Reproductive behavior of Atlantic halibut (Hippoglossus hippoglossus) interpreted from electronic tags
Abstract
Reproductive timing, location, and behavior are important characteristics that determine marine population dynamics, structure, and resilience to threats, including fishing and climate change. It is challenging to evaluate factors driving variability in these reproductive traits in wild fishes because of the difficulty observing individuals in their natural environments. In the present study, we used high-resolution depth, temperature, and acceleration time series recorded by pop-up satellite archival tags to (1) identify and characterize patterns in depth and acceleration that may be indicative of spawning events in large Atlantic halibut (Hippoglossus hippoglossus), and (2) estimate the effects of individual traits (body size and sex) and environmental factors (location and temperature) on spawning time and frequency. Unique rapid rises observed in the winter depth profiles were interpreted as spawning events. The initiation of the first presumed spawning rise was negatively correlated to water temperature experienced during the prespawning season, suggesting that currently increasing water temperature in the Gulf of St. Lawrence may induce phenological change in halibut spawning time. The number of rises of batch-spawning females was unrelated to female body size. The present study demonstrates how electronic tagging can be used for in-depth characterization of timing, location, and behaviors associated with spawning in a large flatfish species. Such information can inform spatiotemporal management and conservation measures aiming to protect species from directed fishing and by-catch during spawning.
1 INTRODUCTION
Reproductive timing, location, and behavior are important factors determining fish population structure, dynamics, and resilience to anthropogenic threats such as fishing and climate change. Understanding variability in these traits is necessary for developing effective management and conservation measures (Le Bris et al., 2013; Zemeckis et al., 2014). However, characterizing the spawning behaviors of marine fish in the wild is challenging, especially for species that are difficult to observe in their natural environments, such as species that are highly migratory, or that inhabit deep and dark environments far offshore. Spawning time and location of marine fish have traditionally been determined by visual and/or histological characterization of the maturity stage of fish gonads (Kohler, 1967; McBride et al., 2022), or by the presence of fish eggs and larvae in planktonic surveys (Ghinter et al., 2023; Pepin & Helbig, 1997). Laboratory tank studies have revealed physiological characteristics of reproduction, including quality and quantity of eggs, spawning frequency, and environmental effects on reproductive timing (Brown et al., 2006; Norberg et al., 2001). Courting and spawning behaviors have also been described using laboratory studies (Brawn, 1961; Smith et al., 1999), as well as active acoustic surveys (Grabowski et al., 2012; Rose, 1993).
Electronic data storage tags have become more prevalent as a method for studying spawning and other behaviors in elusive marine species because of their capacity to record high-resolution time series of individual environmental habitat use (e.g., Grabowski et al., 2015; Siwicke et al., 2022; Tsuda et al., 2006; Yasuda et al., 2013). Time series from electronic tags can reveal unique patterns of depth use that are indicative of potential spawning behaviors, such as the oscillatory diving patterns with prolonged surface intervals exhibited by bluefin tuna (Block et al., 2001), the rapid rises off the seafloor in Pacific halibut (Seitz et al., 2005), or the periodic short-term shallow water activity in small spotted catsharks (Wearmouth et al., 2013). When combined with reconstructed migratory tracks, these patterns can help reveal unknown spawning areas (Gatti et al., 2020) and ultimately inform on the risks of interaction with fisheries or other ocean uses (Marshall et al., 2023).
Pop-up satellite archival tags (PSATs) are a type of electronic archival tag widely used to study marine species, especially large migratory fish. They are attached externally, record ambient oceanographic conditions (temperature, depth, light intensity, etc.), and can be programmed to release from their host fish, float to the surface, and transmit data via satellite, allowing access to data summaries without the need to recapture the tagged fish (Block et al., 2005; Hussey et al., 2015). The physical recovery of PSATs also provides access to the full high-resolution data sets archived in the tags, including three-dimensional acceleration data, which can reveal behavioral patterns not necessarily observable from lower-resolution satellite transmitted data (Fisher et al., 2017; Nielsen et al., 2018).
In recent years, several studies have used PSATs to study the migratory behavior of Atlantic halibut (Hippoglossus hippoglossus) in the Northwest Atlantic (Armsworthy et al., 2014; Gatti et al., 2020; Gatti et al., 2021; James et al., 2020; Le Bris et al., 2018; Liu et al., 2019; Murphy et al., 2017), encouraged by the steady increase in fisheries landings observed in the past two decades (DFO, 2021a, 2021b). Several of these PSAT studies observed rapid ascents and descents during the presumed spawning seasons and suggested that these behaviors were spawning rises (Armsworthy et al., 2014; Le Bris et al., 2018; Murphy et al., 2017) because of their consistency with the spawning rises described from PSAT time series for the closely related Pacific halibut (Hippoglossus stenolepis, Seitz et al., 2005) and directly observed for several other flatfish species (Carvalho et al., 2003; Konstantinou & Shen, 1995; Manabe et al., 2000; Manabe & Shinomiya, 2001; Moyer et al., 1985). Although the reproductive behavior of halibut has not been directly observed in the wild, some inferences have been possible from indirect methods. Egg sampling, gonad histology, and laboratory tank studies have demonstrated that halibut are deep-water batch-spawners, releasing 0.5–7 million mesopelagic eggs (Cargnelli et al., 1999; Haug, 1990; Haug & Gulliksen, 1988a) during varying periods between November and June depending on the region (Armsworthy et al., 2014; Björnsson et al., 1998; Haug, 1990; Kjørsvik et al., 1987; Kohler, 1967; Neilson et al., 1993). Females follow regular batch-spawning intervals based on egg development timing (Finn et al., 2002; Norberg et al., 1991), and males are capable of spawning much more frequently over longer periods (Methven et al., 1992; Norberg et al., 1991). However, despite a rapidly expanding body of research on Atlantic halibut ecology (Shackell et al., 2022), the variability in spawning timing and sex-specific behaviors of the species in the wild remains largely unstudied, in part because of the difficulty observing halibut in the winter spawning season, especially in the Gulf of St. Lawrence that is seasonally covered by sea ice.
In the present study, we used high-resolution depth, temperature, and acceleration data from recovered PSATs to characterize the spawning behavior of Atlantic halibut in the Gulf of St. Lawrence. Building on previous studies observing presumed spawning rises in Atlantic halibut and other flatfish, we identified presumed spawning rises in female and male halibut and determined their dates of occurrence, amplitude, and frequency. We then evaluated the role of temperature, fish body size, and location on both the timing of the first presumed spawning rise and the number of spawning rises per individual female. We were particularly interested in the timing and number of spawning rises because they have direct applications to the management of the fishery. Spawning time can directly inform the timing of fishery closures during the spawning period and may also provide a better understanding of population structure (Zemeckis et al., 2014). Information on the numbers of spawning rises, and whether they are dependent on fish size, is relevant to estimating the total egg production in a population and understanding the capacity of a population to sustain various levels of fishing pressure or patterns of fishing selectivity (Hixon et al., 2014; Le Bris et al., 2015).
2 MATERIALS AND METHODS
2.1 Ethics statement
The care and use of experimental animals complied with the Canadian Council on Animal Care's animal welfare laws, guidelines, and policies as approved by Memorial University's Animal Care Committee, protocol 19-02-AL.
2.2 Electronic tagging
As part of a research effort to understand the ecology of Atlantic halibut in the Northwest Atlantic, PSATs were deployed on 119 individual halibut of legal size (>85 cm) landed in good condition across the Gulf of St. Lawrence in September and October 2013, 2014, 2015, and 2017 (Gatti et al., 2020), several months before the spawning season. Following the methodology developed and used by the International Pacific Halibut Commission (Seitz et al., 2003), PSATs were attached to halibut using sterilized titanium darts and 180-kg-test monofilament tethers. The dart was inserted under the pterygiophores on the dorsal, eyed side of the fish. This methodology was reported to have no long-term effect on the behavior of tagged individuals (Seitz et al., 2003). Tagging operations were carried out exclusively aboard chartered commercial fishing vessels, and fish were caught using commercial demersal longlines (for more information on tagging operations, see Gatti et al., 2020; James et al., 2020; Le Bris et al., 2018; Murphy et al., 2017). Two models of tags were used: X-Tag PSATs from Microwave Telemetry, Inc., which recorded depth (±0.34 to 5.38 m resolution from 0 to 1296 m), temperature (±0.16 to 0.23°C resolution), and light intensity level (±4 × 10−5 lx resolution at 555 nm) every 2 min (Table S1), and MiniPATs from Wildlife Computers Inc., which recorded depth (±0.5 m), temperature (±0.1°C), and light intensity (5 × 10−12 to 5 × 10−2 W/cm2) at 5–15 s resolution throughout the deployment period (Table S1). MiniPATs used in the 2017 deployment also recorded acceleration (n = 36). Tags were preprogrammed to release from their host fish at a specific date ~1 year following tag deployment. High-resolution archived data sets were available from tags that were physically recovered. Tag recoveries were conducted aboard chartered commercial fishing vessels using a CLS ARGOS RXG-134 goniometer (CLS America Inc., Lanham, MD, USA) (Fisher et al., 2017; Gatti et al., 2020). Of the 119 tags deployed, 99 popped off and transmitted to satellite, and 64 of those were physically recovered. Two recovered tags showed stationary depth time series after a few days, and the tagged fish was presumed dead. Two recovered tags also popped off early, so the time series were removed from analyses.
The depth time series from 60 physically recovered tags were visually inspected for evidence of spawning behavior: consecutive rapid ascents and descents similar to patterns previously documented as presumed spawning behavior for halibut species (Murphy et al., 2017; Seitz et al., 2005). Forty of the 60 physically recovered tags showed potential spawning behavior and were selected for sex determination analyses.
2.3 Sex determination
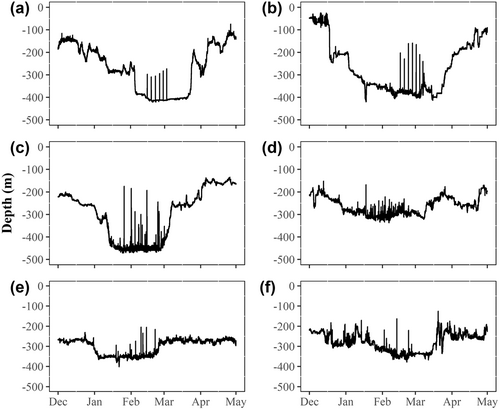
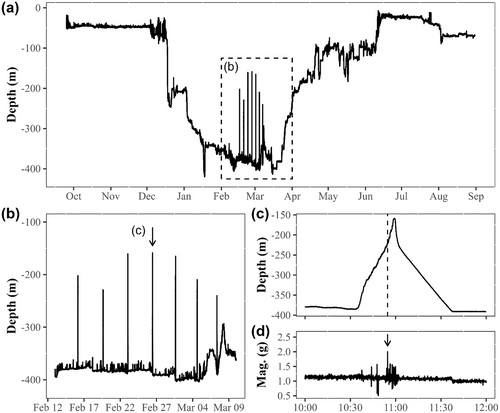
Sex was determined in one of three ways (Table 1). (1) A veterinary ultrasound, available only during the 2014 and 2015 tagging studies, was used during tagging operations to non-invasively inspect the shape of the gonads (Loher & Stephens, 2011) for nine halibut tagged in 2014 and 2015. (2) Taking fin clip samples for genetic analysis was introduced into the tagging protocol in 2017. Fin clip samples were not taken before 2017 because the technology to identify sex genetically from fin clip samples was not yet developed (Einfeldt et al., 2021). Genetic analysis using RADseq-derived single nucleotide polymorphisms associated with a region of chromosome 12 containing the presumed master sex-determining gene GSDF was used to assign sex for 11 halibut displaying presumed spawning rises on PSAT depth profiles (Einfeldt et al., 2021). (3) Sex of 16 additional halibut was identified based on visual inspection of the PSAT depth profiles following the description by Murphy et al. (2017) of distinctive female and male patterns: females show a very regular pattern of one clear rise every few days, whereas males are characterized by a greater number of rises at irregular intervals over a longer period (Figure 1). Depth profiles, including those with confirmed sex from ultrasound or genetics, were distributed to nine research collaborators without communicating the known sex of any profiles. By using this approach, the 20 profiles with known sex could be used to validate the visual inspection method. Responses were reviewed, and sex was assigned to 16 profiles for which there was greater than 75% agreement among the participants. Eighteen of the 20 profiles with known sex were also correctly assigned with greater than 75% agreement among visual inspection responses, reinforcing confidence in the visual assignment method. The final tally of sexed halibut used for further analyses was 36 (Table 1; Table S1).
| Female | Male | Total | |
|---|---|---|---|
| Ultrasound | 4 | 5 | 9 |
| Genetics | 10 | 1 | 11 |
| Visual consensus | 12 | 4 | 16 |
| Total | 26 | 10 | 36 |
2.4 Spawning rise detection
Halibut presumed spawning rises have been identified as rises off of and returning to the seafloor that are extreme both in distance traveled and in rate of ascent and descent, compared to other vertical movement events during the year (Fisher et al., 2017; Seitz et al., 2005). Building on this idea that spawning rises are outliers in the movement of halibut, several methods were attempted to detect spawning rises as objectively as possible (Marshall, 2020: Appendix A). Because of the marked difference in spawning behavior between females and males, two different approaches were chosen for females and males. For the 26 female halibut, we favored a four-step automated procedure using the rate of change in depth. (1) Rates of depth change were estimated by calculating first-order difference in the depth time series. (2) Absolute values of the rates of depth change were filtered using a 5-min moving average to remove noise caused by tag sensor errors or random vertical movements. (3) The mean and standard deviation of the filtered time series of depth changes were calculated. (4) Spawning events were identified as any depth change (absolute value) per time step greater than the mean rate of depth changes plus 12 standard deviations (an arbitrary limit selected after extensive preliminary analysis) that occurred between November 1 and April 30, the period of halibut spawning in the Northwest Atlantic suggested by previous studies (Kohler, 1967; McCracken, 1958; Neilson et al., 1993).
Automated spawning rise detection, including the rate of depth change method described earlier for females, was also attempted for males, but because spawning behavior of males was more irregular (Figure 1), likely due to the ability of males to spawn more readily without the physiological constraints associated with developing eggs (Coleman & Jones, 2011), as well as possible activity associated with courting, the automated detection did not work. Instead, presumed spawning rises of males could be detected only by visual inspection of the depth profiles. Any ascents, of duration less than 4 h from leaving the seafloor to returning to the seafloor, reaching distances ≥25 m from the seafloor, occurring between November 1 and April 30 were considered spawning rises. The 25-m threshold was somewhat arbitrary, but it was consistent with characterization of halibut spawning rise minimum distances by Armsworthy et al. (2014) and Fisher et al. (2017), and with the minimum spawning rise distances detected for females using the aforementioned automated process.
2.5 Spawning rise characterization
To provide a complete characterization of each presumed spawning rise, the depths and times were recorded at the start, end, and peak of every rise for both female and male halibut. Time between successive rises was calculated by subtracting the time at the peak of one spawning rise from the time at the peak of the next spawning rise. Furthermore, the effects of several environmental and individual factors on two characteristics of special interest, the date of the first presumed spawning rise and the total number of spawning rises, were evaluated using generalized linear models (GLM). GLMs were run only on the female halibut spawning rise data because of the higher confidence in the female spawning rise detection, and the understanding that females constrain reproductive potential more than males due to greater physiological requirements for producing eggs (Bateman, 1948; Coleman & Jones, 2011; McBride et al., 2015).
Degree days were calculated using the temperature maximum (Tmax) and minimum (Tmin) recorded by the PSATs for each day (i) of the specified period. The reference temperature (T0) was set at 0°C. Presumed spawning locations were estimated using a hidden-Markov geolocation model that inferred daily fish position by comparing the PSAT-recorded depth data with known Gulf of St. Lawrence bathymetry (a full description and validation of the geolocation model is available in Gatti et al., 2021). The geolocation model estimated the probability distribution of an individual fish across the study grid for each day. Probability distributions were averaged for the numbers of days during the presumed spawning period, and the cell with the highest probability was considered to be the spawning location. Then these locations were categorized based on the Gulf of St. Lawrence oceanographic regions described by Galbraith et al. (2020). The GLM error was assumed to follow a normal distribution, and this assumption was confirmed during model validation by plotting residuals versus fitted values, and versus each predictor in and not in the model (Zuur & Ieno, 2016). Model selection was implemented using the AIC (Akaike, 1974) corrected for a small sample size (AICc; Burnham & Anderson, 2002).
A second GLM was used to analyse the variability in the number of presumed spawning rises per female halibut. The model tested the effect of mean water temperature experienced by the individual during its spawning period (defined as the period between and including the dates of the first and last presumed spawning rises), the date of the first spawning rise, fish size, spawning location, year, and tagging location on the number of spawning rises. The GLM error was assumed to follow a Poisson error distribution with a log link function, and this assumption and risk of overdispersion were confirmed during model validation (Zuur & Ieno, 2016). Model selection was implemented using the AICc (Akaike, 1974; Burnham & Anderson, 2002).
2.6 Acceleration
The time series of the acceleration magnitudes for female halibut showing presumed spawning rises (n = 17) were visually inspected to quantify the frequency of spawning rises accompanied by a sudden increase in magnitude of acceleration. To further explore whether there were consistent patterns of acceleration relative to spawning rises, the maximum magnitude of acceleration between the start time and end time of each rise was recorded. Acceleration magnitude in relation to male halibut presumed spawning rises was not explored because of lower confidence in the spawning rise detection for males than for females.
3 RESULTS
3.1 Spawning behavior characteristics
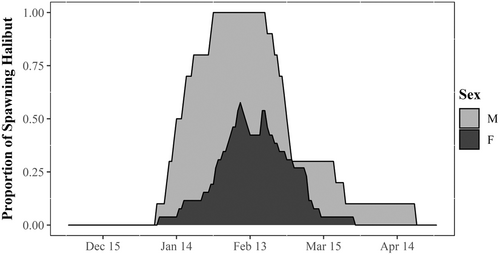
The depth profiles from 36 PSATs, 26 females and 10 males, were examined for spawning rises (Table S1). A total of 830 presumed spawning rises were identified. Females exhibited between 5 and 13 spawning rises (median: 7) per individual, and males exhibited between 22 and 182 spawning rises (median: 57) per individual. Presumed spawning periods for females and males overlapped (Figure 2). The date of the first spawning rise ranged from January 7 to February 26 among females and from January 6 to 29 among males. Spawning period per individual lasted between 9 and 37 days (median: 16 days) for females, and between 31 and 82 days (median: 61 days) for males.
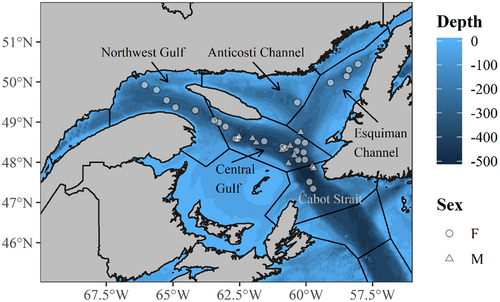
The spatial distribution of females and males during the period of presumed spawning, estimated from the geolocation model, also overlapped, with greater than 50% of total females and males co-occurring in the same oceanographic region (Figure 3). Grouping the spawning locations by oceanographic region (Galbraith et al., 2020) put 4 females in the Esquiman Channel, 1 female in the Anticosti Channel, 6 females in the Northwest Gulf, 13 females in the Central Gulf, and 2 females in the Cabot Strait. One male was estimated to be in the Esquiman Channel region just on the border of the Central Gulf region, two males were estimated to be in the Cabot Strait region, and the other seven males were in the Central Gulf.
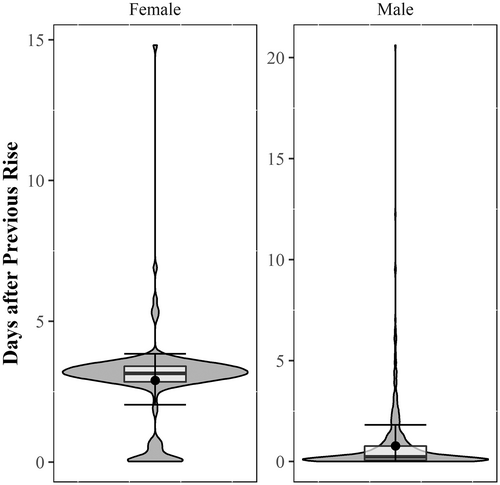
The duration of individual presumed spawning rises from start to end took between 3.25 and 201.58 min (median: 21.00 min) for females, and between 3.00 and 237.25 min (median: 32.12 min) for males. Data exploration did not show any obvious associations with specific periods of the day (day, night, dawn, and dusk). Spawning rises occurred at any time of day, between 00:11:40 and 23:57:45 (median: 12:51:22) for females and between 00:03:15 and 23:55:30 (median: 11:46:47) for males. The amount of time between rises ranged from 0.02 to 14.80 days (median: 3.15 days) for females and from 0.01 to 20.61 days (median: 0.23 days) for males (Figure 4).
The starting depths of presumed spawning rises were between 250.00 and 503.50 m (median: 396.25 m) for females, and between 278.39 and 459.50 m (median: 417.00 m) for males. The depths at the peaks of spawning rises were between 122.50 and 472.00 m (median: 267.00 m) for females, and between 144.00 and 433.50 m (median: 356.75 m) for males. Summaries of depth use at each spawning location are presented in Table 2. Rise distances ranged from 24.54 to 234.50 m (median: 111.25 m) for females and from 25.00 to 284.00 m (median: 40.00 m) for males. The median rise distance for males is expectedly low compared to the female median because the 25-m minimum rise distance criteria strongly influenced male spawning rise detection.
| Sex | Presumed spawning location | Fish count | Maximum | Minimum | Mean | |||
|---|---|---|---|---|---|---|---|---|
| Start depth | Peak depth | Start depth | Peak depth | Start depth | Peak depth | |||
| Female | Anticosti Channel | 1 | 326.0 | 213.0 | 250.0 | 153.5 | 295.1 | 185.1 |
| Cabot Strait | 2 | 503.5 | 472.0 | 476.5 | 279.5 | 490.7 | 386.2 | |
| Central Gulf | 13 | 482.5 | 403.5 | 303.5 | 158.5 | 407.0 | 274.5 | |
| Esquiman Channel | 4 | 376.5 | 289.5 | 253.5 | 151.0 | 299.9 | 223.6 | |
| Northwest Gulf | 6 | 421.5 | 333.0 | 251.5 | 122.5 | 361.3 | 256.7 | |
| Male | Anticosti Channel | 0 | – | – | – | – | – | – |
| Cabot Strait | 2 | 449.0 | 421.5 | 328.0 | 259.0 | 422.8 | 371.4 | |
| Central Gulf | 7 | 459.5 | 433.5 | 283.0 | 144.0 | 412.3 | 353.4 | |
| Esquiman Channel | 1 | 329.8 | 293.2 | 278.4 | 168.1 | 308.1 | 265.4 | |
| Northwest Gulf | 0 | – | – | – | – | – | – | |
- Note: Starting depth is assumed to indicate the seafloor depth at an individual's location.
3.2 Effects on timing and number of spawning rises
The date of the first presumed spawning rise was best explained by a GLM that included only the effect of preseason GDD based on model selection with AICc (Table S2). The model predicted a significant negative relationship between the date of the first female presumed spawning rise and preseason GDD (β = −5.318 ± 2.345, t1,24 = −2.268, p < 0.034), suggesting that female halibut spawn earlier when they experience warmer temperature during the prespawning season. No covariates were found to be significant predictors of the number of female presumed spawning rises. Four models with one covariate each, including mean temperature during the spawning period, fork length, date of the first spawning rise, and tagging location, were selected as the lowest AICc models, comparable within two units of each other (Burnham & Anderson, 2002; Table S2), but in no case was there any significance (Table S2).
3.3 Acceleration
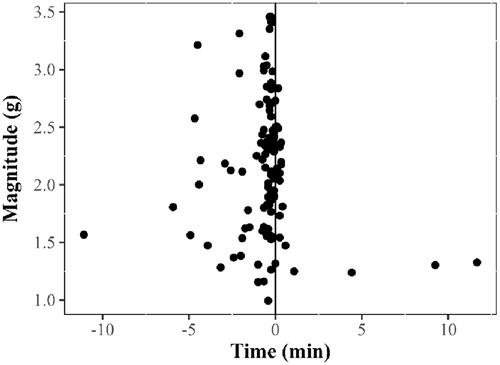
Visual inspection of the magnitude of the acceleration of 17 females showed that 97.5% (115 out of 118) of the presumed spawning rises were accompanied by spikes in acceleration magnitude (Figure 5). Various spikes in acceleration magnitude were observed for each single spawning rise, but the most pronounced acceleration was often observed close to the peak of the spawning rises (Figure 6).
4 DISCUSSION
In the present study, we used the high-resolution time series recorded by PSATs, geolocation of individual tagged fish, and information on halibut sex to characterize the presumed spawning behavior of Atlantic halibut in the Gulf of St. Lawrence. An inherent limitation of electronic tagging data to study fish behavior is the necessity to infer behaviors from patterns observed in the time series data. Analyses of depth time series recorded by PSATs deployed on Atlantic halibut revealed unique rises (up to 284.00 m) off the seafloor by females and males with peak occurrence in February. We interpreted these unique ascents as spawning rises following previous interpretations of similar rises observed in depth time series recorded by electronic tags deployed on several groundfish species, including Pacific halibut (Seitz et al., 2005), Atlantic halibut (Armsworthy et al., 2014; Murphy et al., 2017), Greenland halibut (Siwicke et al., 2022), and Atlantic cod (Grabowski et al., 2014). Furthermore, spawning rises have been directly observed for several flatfish (Carvalho et al., 2003; Konstantinou & Shen, 1995; Manabe et al., 2000; Manabe & Shinomiya, 2001; Moyer et al., 1985).
Although direct observations of halibut spawning would be necessary to validate our interpretation that the unique rises in the PSAT depth time series are spawning behavior, several characteristics of the rises provide strong support. First, the timing and location of the presumed spawning rises identified in the present study match with the recent first capture of larval Atlantic halibut in the Gulf of St. Lawrence (Ghinter et al., 2023). Second, the occurrences of female and male spawning rises overlap spatially and temporally, a necessity for successful mating and egg fertilization. The third piece of evidence is the consistency between the frequency and period of rises observed in the present study and the reproductive physiology of female and male halibut documented in laboratory and aquaculture studies. Female presumed spawning rises generally occurred at 2–4 day intervals, which is consistent with a documented mean ovulatory rhythm of 72–80 h (Norberg et al., 1991) and a mean hydration cycle of sequential egg batches of 50 h at 7°C (Finn et al., 2002). The periodic rises seen on the depth time series of female halibut may therefore correspond to the releases of egg batches at intervals constrained by ovulation and oocyte hydration. Male halibut do not show comparable fluctuations in levels of androgenic steroids and have a more continuous spawning capacity (Methven et al., 1992; Norberg et al., 1991), which is consistent with the greater number and higher frequency of presumed spawning rises by males than by females observed in the present study. Additionally, milt release in male halibut can continue for several months (Methven et al., 1992), which is consistent with our observation of a longer period of presumed spawning in males (median: 61 days) than females (median: 16 days).
The accelerometer data available from physically recovered tags may provide additional support to the spawning rise interpretation. We observed sudden bursts in the magnitude of acceleration recorded by PSATs close to the peak of female presumed spawning rises in 97.5% of cases (115 out of 118). Although little is known about halibut mating behavior, detailed description of Atlantic cod mating behavior showed that cod release eggs while at the top of a rapid rise (Brawn, 1961). Furthermore, previous studies of flatfish spawning have observed eggs being released at the peaks of spawning rises (Carvalho et al., 2003; Konstantinou & Shen, 1995). Acceleration bursts may therefore correspond to release of eggs by female Atlantic halibut. These bursts occurred while the halibut were in the water column, at water depths between 404.5 and 141 m, consistent with observations from Haug et al. (1984, 1986), who found a mesopelagic distribution of Atlantic halibut eggs in Norwegian Fjords. In the present study, we simply explored a general correspondence between sudden changes in the magnitude of acceleration and timing of presumed spawning rises, although accelerometer data archived in PSATs have previously been used to characterize swimming activity of Pacific (Nielsen et al., 2018) and Atlantic halibut (Gatti et al., 2021). Future studies could further investigate the potential of using accelerometer data to identify and characterize the spawning events of wild marine fish, but this may require the use of higher-frequency accelerometers than the one used in the present study (i.e., 0.2 Hz).
Directly observed flatfish have been seen completing spawning rises during which one female and one male swim together up into the water column, simultaneously or sequentially release eggs and a milt cloud, and then immediately return to the seafloor (Carvalho et al., 2003; Konstantinou & Shen, 1995; Manabe et al., 2000; Manabe & Shinomiya, 2001; Moyer et al., 1985). If halibut are performing spawning rises in pairs, the much higher number of male rises than female rises observed in the present study and the observation that male rises are not restricted to female egg batch intervals would support the interpretation that males are engaging in spawning rises with multiple females over the course of the presumed spawning period. Males may also be employing alternative reproductive tactics such as attempting to “sneak in” and fertilize eggs being spawned as a result of a paired rise between a female and another male, as has been seen in other species (Bekkevold et al., 2002; Habrun & Sancho, 2012; Taborsky, 2008). It is also possible that some of the male rises seen on the time series may be part of courting behavior to attract females, as observed for the flounder Bothus podas (Carvalho et al., 2003).
An important question in the reproductive biology of batch-spawning species, relevant to fisheries assessment and management, is whether the number of eggs released by a female during the spawning season depends on fish size. Batch-spawning in marine species may be an adaption to increase individual fecundity otherwise limited by body cavity size (Fordham & Trippel, 1999), or a bet-hedging strategy to increase long-term fitness by exposing offspring to a broader range of environmental conditions and thus reducing the risk of recruitment failure caused by variable environmental conditions (Lambert & Ware, 1984). Population dynamics predict that if the number of egg batches increases with female size, then a population with preserved size structure (i.e., presence of larger older females) should have higher relative total egg production and/or be more resilient to environmental fluctuations (Hixon et al., 2014; Le Bris et al., 2015). A review of 41 articles evaluating relationships between the number of egg batches and species age or size found a positive relationship for 21 out of 33 species investigated (Fitzhugh et al., 2012). In the present study, we did not find a statistically significant relationship between the number of presumed spawning rises, assumed to correspond to the release of egg batches, and female body size, although our results do not reject the idea that spawning potential increases with fish size. Exponentially higher fecundity at larger body sizes (Barneche et al., 2018) may be expressed in other ways not interpretable on PSAT time series, such as a higher number of eggs per batch, larger egg size, or improved egg viability (Hixon et al., 2014). The size of presumed spawning females in the present study ranged from 130 to 202 cm fork length, and it is possible that the lack of females potentially maturing at a smaller size (halibut females mature at ~119 cm; DFO, 2021b) may have biased the analysis. However, previous studies of flatfish reproduction have demonstrated that fish size is linked to fecundity (Cooper et al., 2007; Rideout & Morgan, 2007, 2010; Schmitt & Skud, 1978), including for Atlantic halibut (Haug & Gulliksen, 1988a).
Phenological changes in fish reproduction caused by warming waters are increasingly documented, with warmer waters accelerating gonadal development, inducing earlier spawning (Haug & Gulliksen, 1988b; Kjesbu et al., 2010), and possibly shortening the spawning season (Rogers & Dougherty, 2019; Slesinger et al., 2021). In the present study, the date of the first presumed spawning rise observed in female halibut was negatively correlated with the temperature experienced during the prespawning season. This result contradicts the findings of the aquaculture study by Brown et al. (2006), which found halibut spawning period to be delayed after warmer ambient conditions. However, the halibut studied by Brown et al. (2006) were kept in overall warmer water than that which was recorded by PSATs for halibut in the wild, suggesting a possible threshold water temperature above which additional warming has no effect on spawning phenology (Rogers & Dougherty, 2019). The present study therefore suggests that the current rapid and steady increase in water temperature (~2°C increase in sea surface temperature since the 1990s) observed in the Gulf of St. Lawrence (Galbraith et al., 2012; Galbraith et al., 2022) might lead to an earlier spawning season for Atlantic halibut, which could have consequences for larvae survival and subsequent recruitment to the fishery (Durant et al., 2007). However, our model of presumed spawning start date as a function of preseason temperature, while significant, explained little of the total variance, suggesting that other factors are likely contributing to the variation in spawning time, including natural interindividual variability. This limited explanatory power might also reflect the fact that bottom temperatures in the Gulf channels are relatively constant with little seasonal variation throughout the year (Galbraith et al., 2022). Additionally, we also tested for the effects of temperature during the spawning period on the number of presumed spawning rises and found no relationship, suggesting that temperature does not affect the duration of the spawning period in halibut. This result is consistent with the results from Brown et al. (2006), who found a non-significant difference in annual spawning period length between two groups of Atlantic halibut kept at ambient and chilled water temperatures.
Importantly, our analysis of presumed spawning rises indicates a later peak spawning period (mid-February) within the Gulf of St. Lawrence than has been reported for the adjacent and separately managed stock on the Scotian Shelf and Grand Banks (October–January) (Armsworthy et al., 2014; Neilson et al., 1993). This temporal difference in peak spawning may be a mechanism contributing to the maintenance of subtle but statistically significant population genetic structuring quantified between these adjacent stocks in comparison across the Northwest Atlantic (Kess et al., 2021).
The findings of the present study demonstrate that although the spawning behavior of marine fish species can remain elusive due to the difficulty observing fish in their often dark and deep natural habitats, high-resolution time series recorded by electronic tags can enable a detailed characterization of otherwise unobservable presumed spawning behaviors, such as the unique rapid rises off the seafloor undertaken by female and male Atlantic halibut during the winter in the Gulf of St. Lawrence. By interpreting these rises as spawning behavior, we were able to identify with unprecedented precision the spawning period for Atlantic halibut in the Gulf of St. Lawrence, and also to evaluate some of the factors driving interindividual variability in halibut spawning time and duration. However, although ample evidence exists to support the assumption that these rises are spawning behaviors, without direct observation, there is always uncertainty. Using promising novel telemetry technologies, future work may validate interpretations of spawning behavior. By this, fisheries and ocean managers can gain the understanding of the reproductive behavior of valuable marine species that is necessary to the development of effective management and conservation measures.
AUTHOR CONTRIBUTION
Project conceptualization was undertaken by Arnault Le Bris, Jonathan A. D. Fisher, and Rachel C. Marshall. Data collection was overseen and performed by Arnault Le Bris and Jonathan A. D. Fisher; formal data analysis was done by Rachel C. Marshall, with tag geolocation done by Paul Gatti and Arnault Le Bris. The original manuscript was written by Rachel C. Marshall, with Arnault Le Bris, Jonathan A. D. Fisher, Paul Gatti, and Dominique Robert contributing to the review and editing process. Dominique Robert was responsible for funding acquisition.
ACKNOWLEDGMENTS
We thank all the Gulf of St. Lawrence fish harvesters involved in project planning, tag deployment, and tag recovery. Major funding for this research was provided by the Natural Sciences and Engineering Research Council of Canada via the Strategic Partnership Grants for Projects Program's contributions to “Quantifying spatial dynamics and stock structure of Atlantic halibut within the Gulf of St. Lawrence to improve sustainable exploitation and management,” and Dominique Robert was supported by the Canada Research Chairs programme.
FUNDING INFORMATION
Tag deployment and tag recovery operations between 2013 and 2016 were funded by the Newfoundland and Labrador Department of Fisheries and Aquaculture and by the Research and Development Corporation of Newfoundland and Labrador Ignite Grants to Jonathan A. D. Fisher and Dominique Robert. The Gulf-wide tagging deployment and recovery programmes of 2017–2018, and the postdoctoral position held by postgraduate and master's degree programme completed by Rachel C. Marshall, were largely supported by a Strategic Partnership Grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada (grant number: STPGP 506993 002D 17), with further support from the John R. Evans Leaders Fund of the Canada Foundation for Innovation, the Department of Fisheries and Land Resources of Newfoundland and Labrador, Ressources Aquatiques Québec, and the Ministère de l'agriculture, des pêcheries et de l'alimentation du Québec. This research was also supported by the Ocean Frontier Institute, a network funded by the Canada First Research Excellence Fund. Dominique Robert was supported by the Canada Research Chair programme.




