A new species of rheophilic armored catfish of Rineloricaria (Siluriformes: Loricariidae) from the Vaupés River, Amazonas basin, Colombia
Abstract
A new rheophilic species of the genus Rineloricaria is described for the Amazon basin in Colombia. Rineloricaria cachivera n. sp. differs from its congeners by having anterior to the first predorsal plate, an inconspicuous saddle-like mark; the presence of dark, diffuse blotches, present as unified dark colouration along most of the dorsal portion of the head, without bands or spots on the head; a long snout that occupies more than half the head length (HL), between 58.0% and 66.3% HL; a naked portion on the cleithral area from the border of lower lip reaching the origin of pectoral fin; and by having five series of lateral plates in longitudinal rows below the dorsal fin. The new species is morphologically similar to Rineloricaria daraha; however, it can be distinguished by the presence of six branched pectoral fin rays (vs. seven) and the lower lip surface with short thick papillae (vs. long finger papillae). An identification key to the Rineloricaria species of the Amazon River basin in Colombia is provided. The new species is herein categorized as Least Concern, following the IUCN criteria.
1 INTRODUCTION
The armored catfish Rineloricaria Bleeker, 1862, has 71 valid species, being the richest genus in the family Loricariidae (Fricke et al., 2023). The genus is diagnosed by a combination of characteristics such as the presence of postorbital notch; lower lip with short round papillae; premaxilla with 7 to 15 teeth on each ramus; dentary teeth strong, deeply bicuspidate, and larger than premaxillary; colouration of the dorsal region with dark-brown bars or blotches; abdomen with a conspicuous polygonal pre-anal plate, usually bordered by three other large trapezoidal plates (Fichberg & Chamon, 2008) and some features of sexual dimorphism, which are traits not always present in the individuals available for examination (Londoño-Burbano & Urbano-Bonilla, 2018). Progress has been made in the taxonomic and phylogenetic relationships between Rineloricaria species (Covain & Fisch-Muller, 2007), and it is now demonstrated to be a monophyletic group based on molecular data (Costa-Silva et al., 2015; Covain et al., 2016) with wide interspecies morphological variation (e.g., body color and shape, arrangement of abdominal plates, shape of head, and distribution of hypertrophied odontodes; Vera-Alcaraz et al., 2012).
The wide distribution of Rineloricaria in the main Neotropical basins and environments reflects the diversity and morphological adaptations of its species (van der Sleen & Albert, 2017; Vera-Alcaraz et al., 2012). Some species occur in small drainages of slow to moderate-flowing waters, associated with sand, vegetation, and organic matter; others are rheophilic inhabiting fast-flowing rivers associated with rocks (Costa-Silva et al., 2021; Lima et al., 2005; Londoño-Burbano & Urbano-Bonilla, 2018; Rapp Py-Daniel & Fichberg, 2008; Rodriguez & Reis, 2008). Rheophilic environments have driven the evolution of armored catfish lineages in the family Loricariidae (Lujan & Conway, 2015); the rheophilic species of Rineloricaria exhibit consistent ecomorphological patterns and that is evidenced in the shape of the body, mouth, and buccal papillae (Bressman et al., 2020).
In Colombia, 11 species are present in different hydrographic basins: Pacific and Caribbean: Rineloricaria jubata (Boulenger 1902); Pacific: Rineloricaria sneiderni (Fowler 1944); Caribbean Rineloricaria rupestris (Schultz 1944) and Magdalena-Cauca and Caribbean: Rineloricaria magdalenae (Steindachner 1879); Orinoco: Rineloricaria eigenmanni (Pellegrin 1908) and Rineloricaria formosa Isbrücker & Nijssen 1979; Amazonas: Rineloricaria castroi Isbrücker & Nijssen 1984, Rineloricaria daraha Rapp Py-Daniel & Fichberg, 2008, Rineloricaria phoxocephala (Eigenmann & Eigenmann 1889), Rineloricaria lanceolata (Günther 1868) and Rineloricaria jurupari Londoño-Burbano & Urbano-Bonilla, 2018 (DoNascimiento et al., 2021). The diversity of species in Colombia may have been underestimated due to a lack of data and sampling, especially in the Amazon basin (Jézéquel, Tedesco, Bigorne, et al., 2020a). Of the main rivers that drain to the Amazon (e.g., in Colombia: Caquetá, Putumayo, Apaporis, and Vaupés), the Vaupés is located in a Miocene Andean tectonic upheaval known as the Vaupés Arch (10 Ma), which acts as a semi-permeable barrier for the dispersal of fish (Winemiler & Willis, 2011) dividing the Amazon and Orinoco basins (Mora et al., 2010). Located on its border with Brazil, this river has numerous rocky rapids (locally known as “Cachiveras,” or “Raudales”) along its course that serve as a habitat and act as hydrogeographic barriers for fish. In the exploration of these environments, a new species of the genus Rineloricaria was identified, and it is described herein. Additionally, an updated identification key for species present at the Colombian Amazon is provided.
2 MATERIALS AND METHODS
Fish collection follows animal care guidelines provided by the American Society of Ichthyologists and Herpetologists (2013) -https://www.asih.org/resources. The biological material of MPUJ collected in this expedition in the río Vaupés went through a process of amnesty by the Instituto de Investigación Alexander von Humboldt under Colombian law “article 6 of law 1955 of 2019.” Fishes were captured using hand-nets or hand-captured by active snorkeling dives in polls or rapids of the Vaupés River. Specimens were photographed in life following the scientific documentation protocols of Photafish (Garcia-Melo et al., 2019). The holotype was also photographed in the laboratory following similar protocols. When the collected specimens were euthanized, doses of 0.3 mL/0.25 L of clove oil were added (Syzygium aromaticum; Lucena et al., 2013) before fixation. Fishes were fixed in 10% formaldehyde and later preserved in 70% ethanol for storage. Counts and measurements were made on the left side of specimens when possible, using digital calipers to the nearest 0.1 mm. Measurement, plate series count, and nomenclature followed Vera-Alcaraz et al. (2012). The terms “main cusp” and “lateral cusp” follow Muller and Weber (1992). Institutional acronyms follow Sabaj (2020). Characteristics used to diagnose the new species from species that are not included in the item “Additional specimens examined” were analysed and compared using original and subsequent descriptions of each species. In the description, counts are followed by their frequency in parentheses, and an asterisk (*) indicates the count of the holotype. Conservation Assessment Tool-GeoCAT was used to assess the geographic range of the taxon in two approaches: (i) extent of occurrence (EOO) and (ii) area of occupancy (AOO). Both metrics are part of the IUCN Red List categories and criteria (IUCN Subcommittee on Standards and Petitions, 2022). This study adjusted the grid to 1 km2, following the criteria of Bachman et al. (2011) for aquatic ecosystems.
3 RESULTS
3.1 R. cachivera new species
urn:lsid:zoobank.org:pub:7E3CCD7A-6118-4D3C-ADD1-F89A06ADF735.
urn:lsid:zoobank.org:act:FA5DEFCE-5666-46DD-9D3E-1F6E8E138668.
(Figures 1 and 2; and Table 1).


| Holotype | Minimum | Maximum | Mean | S.D. | |
|---|---|---|---|---|---|
| Standard length | 122.8 | 77.2 | 122.8 | 107.2 | - |
| Percentage of standard length | |||||
| Head length | 24.7 | 21.9 | 25.1 | 24.1 | 1.48 |
| Predorsal length | 37.1 | 37.1 | 37.8 | 37.4 | 0.33 |
| Postdorsal length | 62.2 | 62.2 | 65.4 | 63.9 | 1.30 |
| Prepectoral length | 22.4 | 21.3 | 22.4 | 21.8 | 0.48 |
| Postpectoral length | 82.1 | 80.2 | 84.3 | 82.3 | 1.66 |
| Prepelvic length | 36.0 | 36.0 | 36.7 | 36.3 | 0.28 |
| Postpelvic length | 65.5 | 65.4 | 66.7 | 65.8 | 0.61 |
| Pre-anal length | 50.9 | 50.0 | 51.4 | 50.9 | 0.62 |
| Postanal length | 50.3 | 47.2 | 50.3 | 49.2 | 1.41 |
| Unbranched dorsal-fin ray | 19.4 | 17.0 | 20.9 | 19.4 | 1.67 |
| Unbranched pectoral-fin ray | 17.7 | 17.8 | 20.6 | 19.0 | 1.18 |
| Unbranched pelvic-fin ray | 18.3 | 18.3 | 19.3 | 18.9 | 0.42 |
| Unbranched anal-fin ray | 17.7 | 17.7 | 20.6 | 19.4 | 1.26 |
| Thoracic length | 16.7 | 15.8 | 18.0 | 16.7 | 0.92 |
| Abdominal length | 17.0 | 16.2 | 17.8 | 17.1 | 0.71 |
| Cleithral width | 19.6 | 19.6 | 20.6 | 19.9 | 0.47 |
| Depth at dorsal-fin origin | 13.3 | 11.3 | 14.4 | 12.6 | 1.45 |
| Width at anal-fin origin | 14.7 | 13.1 | 15.0 | 14.2 | 0.89 |
| Caudal peduncle depth | 1.5 | 1.4 | 1.7 | 1.5 | 0.11 |
| Caudal peduncle width | 2.7 | 2.7 | 3.2 | 3.0 | 0.24 |
| Percentage of head length | |||||
| Snout length | 58.0 | 58.0 | 66.3 | 60.4 | 3.96 |
| Eye diameter | 10.2 | 10.2 | 13.0 | 11.2 | 1.22 |
| Maximum orbital diameter | 14.5 | 13.2 | 18.3 | 15.2 | 2.19 |
| Interorbital width | 25.0 | 23.3 | 29.0 | 25.4 | 2.47 |
| Internarial width | 9.6 | 9.6 | 15.4 | 11.8 | 2.73 |
| Head depth | 44.8 | 36.2 | 51.5 | 43.2 | 6.54 |
| Head width | 76.4 | 73.5 | 93.5 | 81.5 | 8.82 |
| Free maxillary barbel | 5.6 | 5.3 | 8.3 | 6.6 | 1.42 |
| Ventrorostral length | 9.9 | 8.3 | 10.5 | 9.6 | 0.94 |
| Lower lip length | 22.4 | 20.1 | 23.7 | 21.8 | 1.57 |
- Abbreviation: S.D., standard deviation.
3.2 Holotype
MPUJ 14451, 122.8 mm standard length (LS), Colombia, Vaupés Department, Mitú municipality, Negro River drainage, río Vaupés upstream Cachivera Tapira-llerao, Comunidad de Matapí, 1° 4′ 49.63″ N, 69° 22′ 20.82″ W, 143 m a.s.l., coll, J. A. Maldonado-Ocampo et al., February 28, 2019.
3.3 Paratypes
MPUJ 14375, 114.5, mm LS. Colombia, Vaupés Department, Mitú municipality, Negro River drainage, río Vaupés at Resguardo Trubón, 1° 12′ 8.38″ N, 70° 2′ 20.67″ W, 176 m a.s.l., coll, J. A. Maldonado-Ocampo et al., February 22, 2019. MPUJ 14481, 114.4 mm LS, Colombia, Vaupés Department, Mitú municipality, Negro River drainage, río Vaupés, Laguna Arcoiris small rocky bottom isolated lagoon from the river, Comunidad de Matapí, 1° 4′ 48.30″ 14495, 77.2 mm LS Colombia, Vaupés Department, Mitú municipality, Negro River drainage, río Vaupés at Comunidad de Matapí, 1° 4′ 49.16″ N, 69° 21′ 50.44″ W, 119 m a.s.l., coll, J. A. Maldonado-Ocampo et al., March 2, 2019.
3.4 Diagnosis
The new species differs from all its congeners by the following combination of characters: presence of a transverse dorsal band that is not well defined and is curved, similar to that band observed on anterior border of snout, anterior to the first predorsal plate (vs. transversal band absent; when present, first dorsal transversal band well defined, straight, not curved); presence of dark, diffuse blotches, present as unified dark colouration along most of dorsal portion of head, without bands or spots on head (vs. absence of dark, diffuse blotches, present as unified dark colouration along most of dorsal portion of head, without bands or spots on head); a long snout that occupies more than half the HL, between 58.0% and 66.3% HL (vs. short snout, occupying half or less than half of HL, usually less than 56% HL; except in R. daraha, R. lanceolata, R. malabarbai Rodriguez & Reis, 2008, Rineloricaria microlepidogaster [Regan 1904], and Rineloricaria osvaldoi Fichberg & Chamon, 2008); and naked portion on the cleithral region from border of lower lip reaching origin of pectoral fin (vs. naked portion of cleithral region not reaching origin of pectoral fin, beyond pectoral-fin origin, or portion totally covered by plates). Rineloricaria cachivera n. sp. is further distinguished by having five series of lateral plates in longitudinal rows below the dorsal fin (vs. four series of lateral plates in longitudinal rows below the dorsal fin in R. aurata (Knaack 2002), Rineloricaria beni (Pearson 1924), Rineloricaria cadeae (Hensel 1868), R. castroi, Rineloricaria catamarcensis (Berg 1895), Rineloricaria cubatonis (Steindachner 1907), Rineloricaria felipponei (Fowler 1943), Rineloricaria henselli (Steindachner 1907), R. jurupari, R. lanceolata, Rineloricaria langei Ingenito, Ghazzi, Duboc & Abilhoa 2008, Rineloricaria lima (Kner 1853), Rineloricaria longicauda Reis 1983, Rineloricaria magdalenae, Rineloricaria microlepidota (Steindachner 1907), Rineloricaria misionera Rodriguez & Miquelarena, 2005, Rineloricaria nigricauda (Regan 1904), Rineloricaria pareiacantha Mirande & Koerber 2015, Rineloricaria parva (Boulenger 1895), Rineloricaria quadrensis Reis 1983, Rineloricaria sanga Ghazzi 2008, Rineloricaria setepovos Ghazzi 2008, Rineloricaria sneiderni, Rineloricaria stellata Ghazzi 2008, Rineloricaria thrissoceps (Fowler 1943), Rineloricaria uracantha (Kner 1863), and Rineloricaria wolfei Fowler 1940). The new species is morphologically similar to R. daraha, a congener distributed in the río Negro basin (Brazil and Colombia); however, it can be easily distinguished by the presence of six branched pectoral fin rays (vs. seven) and the lower lip surface with short thick papillae (vs. long papillae).
3.5 Description
Morphometric data in Table 1. Largest specimen reaching 122.8 mm LS. Snout straight in lateral view, slightly raised on its tip. Dorsal profile straight to slightly convex from orbits to nuchal plate, and straight from dorsal origin to base of caudal-fin origin. Ventral profile of head straight; convex from anterior abdominal plates to anal-fin base, straight from that point to caudal-fin origin. Body and head wide, widening strongly at about origin of first infraorbital. Posterior portion of body with a noticeable narrowing at about half length of caudal peduncle. Five plates in infraorbital series, with sensory pores exposed ventrally. Snout tip with large oval naked area, but not extending laterally surpassing sensorial pore of first infraorbital. Poorly developed odontodes on head and trunk. Dorsum of head smooth, not presenting ridges on head and predorsal plates. Posterior margin of parieto-supraoccipital triangular. Dorsal margin of orbit not raised; postorbital notch shallow, not well developed. Eye large, round to slightly oval horizontally. Lower lip large, almost reaching anterior limit of abdominal plates, with lower border covered by 10 to 12 fringes on margin of each lobe. Round papillae covering lower lip, increasing in size toward dentary ramii, a line of more developed papillae near line with maxillary barbel. Teeth bicuspid, long, main cusp greater and wider than lateral, dentary teeth larger than premaxillary teeth, main cusp about twice length of lateral cusp. Premaxilla with 6(3*) or 8(1) teeth; dentary with 5(1*), 6(1), 7(1), 8(1) teeth. Five lateral plate series. Median lateral plates 27(2), or 28(2*). Lateral line complete. Lateral abdominal plates 9(2), 10(1), or 11(1*). Central abdominal plates well developed, slightly smaller anteriorly, having about 4–5 rows irregularly distributed. Abdominal plates covering entire abdomen, without naked areas. Posterior abdominal plates surrounding a well-defined pre-anal area, with three plates surrounding anus, one anterior and two lateral. Anterior margin of anterior abdominal plates slightly rounded or concave on its central portion. Dorsal fin I,7(4), dorsal-fin spinelet present, locking mechanism not functional; tip of depressed unbranched ray reaching fourth or fifth plate posterior to dorsal-fin base; tip of depressed last branched ray reaching third or fourth plate; distal margin falcate with unbranched and first branched rays longer than remaining, dorsal-fin base occupying 4(4) plates; pectoral fin I,6(4); tip of depressed unbranched ray reaching and slightly surpassing dorsal-fin origin; distal margin truncate. Pelvic fin i,5 (4), depressed unbranched ray slightly surpassing anal-fin origin. Anal fin i,5(4), with tip of depressed unbranched ray reaching sixth plate posterior to its base, depressed last branched ray reaching third or fourth plate posterior to its base; anal-fin base with three plates; distal margin truncate. Caudal fin emarginate, i,10,i (3) and i,9,i(2*); dorsal principal ray extended as long filament, filament about 2−4 times length of lower unbranched one (Figure 2a).
3.6 Colouration
Overall ground colouration yellowish, presenting dark-brown portions, especially on dorsal surface (Figure 1). Dorsal surface of body mostly yellowish contrasting with darker saddle-like pigmentation areas, ventral portion of body lighter. Dorsal body with five wide saddle-like dark marks; first one at base of dorsal fin, second starting just posterior to end of adnate last branched dorsal-fin ray and three marks between adnate end of anal fin and caudal peduncle. Lighter areas between dark saddles presenting dark scattered spots. Posterior region of parieto-supraoccipital darker with an inconspicuous saddle-like mark; ventral surface of head light yellowish with dark spots on cheek plates and snout area. Dark-brown irregular spots covering the lateral margin of abdomen, the remaining portion with yellow ground colouration (Figures 1 and 2). All fins with a dark band occupying almost their entire surfaces. Colouration in life similar as in preserved specimens except for brighter yellowish ground colouration (Figure 2).
3.7 Sexual dimorphism
In adult males, the first ray (unbranched) of pelvic fins has an extension equal in width to the rest of the ray; it is filament-shaped but very thick (Figure 2a; this individual was lost in the expedition accident and the photograph).
3.8 Distribution, habitat, and physicochemistry of water
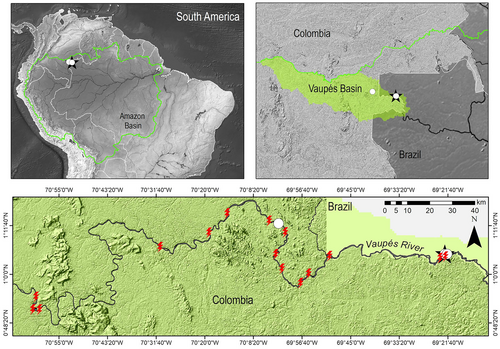
Rineloricaria cachivera n. sp. is known from four localities in the middle Vaupés River, downstream from the municipality of Mitú in Colombia (Figures 3 and 4a–d). With dark waters, little transparency (Secci disk values: 116 ± 11.31 S.D. and 122.50 ± 10.61 S.D.), and deep zones in the area of the rapids (up to 18.6 m). The mean values with standard deviation (mean ± S.D.) of the temperature (28.15 ± 0.21°C–29.65 ± 0.21°C), dissolved oxygen in the water (7.63 mg/L ± 0.25) and the surface (6.41 mg/L ± 0.01) are variable. In subaquatic dives with a diving mask, the specimens were collected by hand. In these rheophilic environments that are characterized by having aquatic plants (Podostemaceae) attached to the rocks, some fish were observed in low abundance (Leporinus fasciatus [Bloch, 1794], Characidium declivirostre Steindachner, 1915, Ancistrus patronus de Souza, Taphorn & Armbruster, 2019, and Hemiancistrus sp.), living in sympatry with R. Cachivera n. sp.

3.9 Conservation assessment
Rineloricaria cachivera n. sp. is currently known from four localities in the middle river Vaupes basin, and despite the small known distribution area (i.e., EOO: 51.752 km2/AOO: 4.000 km2), no significant threats to the species were detected. For this reason, R. cachivera can be preliminarily categorized as Least Concern (LC) according to the IUCN categories and criteria (IUCN Standards and Petitions Committee, 2022).
3.10 Etymology
The specific name cachivera refers to a flow of water that runs violently between the rocks. In the cosmology of the indigenous peoples of the Vaupés, the waters of its rivers are inhabited by various supernatural creatures that must be venerated, consulted, and appeased in the rituals of the shamans; these creatures live and guard mainly the cachiveras of the rivers where humans are more fragile and face the greatest danger (Schultes & Raffauf, 2004) (e.g., Figure 4e,f). The species was named in memory of Javier Alejandro Maldonado-Ocampo “Nano,” who collected the new species in the cachivera of “Trubón” and “La Mojarra”; in the latter, on March 2, 2019, Nano stayed forever swimming in peace and happy with the rheophilic fish of the cachiveras of the Vaupés River.
3.11 Key to the Rineloricaria species of the Amazon River basin in Colombia
1 Abdomen covered by brown dark spots; presence of dorsolateral stripes on both sides of the head.………2.
1' Abdomen without blotches and/or spots; absence of dorsolateral stripes on both sides of the head.………3.
2 Four or five premaxillary teeth; anterior abdominal plates the same size and equally numerous as central abdominal plates; anterior dorsal portion of body dark without transverse bands; two or three dark-brown narrow transverse bars restricted to the caudal peduncle.………R. jurupari (Londoño-Burbano & Urbano-Bonilla, 2018).
2' Five to eight premaxillary teeth; anterior abdominal plates smaller and more numerous than central abdominal plates; anterior dorsal portion of body with transverse band; five or six dark-brown broad transverse bars on caudal peduncle and predorsal region.………R. lanceolata (Günther, 1868).
3 Shallow posterior orbital notch; all fins (pectoral, pelvic, anal, and caudal) without a color pattern of “dark and light,” with spot occupying almost entire fin; pre-anal plate, with three polygonal scutes, of which the median one is the same size than those at either side; five series of lateral plates in longitudinal rows below the dorsal fin (Figure 5b) ………4.

3' Conspicuous posterior orbital notch; all fins (pectoral, pelvic, anal, and caudal) with a color pattern of “dark and light” vertical stripes; pre-anal plate, with three polygonal scutes, of which the median one is much smaller than those at either side; four series of lateral plates in longitudinal rows below the dorsal fin (Figure 5a) ………R. castroi (Isbrücker & Nijssen, 1984).
4 Six branched pectoral fin rays and lower lip surface button-like papillae.………5.
4' Seven branched pectoral fin rays and lower lip surface long finger papillae.………R. daraha (Rapp Py-Daniel & Fichberg, 2008).
5 Five dark-brown broad transverse bars on caudal peduncle and predorsal region; eye large, round to slightly oval horizontally and shallow postorbital notch; pre-anal plate with three plates surrounding the anus, one anterior and two lateral.………R. cachivera n. sp.
5' Six dark-brown broad transverse bars on caudal peduncle and predorsal region; eye large, round to slightly oval horizontally and conspicuous orbital notch; pre-anal plate, preceded by three polygonal scutes, of which the median one is much smaller than those at either side.………R. phoxocephala (Eigenmann & Eigenmann, 1889).
4 DISCUSSION
Rineloricaria has not been the subject of a complete taxonomic study (neither taxonomy alpha, nor integrative taxonomy) that could offer a delimitation between valid species, and offer updated diagnostic characters for each of the species. The only phylogenetic analysis using morphological evidence, including a significant number of valid species, is the unpublished study by Fichberg (2008); even though the author found the genus as a monophyletic assemblage, the delimitation of the species remains a problem. On the contrary, Costa-Silva et al. (2015) published a work aiming at delimiting species of the genus using molecular evidence, through the COI marker. The authors found that using different species delimitation methods, different outcomes regarding the number of species were found (i.e., lineages). This result reflects what has been happening to the taxonomy of the genus, in that descriptions of new species are published often, and it appears that there is a hidden diversity of the genus, in diverse environments, far from being discovered, fully known, or understood. Finally, Covain et al. (2016) published the most comprehensive phylogenetic analysis, through molecular evidence, regarding the genus, including representatives from its entire distribution, from the trans-Andean regions to the southeast of South America, Shields, and the Amazonian region. The authors in fact divided the genus into different groups, mostly finding a component of geographic distribution for the monophyletic assemblages present within Rineloricaria. As a result, they maintained the genus as a single monophyletic unit, did not split it into different genera, and moreover synonymized several genera to Rineloricaria (i.e., Fonchiiichthys Isbrücker & Michels, 2001; Hemiloricaria Bleeker, 1862; Ixinandria Isbrücker & Nijssen, 1979; and Lelliela Isbrücker, 2001), showing how complex the level of diversity is within the genus at the phylogenetic level. The study by Covain et al. (2016) is an excellent contribution to the delimitation of the different clades found within Rineloricaria for morphological characterization. Thus, the use of molecular evidence could be the first step toward an understanding of the diversity of Rineloricaria, and the number of valid species, which is increasing, could be at least delimited at the molecular level to allow a more approachable morphological work (Costa-Silva et al., 2015).
Rineloricaria cachivera n. sp. is a clear case of the diversity of environments in which species of the genus can be found. The Vaupés River in its headwaters (i.e., Itilla and Unilla rivers) is borne in outcrops of the Craton and crosses very old rocks of the Precambrian age (Botero & Serrano, 2019). Its dark-colored waters (black-brown), acidic, with a large number of humic acids, and poor in dissolved inorganic substances, explain the low levels of nutrients (Cabalzar & Lima, 2005). The substrate is mainly sand (with beaches in the low water period) and rock (Bogotá-Gregory et al., 2022); the latter, in rocky outcrops that give way to innumerable cachiveras that serve as habitat and act as a natural barrier for fish dispersal (Lima et al., 2005). Few species belonging to Rineloricaria are found in environments with such characteristics, and one example is R. daraha, present in the Negro River basin in localities both in Brazil and Colombia. These species are not sympatric; however, they are similar morphologically and can be differentiated by the presence of six branched pectoral fin rays (vs. seven), dark spots along the dorsal portion of the body (vs. dark spots restricted to the head), and the lower lip surface with short thick papillae (vs. long finger papillae). There is also an important similarity between both species, and that is related to their rheophilic nature and the environments in which they are found. Both appear to be adapted to turbulent waters and are capable of supporting strong currents, which contain grazeable aquatic plants, algae, and invertebrates found in the holes between and on the surface of waterfall rocks (Bogotá-Gregory et al., 2016) as resources for the species. These characteristics seem to be reflected in the morphology of both species, mainly regarding head morphology. Both species have an elongated head, with a long snout, and slender bodies, especially when compared to some congeners with stockier bodies (e.g., R. misionera, R. osvaldoi Fichberg & Chamon, 2008, R. rodriguezae Costa-Silva, Oliveira & Silva 2021, Rineloricaria steinbachi [Regan 1906], Rineloricaria zawadzkii Costa-Silva, Silva & Oliveira 2022, and most southeastern and southern distributed species of the genus).
In rheophilic environments, in addition to the morphology of the head and body, loricariids develop wide mouths and thick lips with well-developed papillae that increase friction and prevent drag by the water current (Gradwell, 1971; Ono, 1980). In the lips, collagen is supposed to work to reinforce the oral suction cups and reduce slippage; furthermore, its content correlates with the substrate and the flow of water; species that live on rocky substrates and torrential water current species have larger lips, with high collagen content (Bressman et al., 2020). The Andes mountain range with its water network having innumerable rapids promoted the evolution of oral characteristics (wide mouths and thick lips with well-developed papillae) that can be observed in other genera of loricariids with restricted distribution: Andeancistrus Lujan, Meza-Vargas & Barriga Salazar 2015, Cordylancistrus Isbrücker 1980, Chaetostoma Tschudi 1846, Dolichancistrus Isbrücker 1980, Fonchiiloricaria Rodriguez, Ortega & Covain 2011, and Transancistrus Lujan, Meza-Vargas & Barriga Salazar 2015. In the genus Rineloricaria the size of the mouth, papillae, and distribution range may vary significantly (Fricke et al., 2022; van der Sleen & Albert, 2017). This is evident in some cis- and trans-Andean species, where head shape, mouth size (length/width), maxillary barbels, labial papillae, and fringes on its edge are varied and may reflect adaptations to their environment (Figure 6a–i). The species that inhabit either the rocky rapids of the Paraná sedimentary basin (Costa-Silva et al., 2021) or in drainages of the Guiana Shield in the rapids of the Vaupés-Negro River (Rapp Py-Daniel & Fichberg, 2008), including R. cachivera n. sp., R. jurupari and R. daraha, have wide and high mouths, with thick lips and well-developed papillae that may explain their rheophilic nature (Figure 6a–c), compared to species with a smaller mouth, and which have greater environmental plasticity and distribution (Figure 6d–f,i). Rapids, in addition to promoting endemism and morphological specialization of fish, limit gene flow (Lima et al., 2005; Lujan & Conway, 2015; Torrente-Vilara et al., 2011). The species adapted to these environments have restricted distributions (Bressman et al., 2020) such as R. jurupari that lives in the headwaters of the Vaupes, that is, in the Itilla and Unilla rivers (Londoño-Burbano & Urbano-Bonilla, 2018). In the middle part, R. cachivera n. sp. seems to be exclusive to the rapids of the Vaupés River, and from its type locality we recorded it to occur ~138 km upstream in the same type of environments (Figure 3). Another endemic species is R. daraha; although with a greater distribution in the basin (>700 km), it has only been recorded in rheophilic environments, that is, in the Cachiveras-Cachoeiras of the Vaupés River in Brazil and Colombia (Bogotá-Gregory et al., 2016; Rapp Py-Daniel & Fichberg, 2008). In the Amazon basin, areas of endemism have been identified (Jézéquel, Tedesco, Darwall, et al., 2020b) as basic units of analysis in historical biogeography (Morrone, 2014) and useful in conservation biology (Löwenberg-Neto & de Carvalho, 2004). The Vaupés-Negro basin, in addition to being diverse in fish, exhibits a high degree of endemism (Jézéquel, Tedesco, Darwall, et al., 2020b); consequently, identifying new species (e.g., R. jurupari and R. cachivera n. sp.) or ecosystems (e.g., Raudales-Cachiveras-Cachoeiras) as “possible” conservation targets has proven to be an effective tool for the implementation of comprehensive conservation strategies in the Amazon Colombian (Portocarrero-Aya & Cowx, 2016).

The known diversity of Rineloricaria is growing fast, and with 71 valid species, this diversity is likely to increase. Currently, there are 11 valid species reported for Colombia, with 5 species present in the Colombian Amazon: R. castroi, R. daraha, R. phoxocephala, R. lanceolata, and R. jurupari (DoNascimiento et al., 2022), R. cachivera n. sp. being the sixth valid species recorded for the region. Of those species, one was recently recorded for Colombia (R. daraha by Bogotá-Gregory et al., 2016), and another was recently described (R. jurupari, by Londoño-Burbano & Urbano-Bonilla, 2018), summing up the species described here, that is three species recorded in less than 10 years for the same basin (i.e., Vaupés River). Furthermore, R. lanceolata is a species currently recognized as widespread along the Amazonas River basin (including the upper, middle, and lower portions of the basin, in localities of Colombia, Bolivia, Brazil, and Peru); however, from this wide distribution, a cryptic component could be present in the species. It is important to address such issues within the species as it is likely to result in several cryptic, undescribed species, and to delimit R. lanceolata to a more restricted distribution, with a better delimitation of the species (both morphological and molecular), and to understand and better describe the already great richness of Rineloricaria. This single example shows how complex the genus can be (the most diverse within Loricariinae) and adds a new component to tackle when studying its species, and the possibility of the presence of cryptic species, not only in R. lanceolata, but also for other species considered as widespread.
The description of R. cachivera n. sp. as the fourth species distributed in the Vaupés River reveals the underestimation of the diversity of Rineloricaria, which is already high as mentioned earlier. A revisionary study of the genus, delimiting the poorly characterized type species of the genus, R. lima, examination of type series and topotypic material of all valid species, and inclusion of both morphological and molecular evidence, is needed.
5 SPECIMENS EXAMINED
All the material was examined in Londoño-Burbano and Urbano-Bonilla (2018).
6 ADDITIONAL SPECIMENS EXAMINED
6.1 Rineloricaria daraha
CZUT-IC 3620, Vaupés, Mpio. Yavarate, Río Papuri, comunidad de Piracuara, 0° 4′ 0″ N, 69° 33′ 28″ W; CZUT-IC 3621, Colombia, Vaupés, Mpio. Yavarate, Río Papuri, comunidad de Piracuara, 0° 4′ 11″ N, 69° 28′ 16″ W; CZUT-IC 4954, Colombia, Vaupés, Mitú, Isla Roca, 1° 11′ 29″ N, 70° 17′ 19″ W.
6.2 Rineloricaria eigenmanni
MPUJ, 3281, 6, Colombia, Vichada, Puerto Carreño, Isla Santa helena, Río Orinoco, 5° 59′ 42″ N, 67° 25′ 34″ W, 17/04/2007. Col. González J.
6.3 Rineloricaria formosa
ZSM 25821, paratypes, 2alc, Venezuela, Orinoco River basin, Atabapo River near San Fernando 4° 03′ 0.00″ N, 67° 42′ 0.00″ W, 05/02/1973. Col: H.J. Köpcke & M. Jeschke. MPUJ 3249, 2, Colombia, Vichada, Puerto Carreño, Brazuelo Río Bita, directo al Orinoco, 6° 10′ 46″ N, 67° 38′ 0.15″ W, 10/10/2007. Col. González J.
6.4 Rineloricaria jubata
BMNH 1902.5.27.45, lectotype of Loricaria jubata, Ecuador, Durango. BMNH 1901.3.29.74–76, paralectotypes, 3alc, same data as lectotype. MPUJ 11152, 1, Colombia, Valle del Cauca, Buenaventura, Corregimiento de Sabaletas, confluencia del rio Sabaletas con Río Anchicaya, 3° 44′ 23.1″ N, 76° 58′ 0.00″ W, 12/10/2014. Jorge E. García-Melo.
6.5 Rineloricaria lanceolata
BMNH 1867.6.13.79, holotype of Loricaria lanceolata, Peru, Xeberos. MZUSP 81422, Brazil, Amazonas, Negro River basin, Tiquié River, Açaí stream, near São Pedro community, 0° 16′ 0″ N, 69° 58′ 0″ W; MZUSP 81379, Brazil, Amazonas, Negro River basin, Tiquié River, Onça stream, Onça Igarapé community, 0° 13′ 52″ N, 69° 51′ 4,9″ W; MZUSP 81439, Brazil, Amazonas, Negro River basin, Tiquié River, Caruru community, corridor above Caruru waterfall, 0° 16′ 29″ N, 69° 54′ 54″ W.
6.6 Rineloricaria magdalenae
NMW 45080, lectotype of Loricaria magdalenae, Colombia, Magdalena River basin; NMW 45800, paralectotypes, 6alc, same data as lectotype. MPUJ 7940, 3, Colombia, Antioquia, El Bagre, Quebrada El Guamo, 7° 54′ 48″ N, 74° 46′ 440″ W, 31/05/2015. Col. Jhon E. Zamudio.
Rineloricaria sp. “Orinoco”
MPUJ 2908, 3, Colombia, Meta, San Martin, Caño Camoa, 3° 39′ 47″ N, 76° 36′ 33″ W, 26/08/2017. Col. Saúl Prada-Pedreros.
6.7 Rineloricaria sp.
MZUSP 64690, Brazil, Amazonas, Negro River basin, Tiquié River, São Pedro community, 0° 15′ 41″ N, 69° 57′ 23″ W; MZUSP 66145, Brazil, Amazonas, Negro River basin, Tiquié River, Açaí stream, tributary of Tiquié River, near São Pedro community (opposite margin), 0° 15′ 41″ N, 69° 57′ 23″ W; MZUSP 81159, Brazil, Amazonas, Negro River basin, Tiquié River, between Caruru and Boca de Sal communities, 0° 16′ 0″ N, 69° 54′ 0″ W; MZUSP 81240, Brazil, Amazonas, Negro River basin, Tiquié River, Açaí stream, near former São Pedro community; MZUSP 81345, Brazil, Amazonas, Negro River basin, Tiquié River, São Pedro community, Umari Norte stream, from Caruru to Cachoeira da Abelha waterfall, 0° 16′ 0″ N, 69° 58′ 0″ W; MZUSP 81417, Brazil, Amazonas, Negro River basin, Tiquié River, Açaí stream, near São Pedro community, 0° 16′ 0″ N, 69° 58′ 0″ W; MZUSP 81501, Brazil, Amazonas, Negro River basin, Tiquié River, São Pedro community, 0° 16′ 4″ N, 69° 58′ 21″ W; MZUSP 85099, Brazil, Amazonas, Negro River basin, Tiquié River, lower portion of Supiã stream, below Comprida waterfall, 0° 15′ N, 70° 01′ W; ZSM 27058, 4alc, Brazil, Pará, Guamá River near Ourem, Atlantic slope, 10/1988. Col: R. Stawikowski & U. Schliewen.
AUTHOR CONTRIBUTIONS
Initial study design, specimen collection, and processing (A.U.B.). All authors participated equally in collection, analysis, and interpretation of data and in the preparation of the manuscript.
ACKNOWLEDGMENTS
We want to thank the support of several people from the communities of the region: William González-Torres and Arturo Hernández (Trubón community, Cubeo ethnic group), Emilio Marquez and Anderson Marquez (Villa Fátima community, Guanano ethnic group); Adelmo Santa Cruz (Nana community, Guanano ethnic group); Jaider Ramírez-Samaniego (Macucú community, Desano ethnic group), Julio V. Vélez and Silvio Vélez (Matapi community, Desano ethnic group). To Alejandro Campuzano (Fundación Conservando), Luis F. Jaramillo-Hurtado (SINCHI), and Mariana A. Moscoso (Ictiología y Cultura) for their technical support. Sandra Bibiana Correa (Mississippi State University) for her technical support and for providing data on the physicochemical aspects of water. A.U.-B. thanks the Catalog of the Fishes of Colombia, grant BID-CA2020-030-USE by GBIF, for allowing visits to some museums in the country and especially thanks curators or administrators for their unconditional support: Carlos A. García-Alzate (UARC-IC), Francisco A. Villa-Navarro (CZUT), Lauren Raz, Henry Agudelo-Zamora (ICN-MHN), Saúl Prada-Pedreros (MPUJ), Fernando Sarmiento Parra, and Julieth Stella Cárdenas Hincapié (MLS). A.L.-B. thanks James Maclaine (BMNH), Anja Palandačić (NMW), Ulrich Schliewen, Robin Böhmer, and Patricia Schulze (ZSM) for hospitality and assistance during visits to collections under their care, and Marcelo R. Britto (MNRJ) for technical and logistic support at MNRJ, where the manuscript was partially completed. Thanks to Omar Melo for help with the photographs of the holotype; Camila Castellanos, for the photo of R. daraha (Figure 6c); and Jorge García-Melo for taking photographs of live specimens in the field. Please visit the visual catalog of Colombian fish “https://cavfish.unibague.edu.co/”. Financial support was given by Pontificia Universidad Javeriana with the “Carta Encíclica Laudato Si” grant in the project entitled “Ictiología y Cultura: Aproximación biológica y cultural a los datos obtenidos en la expedición en las cachiveras del río Vaupés” (#20112). A.L.-B. was supported by a postdoctoral fellowship from FAPERJ Pós-Doutorado Nota 10 (05/2019 - E-26/202.356/2019).
FUNDING INFORMATION
From Pontificia Universidad Javeriana with the “Carta Encíclica Laudato Si” grant in the project entitled “Ictiología y Cultura: Aproximación biológica y cultural a los datos obtenidos en la expedición en las cachiveras del río Vaupés” (#20112). A.U-B. was supported by the Catalog of the Fishes of Colombia grant BID-CA2020-030-USE by GBIF. A.L.-B. was supported by a postdoctoral fellowship from FAPERJ Pós-Doutorado Nota 10 (05/2019 - E-26/202.356/2019).




