 63.6 | 0.0 | 27.3 | 0.36 | ||
| H. vitt (S) | ||||||||
| H. vitt (L) | 39 | 0.0 | 0.0 | 0.0 | 100.0 | 0.00 | ||
| Total error | 0.17 | |||||||
| Lake Nyamithi | P | 13 | 84.6 | 15.4 | 0.0 | 0.0 | 0.0 | 0.15 |
| I | 7 | 28.6 | 0.0 | 71.4 | 0.0 | 0.0 | 1.00 | |
| F | 62 | 0.0 | 0.0 | 90.3 | 1.6 | 8.1 | 0.10 | |
| H. vitt (S) | 13 | 0.0 | 0.0 | 0.0 | 7.7 | 92.3 | 0.92 | |
| H. vitt (L) | 20 | 0.0 | 0.0 | 30.0 | 0.0 | 70.0 | 0.30 | |
| Total error | 0.29 | |||||||
- Abbreviations: HF, high flow; H. vitt (S), juvenile H. vittatus; H. vitt (L), large H. vittatus. F, grouped fish species; I, invertebrates; LF, low flow; P, primary producers.
3.2 Diet composition of H. vittatus
The isoplots plotted in Figures S1 to S3 indicate where large H. vittatus from lotic systems (Figure S1), lentic systems (Figure S2) as well as juvenile H. vittatus from the Luvuvhu River (Figure S3a,b) and Lake Nyamithi (Figure S3c,d) are feeding within the trophic enrichment corrected (δ15N and δ13C) food source groups.
The median as well as the 5% and 95% confidence intervals for the three major food source contributions to H. vittatus diet are provided in Table 3. The mixing models indicated fish as the predominant food source for large H. vittatus from each of the systems apart from Lake Nyamithi, where the mixing model suggested invertebrates as the main food source. Invertebrates were also indicated to form a small part of the diet in the Luvuvhu River and Lake Jozini H. vittatus. The structure matrices for the lotic and lentic systems suggest some correlation between the fish and invertebrate source groups, −0.70 and −0.76, respectively. The MixSIAR results of the juvenile H. vittatus (Table 3) indicated that primary producers were the predominant food source for those from the Luvuvhu River, whereas invertebrates and fish were the predominant source for the small H. vittatus from Lake Nyamithi. However, it is important to note that the structure matrices indicated a substantial correlation between invertebrates and primary producers as food sources for the juvenile tigerfish from the Luvuvhu River (−0.99), and fish and invertebrates for the small Lake Nyamithi tigerfish (−0.80).
| Percentage (%) contribution of food source to the diet of Hydrocynus vittatus | |||||||
|---|---|---|---|---|---|---|---|
| Broad assessment | |||||||
| Food source | Large | Juvenile | |||||
| Luvuvhu River (HF) | Luvuvhu River (LF) | Olifants River | Lake Jozini | Lake Nyamithi | Luvuvhu River (HF) | Lake Nyamithi | |
| Primary producers | 6.5 (0.0–25.6) | 0.2 (0.0–6.3) | 11.8 (1.1–28.7) | 8.1 (0.5–27) | 2 (0.2–7.8) | 88.8 (71–95.9) | 7.5 (0.7–27.4) |
| Invertebrates | 33.1 (16.0–49.6) | 92 (66.9–100) | 3.1 (0.6–10.6) | 26.5 (12.9–42.6) | 84.2 (68.3–94.7) | 9.6 (1.3–28) | 51.9 (31.3–77.8) |
| Fish | 57.8 (42.6–74.4) | 6.6 (0.0–30.7) | 83.6 (68–96.2) | 62.9 (44.5–80.9) | 12.8 (3.4–29.4) | 1.4 (0.1–4.3) | 37.9 (12–58.4) |
| Detailed assessment | |||||||
| Ephemeroptera | 9.7 (0.9–34.1) | 7.3 (0.7–26.6) | |||||
| Hemiptera | 14.5 (1.0–36.9) | 8.5 (0.7–30.8) | 14.5 (1.8–49) | ||||
| Decapoda | 4.7 (0.4–18.9) | 12.1 (0.8–51) | |||||
| Coleoptera | 27.8 (3.2–62.3) | ||||||
| Odonata | 8.0 (0.7–27.2) | ||||||
| Plankton | 14.2 (1.4–43.4) | ||||||
| Plecoptera | 12.4 (1.0–42.0) | ||||||
| Alestidae | 11.6 (1.0–36.9) | 16.7 (1.7–46.8) | 9.1 (0.8–25.2) | ||||
| Cichlidae 1 (Oreochromis mossambicus) | 14.8 (1.5–40.9) | 6.2 (0.4–20.1) | 7.3 (0.7–24.7) | 60.7 (42.9–76.3) | 58 (6.7–87.1) | ||
| Cichlidae 2 (Coptodon rendalli) | 8.7 (0.9–32.9) | 7.2 (0.6–26.3) | 5.0 (0.5–15) | 6.8 (0.5–35.9) | |||
| Cichlidae 3 (Pseudocrenilabrus philander) | 6.3 (0.5–27.3) | ||||||
Cichlidae 4 (Tilapia sparmanii) |
6.2 (0.5–24.8) | 24.4 (2.7–60.4) | |||||
| Clariidae | 47.1 (12.5–63.5) | ||||||
| Cyprinidae | 24.2 (3.2–53.6) | 8.5 (0.7–29.3) | 7.5 (0.6–23.7) | 7.6 (0.6–26.4) | |||
| Gobiidae | 19.3 (2.2–52.6) | 31.6 (8.8–60.2) | 5.6 (0.4–19.4) | ||||
| Mormyridae | 8.5 (0.7–29.8) | 64.3 (47.9–81.1) | |||||
| Schilbeidae | 16.3 (1.9–35.7) | 4.8 (0.3–18) | |||||
- Abbreviations: HF, high flow; LF, low flow.
The preceding broad analyses indicated that macroinvertebrates and fish were the major dietary elements in the diet of H. vittatus. Subsequently, the secondary analyses focused on the various fish families as food sources for the large and small H. vittatus (refer to Table 3). The analyses revealed that, during the high-flow period, cyprinids made the largest contribution to the diet of the large H. vittatus, followed by gobies, detritivorous cichlids, and alestids. During the low-flow periods, gobies made the largest contribution to the diet of large H. vittatus from the Luvuvhu River, followed by alestids, cyprinids, and mormyrids. The analyses further indicated that mormyrids made the largest contribution to large H. vittatus, followed by schilbeid catfishes, cyprinids, and detritivorous cichlids. Within Lake Nyamithi detritivorous cichlids made the largest contribution to the large H. vittatus, followed by alestids, cyprinids, and gobies. In Lake Jozini, clariid catfishes made the largest contribution to large H. vittatus diet, followed by invertivorous cichlids.
With regard to the small H. vittatus, the detailed secondary assessment from the Luvuvhu River indicated that macroinvertebrates from the order Coleoptera made the largest contribution to their diet, followed by plankton, Plecoptera, and Hemiptera. The detailed assessment of the small H. vittatus from Lake Nyamithi, which included both macroinvertebrates as well as select fish species, revealed that detritivorous cichlids made the largest contribution, followed by hemipterans, decapods, and omnivorous cichlids.
4 DISCUSSION
The results confirmed that H. vittatus are the apex aquatic predators in these freshwater systems and demonstrated distinct ontogenetic shifts in their diet as individuals grow. Large H. vittatus were primarily piscivorous and fed on a variety of fish species within lotic systems but demonstrated some specialization within lentic ecosystems. This trend was also apparent for small H. vittatus that were predominantly invertivorous in the lotic ecosystem and were relatively specialized piscivores within the lentic ecosystem.
4.1 Trophic position
It is expected that the habits of more generalized carnivores and omnivores feeding among multiple trophic levels introduce uncertainty into the assignment of trophic levels within aquatic food webs (Davis et al., 2012). In all of the studied systems, large H. vittatus occupied the highest trophic position, indicative of their role as apex predators. However, in Lake Nyamithi the large H. vittatus occupied a TP lower than in the other systems. This can be attributed to the fact that Lake Nyamithi is part of a floodplain system, an environment that frequently experiences disturbances such as desiccation, which normally results in a food web with few trophic levels, as described by de Necker et al. (2022). The increased range in TP by large H. vittatus from the Luvuvhu River during the low-flow period is likely the result of increased availability of a higher diversity of food items due to the lower water levels and hence concentration of the aquatic biota (refer to Section 4.2 for a detailed discussion).
As expected, the small H. vittatus from the Luvuvhu River occupied a TP lower than their larger counterparts, indicating that these small H. vittatus are not yet piscivorous and feed at a similar level to other omnivorous and invertivorous fish species in the Luvuvhu River. Remarkably, however, the small H. vittatus from Lake Nyamithi occupied a similar trophic position as their larger counterparts, indicating that both large and small H. vittatus are exploiting a similar resource.
4.2 H. vittatus diet and ontogenetic dietary shifts
This is the first study to use stable δ15N and δ13C isotopes to determine the H. vittatus diet in aquatic ecosystems of South Africa and only the second on the continent. The first was a study by Marufu et al. (2017) that evaluated whether H. vittatus in Lake Kariba incorporated an invasive crayfish (Cherax quadricarinatus [Von Martens, 1868]) in their diet. The results of this study indicated that large H. vittatus feed on a narrow range of food items. These food items are likely those which are most readily available within the habitats that H. vittatus occupy. Similarly the small H. vittatus also feed mainly within a narrow niche, albeit very different from the large H. vittatus in the Luvuvhu River.
Carbon fractionation between different trophic levels and predator and prey is generally small, and higher trophic levels are typically δ13C-enriched compared to their diet, although carbon signatures between diet and consumer are almost identical (Peterson & Fry, 1987; Post, 2002). The differences in δ13C isotope signatures between large and small H. vittatus were not substantial and may indicate that larger individuals feed on smaller prey fish in the littoral margins of lentic and lotic systems. This is supported by the fact that the δ13C signatures of large H. vittatus individuals from both the lotic systems as well as one of the lentic systems, Lake Nyamithi, are similar to other fish species that occupy the littoral vegetated margins of these rivers, i.e., O. mossambicus, Enteromius sp. and Glossogobius giuris (Hamilton, 1822). These results concur with studies on large yellow perch (Perca flavescens [Mitchill, 1814]) that were also shown to prey on smaller fish from littoral zones of Rice Lake, south-eastern Ontario, Canada (Kraemer et al., 2012).
The mixing model confirmed the abovementioned findings, as these species were from the three families of fishes that contributed considerably to the large H. vittatus diet. Adebisi (1980) studied the gut contents of larger specimens of Hydrocynus forskahlii (Cuvier, 1819), a species similar to H. vittatus, from the upper Ogun River in Nigeria and found that this species mostly fed on Enteromius sp. (Barbus sp.), M. acutidens, and various cichlids, which agrees with what has been found in this study. Similar findings were reported by Bell-Cross (1965), where Enteromius sp. (Barbus sp.) were predominant in H. vittatus stomachs from the upper Zambezi River. These results are further corroborated by the study by Dalu et al. (2012) that through SCA found that per volume H. vittatus in the Malilangwe Reservoir in Zimbabwe consumed fish in the orders of Cichlidae (35%), Gobiidae (31%), and Cyprinidae (29%).
Although fish from the Cyprinidae and Gobiidae families collectively contributed >40% to the large H. vittatus diet during both flow periods in the Luvuvhu River, the mixing models indicated that the majority of fish species contributed to H. vittatus to varying degrees. In all three other ecosystems the mixing models indicated a level of specialization in feeding by large H. vittatus: Mormyridae in Olifants River H. vittatus, Clariidae in Lake Jozini H. vittatus, and O. mossambicus in Lake Nyamithi H. vittatus. These findings are congruent with the literature as mormyrids and fishes from the family Clariidae were found to contribute significantly to the diet of H. vittatus (formerly H. forskahli) in the Lower Zambezi floodplain (Winemiller & Kelso-Winemiller, 1994), Lake Kariba (Mhlanga, 2003) as well as the Malilangwe Reservoir (Dalu et al., 2012), respectively. Throughout the H. vittatus dietary ecology literature, Cichlids are reported to make up a significant portion of the H. vittatus diet, particularly within lentic ecosystems (Dalu et al., 2012; Marufu et al., 2017), similar to H. vittatus from Lake Nyamithi.
With regard to seasonal variation in H. vittatus diet composition, the greater variety in food availability during periods of low flow is evidenced by the increased range in carbon values found in Luvuvhu River H. vittatus. This in turn suggests that a greater variety of carbon sources are consumed during low flow periods. Additionally, the MixSIAR results demonstrated a more even contribution of each of the families of fishes to H. vittatus's diet. This corresponds to SCA findings by Winemiller and Kelso-Winemiller (1994) that, during low-water periods in the Zambezi River floodplain, H. vittatus fed more evenly across more fish species and specialized by feeding on Cichlids during times with higher water levels. Similarly, during the high flow in the Luvuvhu River, Cichlids contributed more to H. vittatus's diet.
The vast majority of SCA feeding ecology studies conducted on H. vittatus have found that small tigerfish rely heavily on an invertivorous and zooplanktivorous diet (Dalu et al., 2012; Gaigher, 1970; Kenmuir, 1975; Marufu et al., 2017; Mhlanga, 2003; Skelton, 2001; Winemiller & Kelso-Winemiller, 1994). Our SIA data corroborate this as the findings of this study clearly show that small H. vittatus in the Luvuvhu River preyed primarily on a selection of macroinvertebrates. However, small H. vittatus from Lake Nyamithi preyed primarily on the cichlid O. mossambicus. O. mossambicus readily breeds in Lake Nyamithi (likely year-round) and is by far the dominant fish species (Smit et al., 2016). The findings suggest that the small H. vittatus feed on fry of O. mossambicus. Fry frequently have a similar signature to their parents as the C and N signatures of the egg and subsequently yolk sac are identical to the parents. In addition, even after fry are no longer reliant on the yolk sac and become self-sufficient, they are detritivorous and likely consume a similar diet to that of the adult O. mossambicus (Skelton, 2001). Furthermore, O. mossambicus fry and fingerlings inhabit the shallow littoral zones of water bodies, such as Lake Nyamithi, where water temperatures are highest and growth maximized (Skelton, 2001). Small H. vittatus are known to feed in shallower littoral zones (Kenmuir, 1975; Skelton, 2001) and this phenomenon was further apparent in the current study as small H. vittatus were caught exclusively in the littoral zone of the lake.
The range of N values observed in this study suggests that H. vittatus feed at different trophic levels as they grow. However, using δ15N as a proxy for trophic position, dietary shifts may be valid for specialized predators (Post, 2003), as is the case with H. vittatus. The diet of H. vittatus progressively evolves to include larger prey and more fish as they grow larger. Thus, the observed increase in N signatures as tigerfish grow reflects these dietary changes. The changes in the stable carbon data also showed that large and small H. vittatus clearly relied on different carbon sources depending on their size. A similar observation was made for yellow perch from Rice Lake (Kraemer et al., 2012). Clear ontogenetic dietary shifts in yellow perch C and N signatures were recorded as they grew and became more piscivorous. Ontogenetic dietary shifts as fish become larger have also been shown in the predatory Nile perch Lates niloticus (Linnaeus, 1758) from Lake Victoria (Campbell et al., 2003).
Results from the SIA and the mixing models have shown that smaller H. vittatus individuals from the Luvuvhu River are predominantly invertivorous, whilst the larger individuals are principally piscivorous, which is in agreement with other studies (Adebisi, 1980; Kenmuir, 1975; Skelton, 2001). This ontogenetic dietary shift was, however, not evident in the H. vittatus population within Lake Nyamithi as the analyses indicated that all size classes were piscivorous due to smaller H. vittatus feeding on fish fry in Lake Nyamithi. This is contrary to Kenmuir (1975), Marufu et al. (2017), and Skelton (2001), who have all either suggested or found ontogenetic dietary shifts within this species, irrespective of the type of system.
The changes in the stable carbon data not only reflect the change in diet and prey consumed but may also reflect the habitat in which feeding occurs, and therefore the habitats occupied by large and small H. vittatus. In the present study, the C signature of small tigerfish from the Luvuvhu River was similar to the signature seen in food web elements such as Enteromius afrohamiltoni (Crass, 1960), L. molybdinus, and macroinvertebrates of the Coenogrionidae and Libellulidae families, which occupy shallow water and vegetated habitats within the river. Larger H. vittatus individuals are almost exclusively found in deeper, more open water stretches of the river, a finding in congruence with Winemiller and Kelso-Winemiller (1994). The use of these different habitats, and thus different prey items, is clearly indicated by the differences in C and N isotope signatures.
Although the H. vittatus from this study were not aged, it appears that the ontogenetic dietary shift in this species occurs near the end of its first year of life, as H. vittatus from the Okavango Delta (Gerber et al., 2009) were shown to be approximately 200 mm in length at a relative age of 1 year. This would indicate that all larger H. vittatus sampled in this study are 1 year old and older, whereas the small H. vittatus are all less than a year old. Kenmuir (1975) found that the ontogenetic dietary shift in tigerfish from Lake Kariba happened quite early in life and indicated that juvenile tigerfish changed from an invertivorous diet to a piscivorous diet at around 40 mm. Fish that were much smaller than those sampled during this study.
Other unique feeding behaviors have also been recorded in H. vittatus. These include some evidence of cannibalism within the species, although the recorded incidences are low (Marufu et al., 2017; Winemiller & Kelso-Winemiller, 1994). Large H. vittatus have also been documented to display avivory behaviors in lakes (O'Brien et al., 2014). Jackson (1961) further observed that H. vittatus attacked the wound of a Nile crocodile (Crocodylus niloticus Laurenti, 1768) that had been shot, and posited that they had been attracted by the blood in the water. Similarly, in his PhD thesis Dr Francois Jacobs (Jacobs, 2017) reported seeing H. vittatus feeding on the carcass of a hippopotamus (Hippopotamus amphibius Linnaeus, 1758). These limited reports suggest that although H. vittatus have a highly specialized diet, they are opportunistic feeders and may shift their diet in accordance with what is available to survive.
4.3 Limitations of the current study
A notable limitation of the current study is that no complementary SCA was conducted simultaneously. Although no detailed SCA was conducted, the stomachs of the dissected fish were cut open for a rapid assessment of whether any identifiable dietary items could be identified. These data were not quantified or qualified and are not reported here. However, the vast majority of the individuals had empty stomachs and this is in line with what several studies have found. Dalu et al. (2012) found that, depending on sampling time, between 80% and 100% of the H. vittatus individuals dissected during their study had empty stomachs. Even studies that used other capture techniques, such as gillnetting or seine netting, found that at least 50% of H. vittatus stomachs were empty (Gaigher, 1970; Marufu et al., 2017; Winemiller & Kelso-Winemiller, 1994). An additional reason for the empty stomachs may have been that all large individuals were caught using rod and line, and therefore captured individuals may have been hungry and thus actively hunting. The results presented here of the H. vittatus diet from Lake Jozini should be interpreted with caution due to the lack of food web data available to conduct the analysis. However, these results can still be considered as indicative of the H. vittatus diet in the lake as the Bayesian analyses alluded to a piscivorous diet, as was also demonstrated within the other systems studied and throughout the literature, as discussed in previous sections. Furthermore, these results still provide important baseline information on the dietary habits of this important aquatic apex predator.
4.4 Implications of the trophic position for H. vittatus
The understanding of food webs allows the mechanisms of bioaccumulation found within these food webs to be identified. Furthermore, understanding of food webs is an essential constituent in determining food webs that may be at risk to increased bioaccumulation that, in turn, endangers the health of not only upper-trophic predators but also humans (McIntyre & Beauchamp, 2007). It is of the utmost importance to determine the trophic level fish are feeding at, as this may influence their exposure to pollutants through mechanisms such as biomagnification (Kraemer et al., 2012). The shift to piscivory in many fish species has been shown to occur with a concurrent increase in contaminant concentrations (Bowles et al., 2001; McIntyre et al., 2006; McIntyre & Beauchamp, 2007). Even though there is some debate over whether aquatic organisms primarily bioaccumulate pollutants from the ambient environment (water and sediment) or through biomagnification (via dietary uptake) (Gray, 2002), the general consensus is that the dominant pathway in most situations is from food (Borga et al., 2004; Fisk et al., 2003; McIntyre & Beauchamp, 2007). Thus, feeding at higher trophic levels results in greater bioaccumulation (McIntyre & Beauchamp, 2007), as was observed for tigerfish from the Congo River (Verhaert et al., 2013), the Lower Phongolo floodplain (Volschenk et al., 2019, Wepener et al., 2012,) as well as the Luvuvhu, Letaba, and Olifants rivers of the KNP (Gerber et al., 2016).
5 CONCLUSIONS
Tigerfish were shown to occupy the top of the food chains in both of the studied river systems and their stable isotope signatures were shown to be relatively distinctive within both of these ecosystems. The SIA and Bayesian isotopic mixing models revealed substantial differences in feeding across season as well as between aquatic ecosystems, particularly between lotic and lentic systems. The SIA and Bayesian analyses further revealed distinct ontogenetic shifts as fish grew larger and became more piscivorous, but not in cases where a substantial source of fish fry and fingerlings were present. This piscivorous diet and H. vittatus's position as an apex predator may result in increased bioaccumulation of various pollutants. These findings have several implications for future fisheries management due to the differences in feeding preference across aquatic ecosystems and fish development as local depletion of prey or extended disturbance events may potentially affect food availability and hence the diet and/or presence of tigerfish in the system. This study further illustrates the usefulness in the application of methodologies such as SIA and Bayesian analyses in providing a greater holistic understanding of species feeding behavior and, subsequently, phenological processes and how these may be driven either by potential shifts in season or by aquatic ecosystem type.
AUTHOR CONTRIBUTIONS
Ruan Gerber: Conceptualization; data curation; formal analysis; investigation; visualization; writing – original draft preparation. Lizaan de Necker: Writing – original draft preparation; visualization; software; methodology; formal analysis. Johan H. J. van Vuren: Funding acquisition; project administration; supervision; writing – review & editing. Yoshinori Ikenaka: Writing – review & editing; resources; funding acquisition. Shouta M. M. Nakayama: Funding acquisition; resources; review and editing. Mayumi Ishizuka: Writing – review & editing; resources; funding acquisition. Victor Wepener: Conceptualization; investigation; supervision; writing – review & editing. Nico J. Smit: Conceptualization; funding acquisition; investigation; project administration; supervision; writing – review & editing.
ACKNOWLEDGMENTS
The authors would like to thank the Water Research Commission of South Africa (Projects K5-1922 and K5-2185, Nico J. Smit, PI) for the financial support of this study. This work is further based on research and researchers supported in part by the National Research Foundation (NRF) of South Africa (Grant Numbers: 105979 and 85505). The opinions, findings, conclusions, and recommendations expressed in this publication are those of the authors, and the NRF accepts no liability whatsoever in this regard. The financial assistance of the Deutscher Akademischer Austauschdienst and the NRF towards this research is hereby acknowledged (Lizaan de Necker: Grant UID 105122; R.G: Grant UID 86632). Lizaan de Necker acknowledges funding from the NRF Department of Science and Innovation Professional Development Programme (Grant UID 127549) and the use of infrastructure and equipment provided by the NRF-SAIAB Research Platform and the funding channeled through the NRF-SAIAB Institutional Support system. We would also like to thank the Hokkaido University for the use of their laboratory facilities for stable isotope analysis. This work was supported by the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan awarded to M. Ishizuka (No. 22KK0163, 18KK028708), Yoshinori Ikenaka (No. 17K2003807 and 18H0413208), and Shouta M. M. Nakayama (No. 17KK0009 and 20K20633). This research was also supported by JST/JICA SATREPS (Science and Technology Research Partnership for Sustainable Development; No. JPMJSA1501), aXis (Accelerating Social Implementation for SDGs Achievement; No. JPMJAS2001), JST AJ-CORE (PJ36210002), and the JSPS CORE to CORE program. This research was also funded by Hokkaido University SOUSEI Tokutei Research. We also acknowledge Ezemvelo KZN wildlife for provisions that allowed the completion of field sample collections within Ndumo Game Reserve and particularly previous Conservation Manager Amos Tembe and District Ecologist uMkhanyakude Catharine Hanekom. The authors also wish to thank the Scientific Services of the South African National Parks Board for logistical support during the study and Mr. Divan van Rooyen, North-West University, for generating the study site map. This is contribution number 789 from the NWU-Water Research Group.
FUNDING INFORMATION
Nico J. Smit: Water Research Commission of South Africa (Projects K5-1922 and K5-2185, N.J. Smit, PI). Johan H.J. van Vuren: National Research Foundation (NRF) of South Africa (Grant Numbers: 105979 and 85505). Lizaan de Necker: Deutscher Akademischer Austauschdienst and the NRF (Lizaan de Necker: Grant UID 105122). NRF Department of Science and Innovation Professional Development Programme (Grant UID 127549). M Ishizuka: Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 22KK0163, 18KK028708). Yoshinori Ikenaka: Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 17K2003807 and 18H0413208). S.M.M. Nakayama: Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 17KK0009 and 20K20633). This research was also supported by JST/JICA SATREPS (Science and Technology Research Partnership for Sustainable Development; No. JPMJSA1501), aXis (Accelerating Social Implementation for SDGs Achievement; No. JPMJAS2001), JST AJ-CORE (PJ36210002), and the JSPS CORE to CORE program. This research was also funded by Hokkaido University SOUSEI Tokutei Research.




