Inbreeding alters context-dependent reproductive effort and immunity in male crickets
Abstract
Infection can cause hosts to drastically alter their investment in key life-history traits of reproduction and defence. Infected individuals are expected to increase investment in defence (e.g., by increasing immune function) and, due to trade-offs, investment in other traits (e.g., current reproduction) should decrease. However, the terminal investment hypothesis postulates that decreased lifespan due to infection and the associated reduction in the expectation for future offspring will favour increased investment towards current reproduction. Variation in intrinsic condition will likely influence shifts in reproductive investment post-infection, but this is often not considered in such assessments. For example, the extent of inbreeding can significantly impact an individual's lifetime fitness and may influence its reproductive behaviour following a threat of infection. Here, we investigated the effects of inbreeding status on an individual's reproductive investment upon infection, including the propensity to terminally invest. Male crickets (Gryllodes sigillatus) from four genetically distinct inbred lines and one outbred line were subjected to a treatment from an increasing spectrum of simulated infection cue intensities, using heat-killed bacteria. We then measured reproductive effort (calling effort), survival and immune function (antibacterial activity, circulating haemocytes and haemocyte microaggregations). Inbred and outbred males diverged in how they responded to a low-dose infection cue: relative to unmanipulated males, outbred males decreased calling effort, whereas inbred males increased calling effort. Moreover, we found that inbred males exhibited higher antibacterial activity and numbers of circulating haemocytes compared with outbred males. These results suggest that an individual's inbreeding status may have consequences for context-dependent shifts in reproductive strategies, such as those triggered by infection.
1 INTRODUCTION
Infection can lead to profound changes in the life-history investment of hosts (Sheldon & Verhulst, 1996), and these alterations in investment can have cascading effects on the trade-offs between traits. Especially salient is the trade-off between reproduction and defence against infection (Durso & French, 2018; Lawniczak et al., 2007; Lochmiller & Deerenberg, 2000; Reznick, 1985; Zera & Harshman, 2001; Zuk & Stoehr, 2002). Due to limited resources, infected individuals might be expected to shift investment away from reproduction and towards defence, ensuring their continued survival (Norris & Evans, 2000). However, infected hosts often increase reproductive effort, a seemingly paradoxical response that can be explained by terminal investment (Clutton-Brock, 1984). This hypothesis proposes that decreased lifespan (e.g., due to infection), and consequently a reduced expectation for future offspring (i.e., residual reproductive value), favours increased investment in current reproduction (Williams, 1966). Empirical evidence for terminal investment following an actual or simulated threat to survival has been documented across multiple taxa and for various components of reproductive effort (reviewed in Duffield, Bowers, Sakaluk, & Sadd, 2017). Due to assumed trade-offs, these costly increases in reproductive effort are likely to be accompanied by decreases in traits associated with somatic maintenance, such as immunity (Durso & French, 2018; Faivre, Grégoire, Préault, Cézilly, & Sorci, 2003; Harshman & Zera, 2007; Lawniczak et al., 2007; Lochmiller & Deerenberg, 2000; Schwenke, Lazzaro, & Wolfner, 2016; Sheldon & Verhulst, 1996), although this is often not explicitly measured.
The terminal investment hypothesis has classically been viewed as a fixed strategy, with the switch to increased investment in current reproduction occurring when an individual encounters a cue representing a sufficient threat to longevity or future reproduction. However, the intensity of a threat that elicits terminal investment may itself depend on context. Any factor influencing residual reproductive value, beyond the specific threat that cues the terminal investment strategy, may alter an individual's assessment of the threat posed, and consequently, the propensity to terminally invest at any given threat level (Duffield et al., 2017). The threat level required to trigger a strategy of terminal investment can be referred to as the terminal investment threshold. In decorated crickets (Gryllodes sigillatus), for example, there is evidence that males increase their reproductive effort when challenged with an injection of heat-killed bacteria (Duffield, Hunt, Rapkin, Sadd, & Sakaluk, 2015; Duffield et al., 2018). However, this is age-dependent, with younger males not exhibiting terminal investment, even at high threat levels, whereas older males, with reduced residual reproductive value, terminally invest at lower levels of threat (Duffield et al., 2018). The dynamic, context-dependent nature of the strategy of terminal investment may explain why it is not ubiquitously demonstrated in empirical studies (Duffield et al., 2017).
The extent of inbreeding within a population can significantly influence the intrinsic condition of individuals. The negative effects of inbreeding (i.e., inbreeding depression) occur as a result of unmasked deleterious recessive alleles and loss of heterosis (Charlesworth & Charlesworth, 1987; Charlesworth & Willis, 2009; Crnokrak & Roff, 1999; Keller & Waller, 2002; Ralls, Ballou, Templeton, Ralls, & Ballou, 1988), and can manifest as deformities (Räikkönen, Vucetich, Peterson, & Nelson, 2009), decreased lifetime mating success and fecundity (de Boer, Eens, & Müller, 2018; Drayton, Milner, Hunt, & Jennions, 2010; Fox, Xu, Wallin, & Curtis, 2012; Miller, Glasner, & Hedrick, 1993; Radwan, 2003; Simmons, 2011), decreased offspring quality (Huisman, Kruuk, Ellis, Clutton-Brock, & Pemberton, 2016; Kruuk, Sheldon, & Merilä, 2002), and reduced lifespan (de Boer et al., 2018; Drayton et al., 2010). If inbreeding status itself reduces an individual's residual reproductive value, inbred individuals may increase investment towards current reproduction to mitigate the fitness costs associated with inbreeding depression. Indeed, Richardson and Smiseth (2017) recently found that inbred male burying beetles (Nicrophorus vespilloides) increased reproductive effort via increased competitive effort and greater risk-taking during intraspecific competition, relative to outbred males. Although this study suggests that inbreeding itself may induce terminal investment, how the level of inbreeding influences strategic shifts in reproductive effort upon exposure to a further threat to residual reproductive value, such as infection, has not been investigated.
Beyond inbreeding per se, genetic differences between individuals and populations may affect strategies of reallocation towards reproductive effort. Variation in genetically determined life-history characteristics will likely lead to genotype-specific thresholds, resulting in host genotype-by-environment interactions determining reproductive strategies, including the propensity to terminally invest. For example, changes in the reproductive output of water fleas, Daphnia magna, vary across genotypes upon infection with a bacterial pathogen (Vale & Little, 2012). Individuals from some clonal lines increased offspring production, whereas others decreased offspring production, relative to uninfected controls within the same line. A similar pattern was observed in pea aphids (Acyrthosiphon pisum; Leventhal, Dünner, & Barribeau, 2014). In decorated crickets, not only inbreeding status, but also inbred line identity, representing distinct genotypes, has been shown to influence important life-history associated traits (Archer, Sakaluk, Selman, Royle, & Hunt, 2013; Archer, Zajitschek, Sakaluk, Royle, & Hunt, 2012; Duffield et al., 2015; Gershman, Hunt, & Sakaluk, 2013; Gershman et al., 2010a,2010b; Weddle et al., 2013). For example, the number of sperm allocated to a specific mating varied significantly among eight inbred lines (Gershman et al., 2010b), and was significantly higher in inbred males than outbred males in two lines, significantly lower in another, and did not differ in the remaining five lines. These results show genotypic differences in reproductive effort that, together with the results from the other systems mentioned above, suggest that the effect of inbreeding status on the propensity to terminally invest could be genotype-dependent. Therefore, it is important to consider the effect of inbreeding across multiple distinct genotypes.
Here, we investigate how inbreeding status following exposure to an increasing spectrum of infection cue intensities influences allocation to reproductive effort in decorated crickets, G. sigillatus, including the propensity to terminally invest and the terminal investment threshold. We quantify reproductive effort (calling effort) and immunity (antibacterial activity, circulating haemocytes and haemocyte microaggregations) in inbred and outbred males. Because inbred individuals are often in poorer condition and consequently should have lower intrinsic residual reproductive value, we predicted that inbred individuals would terminally invest at lower threat cues (i.e., have a lower terminal investment threshold) than outbred individuals. We replicated infection cue treatments across four genetically distinct inbred lines to further explore the effect that genotype has on the propensity to terminally invest. Due to the genetic underpinnings of investment in reproduction and defence against infection, and their corresponding trade-off, we expected lines to switch to a terminal investment strategy at different threat levels that correspond to their unique life-history investment patterns (e.g., reproductive effort, immune investment and lifespan).
2 METHODS
2.1 Study animals
Inbred male G. sigillatus used in this study were selected from genetically distinct inbred lines that exhibit significant genetic variation in key life-history traits, including lifespan (Archer et al., 2012), body mass (Gershman et al., 2010b), male calling effort (Archer et al., 2012), female egg production (Archer et al., 2012), immune function (Gershman et al., 2010a), spermatophore mass (Gershman et al., 2010b), nuptial gift composition (Gershman et al., 2013) and cuticular hydrocarbon profiles (Weddle, Mitchell, Bay, Sakaluk, & Hunt, 2012). In this study, four lines were used from nine previously established inbred lines. These inbred lines were created by subjecting crickets, randomly selected from descendants of approximately 500 individuals collected at Las Cruces, New Mexico in 2001, to 23 generations of full-sib mating followed by multiple generations of panmixia within lines thereafter (Ivy, Weddle, & Sakaluk, 2005). Measurements made in 2007 revealed evidence of significant inbreeding depression in the inbred lines after 17 generations of inbreeding: inbred lines all showed lower hatching success, decreased offspring production and longer developmental times compared with the outbred population (Sakaluk, S. K., Oldzej, J., Hodges, C., Harper, J., Rines, I., Hampton, K. J., Duffield, K. R., Hunt, J., & Sadd, B. M., unpublished data). For this study, the four lines (A, F, G and I) selected were chosen because they were at the same stage of development at the time that the experiment was initiated. Outbred experimental males were selected from a large outbred colony, maintained at a population size of approximately 5,000 to ensure genetic heterogeneity and initiated with crickets collected from a wild population from Riverside, CA in 2014.
Newly hatched, experimental crickets were reared in 55-L plastic storage bins filled with egg carton to increase rearing surface area and provisioned with food (Envigo© 2018CM Teklad Certified Global 18% protein rodent diet meal) and water (glass vials plugged with cotton) ad libitum. When sex differences became apparent (4th or 5th instar), juvenile males were removed from stock colonies and housed individually in small (450 ml) plastic containers and provisioned with dry food pellets of the same Envigo© formulation and water (glass vials plugged with cotton) ad libitum. A small section of egg carton was also provided as a refuge. All individuals were housed in an environmental chamber at 32°C on a 16 hr:8 hr light:dark cycle. Males were checked twice weekly to determine the date of final moult to adulthood. Calling effort of males was used as a proxy for reproductive effort. Adult male G. sigillatus begin mating 4.5 days after eclosion on average (Burpee & Sakaluk, 1993) and, because calling is essential for mating, it follows that males are also calling from this time. Males were given an infection cue treatment 3 weeks (±2 days) following their adult eclosion, based on a previous study demonstrating that males at this age are more likely to show terminal investment in calling (Duffield et al., 2018). Body mass for each male was measured immediately prior to infection cue administration using an analytical balance (Mettler Toledo AG245).
2.2 Infection cue
A pathogenic infection may signal reduced residual reproductive value to the host, and this may be mediated through the infection-associated immune challenge. The preferred approach to investigate shifts in host life-history strategies as a consequence of infection is to simulate an infection through the use of nonpathogenic immune elicitors, as this technique eliminates the confounding effects of parasite proliferation, pathogenicity or parasite manipulation (Duffield et al., 2017). Earlier, we demonstrated that injection with heat-killed Escherichia coli stimulates an immune response and invokes a terminal investment response in male G. sigillatus (Duffield et al., 2015, 2018). This approach ensures the response is related to the host's strategy and not the result of alternative causes related to a live infection; thus, a similar protocol was implemented here. Males were randomly assigned to one of five treatments on an increasing infection cue spectrum: (a) naïve (unmanipulated control), (b) sham control (injection of 2 μl ringer saline), (c) low-dose infection cue (injection of 2 μl ringer saline with 5 × 105/ml heat-killed E. coli), (d) moderate-dose infection cue (injection of 2 μl ringer saline with 5 × 107/ml heat-killed E. coli) or (e) high-dose infection cue (injection of 2 μl ringer saline with 5 × 108/ml heat-killed E. coli). We consider the sham control treatment not only a control for the effect of injection, but also as a low-level mortality threat because injection causes cuticle damage and induces an immune response that potentially signals a mortality threat to the individual (Ardia, Gantz, Schneider, & Strebel, 2012; Gillespie & Khachatourians, 1992; Wigby, Domanitskaya, Choffat, Kubli, & Chapman, 2008). Injections were performed using a 5-μl syringe with a 1 mm compression fitting (Hamilton® brand) within which a needle formed from a heat-pulled glass capillary tube was inserted. Crickets were injected between the 6th and 7th pleurite of the thorax. Pulled capillaries were cleaned in 70% ethanol, rinsed with ultrapure water and dried between each injection. Capillaries were not reused across treatments nor days. Treatments were always applied at the same time (09:00 hr ± 1 hr) throughout the experiment.
Escherichia coli (ATCC strain 23716) used to create our low, moderate and high infection cues were cultured at 30°C in 7 ml of liquid medium (10 g bacto-tryptone, 5 g yeast extract, 10 g NaCl in 1,000 ml of distilled water, pH 7). To prepare bacterial suspensions for challenge injections, 1 ml of an overnight culture was centrifuged (850 g, 4°C, 10 min), and the supernatant was discarded and replaced with sterile ringer saline. This procedure was repeated three times. The bacteria were then heat-killed (90°C, 5 min), and the concentration of bacterial cells was adjusted to the concentrations described earlier for each infection cue dose. Efficiency of the heat killing was confirmed by plating out samples of the suspension on media agar.
2.3 Assessing reproductive effort
We quantified calling effort (i.e., the amount of time males spent calling) over two consecutive nights following infection cue treatment. Calling effort was measured using a custom-built high-throughput sound monitoring array (Bertram & Johnson, 1998; Duffield et al., 2018) in which each male-containing individual container (250 ml) was fitted with a lid-mounted microphone (C1163, Dick Smith Electronics) and placed within a small Styrofoam box to prevent crosstalk between containers. Following administration of infection cues, males were isolated and given 7 hr (±1 hr) to acclimate prior to the start of recording trials and provisioned with water and a small piece of egg carton for refuge. Recording periods started at 17:00 hr and ended at 09:00 hr and ran again for 24 hr starting at 10:30 hr the day following treatment; these periods capture calling effort at a time most relevant to female mate attraction (Burpee & Sakaluk, 1993; Sakaluk, 1987). The sound monitor sampled each microphone throughout the recording periods every 2 s, and based on the binary output resulting from this protocol, total calling time was calculated for each male across recording periods (Duffield et al., 2018; Hunt et al., 2004).
2.4 Survival and post-mortem measurements
Following the end of the two-night calling trials, males were returned to their individual rearing boxes where they were provided water ad libitum. To make monitoring survival tractable, individuals were food-deprived during this time, as laboratory-reared G. sigillatus are known to live for 2–3 months in the laboratory when fed ad libitum (Burpee & Sakaluk, 1993; Ivy & Sakaluk, 2005; Sakaluk, 1987), far longer than their longevity under more natural field conditions (Sakaluk, Schaus, Eggert, Snedden, & Brady, 2002). Mortality was monitored and recorded daily. Upon their death, the pronotum width of experimental subjects was measured as a proxy for structural body size, using a stereomicroscope (Nikon SMZ800) equipped with a digital camera and imaging software (Nikon NIS-Elements Documentation v. 4.20).
2.5 Statistical analyses for reproductive effort and survival
The distribution of male calls indicated that calling effort measurements had an excess of zeroes and were over-dispersed. Therefore, the calling effort data were analysed in the same way as previously done in this system (Duffield et al., 2018). We analysed calling effort data with Bayesian methods using the R package MCMCglmm (Hadfield, 2010), fitting a zero-altered Poisson (ZAP) model in R (version 3.4.2, R Core Team, 2016). The ZAP model includes a logistic regression for the zeroes in the data and an over-dispersed Poisson regression for the zero-truncated counts. This type of model enabled us to answer two distinct questions within a single statistical structure: (a) what factors affect whether a male chooses to call and (b) if a male calls, what factors affect the amount of calling (Duffield et al., 2018; Houslay, Houslay, Rapkin, Hunt, & Bussière, 2017; Houslay, Hunt, Tinsley, & Bussière, 2015)?
We first wanted to know whether inbreeding influences an individual's propensity to terminally invest. For this “Inbreeding model,” we included the full data set and our predictors were treatment (naïve, sham, low, moderate and high infection cue doses), inbreeding status (inbred versus outbred) and the interaction between infection cue and inbreeding status. We then wanted to know whether genotype itself influenced an individual's terminal investment threshold. For this “Genotype model,” we included only inbred individuals and our predictors were treatment (naive, sham, low, moderate and high infection cue doses), inbred line (lines A, F, G and I) and the interaction between infection cue and inbred line. For both models, we used binary indicator variables (0/1) to specify whether or not an individual belonged to each treatment group (Gelman & Hill, 2007), using “naïve/inbred” and “naïve/line A” as our reference levels for the Inbred model and Genotype model, respectively. We ran each model for a total of 850,000 iterations, with a “burn-in” of 50,000 and retaining every 400th iteration thereafter, yielding an effective sample size of around 2,000 for each coefficient in the model. Fixed effects are considered statistically significant if the 95% credible intervals do not include 0. We checked for a lack of autocorrelation through plots of the thinned chains and ran each model three times to ensure convergence to a similar posterior distribution (tested using the Gelman-Rubin diagnostic; Gelman, Rubin, Gelman, & Rubin, 1992). Our main model used an uninformative prior, and we checked that results were robust to different prior specifications.
For both Inbreeding and Genotype models, male survival was analysed using a Cox proportional hazards model (SAS v. 9.4), with infection cue and inbreeding status (for Inbreeding model) or inbred line (for Genotype model), and the interaction between the two included as main effects and body condition (residuals derived from a regression of body mass on pronotum width) included as a covariate. Reported results derive from the best models as determined by corrected Akaike's information criterion (AICc; Hurvich & Tsai, 1989; Sugiura, 1978).
2.6 Quantifying immune function
We quantified immune function in a separate group of male crickets originating from the same inbred and outbred lines and subject to the same treatments described in the calling effort portion of the study; this was done so that we could quantify both immunity and calling effort at similar time points post-infection. We measured multiple immune parameters in an attempt to capture the suite of immune pathways that may differ between lines or be altered following infection cue treatment. Here, we analysed antibacterial activity, total circulating haemocytes and haemocyte microaggregations from haemolymph that had been collected 4 hr after treatment administration. General cell-free antimicrobial activity of haemolymph is a component of the humoral immune response of insects and includes the action of both lysozyme-like enzymes and antimicrobial peptides (Lemaitre, Reichhart, & Hoffmann, 1997). Additionally, haemocytes are a component of the cellular response of insect immunity and are involved in core processes that include coagulation, nodulation, phagocytosis and encapsulation (Lavine & Strand, 2002). Finally, the microaggregation of haemocytes is an early step in the process of nodule formation (Miller, Howard, Rana, Tunaz, & Stanley, 1999; Miller & Stanley, 2004), the predominant insect cellular defence reaction to bacterial challenges and responsible for clearing a large proportion of bacteria from circulation (Howard, Miller, & Stanley, 1998).
To collect haemolymph, males were cold-anesthetized, the membrane was pierced under the dorsal pronotum plate with a sterile 25-G needle, and 5 μl of outflowing haemolymph was taken with a prechilled glass microcapillary tube positioned at the puncture site. Collected haemolymph was then expelled into 11 μl of Grace's insect medium (MilliporeSigma) to be used in antibacterial assays; 5 μl of this mixture was then added to 15 μl of Grace's insect medium which was immediately used for circulating haemocyte and microaggregation counts. The samples for antibacterial assays were snap-frozen in liquid nitrogen and stored at −80°C for later analysis.
2.7 Zone of inhibition assay
Although immune-challenged individuals in this study were injected with E. coli, previous assays resulted in no measurable antibacterial activity on plates seeded with E. coli. Therefore, antimicrobial activity was assayed from zones of inhibition induced by samples on petri dishes containing agar seeded with Micrococcus luteus (ATCC 4698; see Sadd & Schmid-Hempel, 2007 for methodological details). Briefly, M. luteus from a single colony on a streak plate were incubated overnight at 30°C in 7 ml of media (2.5 g peptone and 1.5 g meat extract in 500 ml of nanopure water, pH 7.0). From this culture, bacteria were added to liquid media containing 1% agar held at 40°C to achieve a final density of 1.5 × 105 cells/ml. Six millilitres of seeded medium was poured into a 100-mm diameter petri dish to solidify. Sample wells were made using a Pasteur pipette (Volac D810) fitted with a ball pump, 2 μl of sample solution thawed on ice was added to each well and negative (PBS) control wells were included on each plate. Plates were inverted, incubated for 48 hr at 30°C and then the diameter of inhibition zones was measured for each sample. Two diameter measurements of zones, perpendicular to one another, were measured for each sample using ImageJ (Schneider, Rasband, & Eliceiri, 2012) and averaged (measurements were performed blind to treatment). Because zone of inhibition diameter does not increase linearly with antibacterial activity, measured zone diameters were converted using a standard curve, to units (mg/ml) of lysozyme (from hen egg white, MilliporeSigma, CAS: 12650-88-3). Each haemolymph sample was tested in duplicate, with the mean of the duplicates being used in subsequent analyses.
2.8 Circulating haemocyte and microaggregation counts
Haemocytes and microaggregations were counted at 400× magnification under a phase-contrast microscope with a haemocytometer (Fast-Read 102® plastic counting chamber) to assess their numbers per individual as a proxy for cellular immunity (Duffield et al., 2018; King & Hillyer, 2013; Stoepler, Castillo, Lill, & Eleftherianos, 2013). Counting was performed blind to treatment.
2.9 Statistical analysis of immune measures
As with our analysis of reproductive effort, we compared inbreeding status (for Inbreeding model) and inbred lines (for Genotype model) for all immune measures. Infection cue and the interaction with inbreeding status or inbred line were also included as main effects, and body condition (residuals derived from a regression of body mass on pronotum width) included as a covariate for all immune measures. Total circulating haemocyte and zone of inhibition values were log-transformed to meet the assumptions of normality (reported means and confidence intervals were back-transformed) and analysed with general linear models. For microaggregation counts, we implemented a generalized linear model with a negative binomial error distribution. Reported results derive from the best models as determined by corrected Akaike's information criterion (AICc).
3 RESULTS
3.1 Reproductive effort
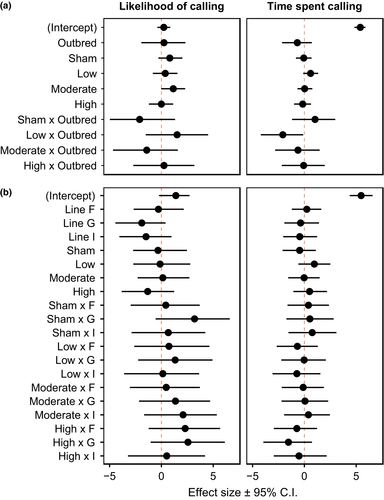
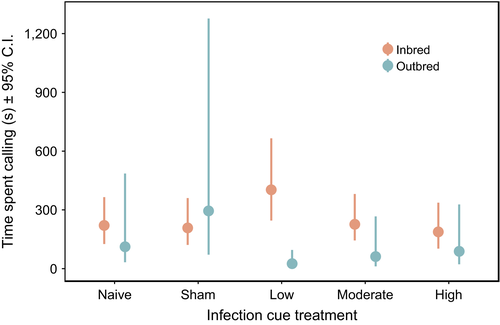
The calling effort of 609 males was measured over two consecutive nights following infection cue treatment (sample sizes included in Table S1). We found a significant interaction between the effects of inbreeding and infection cue on the time spent calling of males (Figure 1a). Inbred and outbred males diverged in how they responded to a low dose of heat-killed E. coli: outbred males decreased calling effort and inbred males increased calling effort (although nonsignificantly) relative to the naïve reference group (Figure 2), and there was a significant increase in the likelihood that a male would call at the moderate-dose treatment level (Figure 1a). Within inbred lines, we found no effect of line identity or any of the other predictors on either the time spent calling by males or the likelihood of calling (Figure 1b). Parameter estimate, confidence interval and p values are reported in Table S2.
3.2 Survival
The nonsignificant interaction term of inbreeding status by infection cue (Wald  = 1.90, p = 0.7544) was removed from the final Inbreeding model for survival, and there was also no significant effect of inbreeding status (Wald
= 1.90, p = 0.7544) was removed from the final Inbreeding model for survival, and there was also no significant effect of inbreeding status (Wald  = 0.48, p = 0.4903) or infection cue (Wald
= 0.48, p = 0.4903) or infection cue (Wald  = 0.88, p = 0.9271). There was, however, a significant effect of body condition (Wald
= 0.88, p = 0.9271). There was, however, a significant effect of body condition (Wald  = 85.09, p < 0.0001), with males in better condition living longer than those in poorer condition (hazard ratio [lower, upper bounds of 95% confidence intervals] = 0.983 [0.980, 0.987]).
= 85.09, p < 0.0001), with males in better condition living longer than those in poorer condition (hazard ratio [lower, upper bounds of 95% confidence intervals] = 0.983 [0.980, 0.987]).
For the final Genotype model, we removed the nonsignificant interaction between inbred line and infection cue (Wald  = 13.85, p = 0.3106). Infection cue did not influence survival (Wald
= 13.85, p = 0.3106). Infection cue did not influence survival (Wald  = 0.83, p = 0.9348), but survival varied significantly among inbred lines (Wald
= 0.83, p = 0.9348), but survival varied significantly among inbred lines (Wald  = 44.34, p < 0.0001), with lines F (hazard ratio [lower, upper bounds of 95% confidence intervals] = 0.539 [0.414, 0.703]) and G (hazard ratio [lower, upper bounds of 95% confidence intervals] = 0.438 [0.331, 0.579]) having increased survival relative to the reference line I and line A (hazard ratio [lower, upper bounds of 95% confidence intervals] = 0.904 [0.658, 1.234]). Body condition (Wald
= 44.34, p < 0.0001), with lines F (hazard ratio [lower, upper bounds of 95% confidence intervals] = 0.539 [0.414, 0.703]) and G (hazard ratio [lower, upper bounds of 95% confidence intervals] = 0.438 [0.331, 0.579]) having increased survival relative to the reference line I and line A (hazard ratio [lower, upper bounds of 95% confidence intervals] = 0.904 [0.658, 1.234]). Body condition (Wald  = 73.17, p < 0.0001) significantly affected survival, with males in better condition living longer than males in poorer condition (hazard ratio [lower, upper bounds of 95% confidence intervals] = 0.984 [0.980, 0.988]).
= 73.17, p < 0.0001) significantly affected survival, with males in better condition living longer than males in poorer condition (hazard ratio [lower, upper bounds of 95% confidence intervals] = 0.984 [0.980, 0.988]).
3.3 Immune function
Immune function, based on antibacterial activity, circulating haemocytes and haemocyte microaggregations, was measured in 265 males 4 hr post-infection cue treatment (sample sizes included in Table S1).
3.3.1 Antibacterial activity of haemolymph
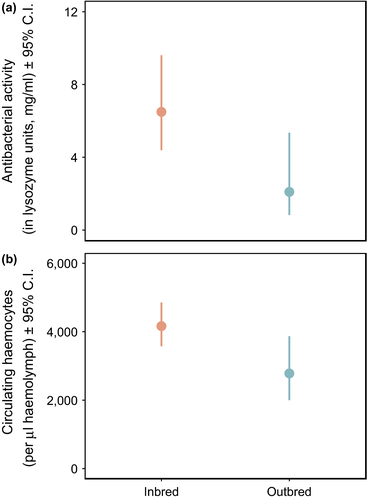
The interaction between inbreeding status and infection cue was nonsignificant (F4,179 = 1.02, p = 0.3984) and was not included in the final Inbreeding model. We found a significant effect of inbreeding status on haemolymph antibacterial activity (F1,183 = 4.77, p = 0.0303), with inbred males having greater antibacterial activity compared with outbred males (Figure 3a). There was also a significant effect of body condition (F1,183 = 5.63, p = 0.0186), with males in better condition having increased activity. Infection cue did not influence antibacterial activity (F4,183 = 0.19, p = 0.9457).
In the Genotype model, a nonsignificant line by infection cue interaction term (F12,140 = 0.61, p = 0.8309) was removed from the final model. Within inbred lines, neither inbred line (F3,152 = 0.78, p = 0.5096) nor infection cue treatment (F4,152 = 0.37, p = 0.8499) significantly affected antibacterial activity, but we again found a significant effect of body condition (F1,152 = 4.36, p = 0.0384), with males in better condition having greater activity.
3.3.2 Circulating haemocytes
A nonsignificant interaction between inbreeding status and infection cue (F4,246 = 2.17, p = 0.0727) was removed from the final Inbreeding model for circulating haemocytes. We found an effect of inbreeding status on total circulating haemocytes 4 hr post-treatment (F1,250 = 4.66, p = 0.0318) with inbred individuals having more circulating haemocytes than outbred individuals (Figure 3b). However, we did not find a significant effect of infection cue treatment (F4,250 = 0.94, p = 0.4429) or body condition (F1,250 = 3.24, p = 0.0729).
A nonsignificant interaction between inbred line and infection cue (F12,189 = 1.71, p = 0.3050) was also removed from the final Genotype model for circulating haemocytes. Further, neither inbred line (F4,201 = 0.84, p = 0.4760), infection cue (F4,201 = 1.00, p = 0.4093), nor body condition (F1,201 = 3.07, p = 0.0812) significantly influenced the number of circulating haemocytes.
3.3.3 Microaggregations of haemoctyes
The nonsignificant interaction between inbreeding status and infection cue (Wald  = 0.51, p = 0.9729) was removed from our final Inbreeding model for microaggregations. There was also no significant effect of inbreeding status (Wald
= 0.51, p = 0.9729) was removed from our final Inbreeding model for microaggregations. There was also no significant effect of inbreeding status (Wald  = 1.27, p = 0.2593), infection cue (Wald
= 1.27, p = 0.2593), infection cue (Wald  = 2.97, p = 0.5621) or body condition (Wald
= 2.97, p = 0.5621) or body condition (Wald  = 1.64, p = 0.2004) on microaggregation counts. Within inbred lines, however, we found a significant interaction between inbred line and infection cue on circulating microaggregations (Wald
= 1.64, p = 0.2004) on microaggregation counts. Within inbred lines, however, we found a significant interaction between inbred line and infection cue on circulating microaggregations (Wald  = 32.27, p = 0.0013).
= 32.27, p = 0.0013).
4 DISCUSSION
Our results show that inbreeding status interacts with infection cue to alter calling effort in male decorated crickets, G. sigillatus. Specifically, at a low-dose infection cue, inbred and outbred males diverged in their calling effort. Relative to the naïve reference group, inbred males showed a trend for increased calling effort, whereas outbred males decreased calling effort. The relative difference in calling effort between inbred and outbred males at a low infection cue dose is consistent with predictions of inbred males maintaining reproductive effort or terminally investing (Duffield et al., 2017; Williams, 1966), while outbred males decrease effort as a consequence of a trade-off between investment into immune defence and reproduction (Harshman & Zera, 2007; Lochmiller & Deerenberg, 2000). This suggests that intrinsic differences between inbred and outbred males influence how they respond to infection cues, at least in the form of time spent calling. Importantly, we did not find an effect of inbred line, which suggests that differences in calling effort are driven by an effect of inbreeding per se, and not by differences in genotype. This is consistent with a previous study that assessed terminal investment in another form of reproductive effort in this species, the gustatory appeal of males’ nuptial food gifts (Duffield et al., 2015). In that study, immune-challenged males increased the attractiveness of their nuptial gifts relative to controls consistently across three inbred lines (i.e., there was no evidence of a genotype-by-environment response among inbred lines).
We found no evidence that a male's likelihood of calling was affected by the interaction between inbreeding status and infection cue, although the likelihood of calling was slightly greater under the moderate-dose infection cue. In a previous study (Duffield et al., 2018), terminally investing males altered their time spent calling, but not their propensity to call. Together, these results suggest that time spent calling is a plastic trait with respect to its expression in response to an infection cue, whereas the likelihood of calling is less labile. Although inbred and outbred males in the current study diverged in their calling effort at the low infection cue dose relative to naïve individuals, there was no difference in calling effort following moderate or high infection cues in either inbred or outbred groups, contrary to earlier work (Duffield et al., 2018). Both studies show reproductive investment can be context-dependent, but there are a couple of important differences that might explain the discrepancy in the specific findings. First, the increased number of factors considered in the current study decreased the number of males within groups, and the concomitant decrease in statistical power may have constrained our ability to detect more subtle differences across treatment levels. Second, it could be that the crickets used in the current experiment differed in some component of their condition or health from those measured in the previous study, which caused them to respond to high infection cues differently. For example, the outbred crickets used here originated from a single geographic location, whereas crickets used in the previous study comprised outbred crickets pooled across three geographic locations. Nevertheless, in the current study, differences in calling effort between inbred and outbred males at the low infection cue dose demonstrate that inbreeding status influences reproductive investment post-treatment.
Baseline calling effort (i.e., calling effort of naïve males) did not differ between inbred and outbred individuals. Similarly, Drayton et al. (2010) found that inbred field crickets (Teleogryllus commodus) called the same amount as outbred crickets, but that the fine-scale structure of the calling song differed between the groups. Specifically, outbred and inbred males differed with respect to the carrier frequency of the song, number of pulses and trills, time between chirps and time between calls (Drayton et al., 2010). Importantly, these calling features are essential for mate recognition and attraction of sexually receptive female crickets (Bentsen, Hunt, Jennions, & Brooks, 2006). Therefore, it is possible that inbreeding and infection cue are influencing calling patterns in additional ways that could dramatically influence male fitness, but that we could not capture using our current methods. Future studies should explore the fine-scale structure of inbred and outbred male calls that are recorded both before and after males have experienced a simulated infection cue.
In this study, inbred males had greater humoral antibacterial activity compared with outbred individuals, and more circulating haemocytes. These elevated immune measures are consistent with earlier findings showing that inbred male G. sigillatus from the same inbred lines as used here exhibited higher levels of melanization of nylon implants (a proxy for macroparasite infection) compared with outbred crickets (Gershman et al., 2010a). Similarly, in T. commodus, inbred individuals had greater numbers of circulating haemocytes than outbred individuals (Drayton & Jennions, 2011). We did not find a significant effect of inbreeding status on microaggregation counts, but response patterns are often not homogenous when multiple immune parameters are measured, as reflected in other studies comparing immunity of inbred and outbred individuals (Drayton & Jennions, 2011; Gershman et al., 2010a). However, within inbred lines, we did find an unexpected interaction between infection cue treatment and inbred line on the number of microaggregations. This suggests that for this component of immunity, at least, a genotype-by-environment interaction may exist, but this requires additional investigation.
The consensus from our study and earlier work in this system (Gershman et al., 2010a) is that immune measures tend to be higher in inbred versus outbred individuals. There are several explanations as to why inbreeding status might lead to higher levels of immunity. A potential explanation is that low-quality inbred individuals are more susceptible to infection, and therefore, higher immune measures are a consequence of greater immune induction by pathogens in inbred compared with outbred individuals. However, inbred crickets used in the current study appeared healthy (i.e., we saw no obvious signs of infection) and higher immunity in inbred individuals includes measurements of immune traits that are considered to be constitutively expressed under the implemented protocols, for example antibacterial activity (Duffield et al., 2018). Secondly, once again if inbred crickets are more susceptible to pathogens, they may prophylactically upregulate constitutive immune function to prevent infection. Thirdly, it could be that inbred individuals are trading off investment in future survival with current maintenance (Gershman et al., 2010a; Stearns, 1992), although inbred males in the current study did not exhibit reduced survival compared with outbred males (but see Archer et al., 2012). Finally, it is important to note that maximal immune function may not be optimal (Sadd & Schmid-Hempel, 2009). Several of the measures of immunity reported to be higher in inbred versus outbred crickets are constitutively expressed. High levels of immunity may be energetically expensive to maintain (Sadd & Schmid-Hempel, 2009) and come with an increased risk of self-damage through autoreactivity (Sadd & Siva-Jothy, 2006). Inbreeding might disrupt the physiological mechanisms responsible for regulating immune function around an optimum, with our results and others therefore potentially being the consequence of an over-active and unrestrained immune system.
In contrast to studies in other crickets (Archer et al., 2012; Drayton, Hunt, Brooks, & Jennions, 2007; Roff, 1998), we found no evidence of reduced survival in inbred males. However, males in this study were food-deprived after calling effort had been quantified, and if they had they been allowed to senesce under ad libitum food conditions, differences between inbred and outbred individuals may have emerged. Consistent with a previous study (Archer et al., 2012), we found a difference in survival of males from different inbred lines. These differences in lifespan could correspond to distinct life-history strategies encompassing differential investment in somatic maintenance and immunity (Gershman et al., 2010a), but we found no evidence of this in the current study.
In conclusion, we show that the reproductive effort of male G. sigillatus is influenced by an interaction between inbreeding status and the intensity of an infection cue. However, within inbred lines, we found no evidence of a genotype-by-environment interaction between line and infection cue treatment in this response. We also found that inbred males had higher levels in two out of three assayed immune parameters, corroborating previous work showing elevated responses of inbred individuals in another measure of immunity. These results suggest that inbreeding influences the allocation of resources to reproduction and immunity, and that, when faced with an infection, the inbreeding status of an individual alters its reproductive strategy.
ACKNOWLEDGMENTS
We thank Savannah Cranford and Emily Bestow for their assistance in the laboratory. This research was funded by a grant from the National Science Foundation to S.K.S., B.M.S., and J.H. (IOS 16–54028), grants from the Sigma Xi Research Honor Society, the Beta Lambda Chapter of the Phi Sigma Biological Honor Society, and Graduate Student Association of Illinois State University to K.R.D., and a grant from the Australian Research Council to J.H. (DP180101708). Research reported in this publication was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R15GM12968 to BMS.
DATA ACCESSIBILITY
All data supporting this manuscript will be deposited in dryad upon acceptance. Data deposited at Dryad: https://datadryad.org/resource/doi:10.5061/dryad.r4s16vs




