Heterosis is common and inbreeding depression absent in natural populations of Arabidopsis thaliana
Abstract
The importance of genetic drift in shaping patterns of adaptive genetic variation in nature is poorly known. Genetic drift should drive partially recessive deleterious mutations to high frequency, and inter-population crosses may therefore exhibit heterosis (increased fitness relative to intra-population crosses). Low genetic diversity and greater genetic distance between populations should increase the magnitude of heterosis. Moreover, drift and selection should remove strongly deleterious recessive alleles from individual populations, resulting in reduced inbreeding depression. To estimate heterosis, we crossed 90 independent line pairs of Arabidopsis thaliana from 15 pairs of natural populations sampled across Fennoscandia and crossed an additional 41 line pairs from a subset of four of these populations to estimate inbreeding depression. We measured lifetime fitness of crosses relative to parents in a large outdoor common garden (8,448 plants in total) in central Sweden. To examine the effects of genetic diversity and genetic distance on heterosis, we genotyped parental lines for 869 SNPs. Overall, genetic variation within populations was low (median expected heterozygosity = 0.02), and genetic differentiation was high (median FST = 0.82). Crosses between 10 of 15 population pairs exhibited significant heterosis, with magnitudes of heterosis as high as 117%. We found no significant inbreeding depression, suggesting that the observed heterosis is due to fixation of mildly deleterious alleles within populations. Widespread and substantial heterosis indicates an important role for drift in shaping genetic variation, but there was no significant relationship between fitness of crosses relative to parents and genetic diversity or genetic distance between populations.
1 INTRODUCTION
With limited gene flow, population differentiation in nature is driven by a complex balance between mutational input, natural selection and random genetic drift. Evolutionary biologists are particularly interested in the mechanisms that contribute to adaptive differentiation, and thus, there is much emphasis on understanding the causes and consequences of selection (Kawecki & Ebert, 2004; Kingsolver et al., 2001). Genetic drift can influence the adaptive process by reducing the efficacy of selection for new beneficial mutations (Dittmar, Oakley, Conner, Gould, & Schemske, 2016; Kimura, 1983) and against deleterious mutations (Kimura, Maruyama, & Crow, 1963). Most new mutations have somewhat deleterious, partially recessive effects (Agrawal & Whitlock, 2011; Eyre-Walker & Keightley, 2007; Halligan & Keightley, 2009). Because genetic drift is expected to fix different deleterious mutations in different populations, crosses between natural populations will be heterozygous for some partially recessive deleterious alleles and are likely to exhibit heterosis (Crow, 1948; Whitlock, Ingvarsson, & Hatfield, 2000), defined here as the increased fitness of crosses between populations relative to crosses within populations.
Heterosis has generated much interest (reviewed in Chen, 2013; Goulet, Roda, & Hopkins, 2017; Hochholdinger & Hoecker, 2007) because of its practical value for breeding, but it also provides insight into the balance between selection and drift in nature (Escobar, Nicot, & David, 2008; Fenster, 1991; Lohr & Haag, 2015; Oakley, Spoelhof, & Schemske, 2015b; Oakley & Winn, 2012; Paland & Schmid, 2003; Pannell & Fields, 2013). Heterosis is ubiquitous in plants (e.g., Busch, 2006; Paland & Schmid, 2003; van Treuren, Bijlsma, Ouborg, & van Delden, 1993) and has been reported in many animal species as well (Armbruster, Bradshaw, & Holzapfel, 1997; Dolgin, Charlesworth, Baird, & Cutter, 2007; Edmands, 1999; Escobar et al., 2008; Hedgecock et al., 1996; Lohr & Haag, 2015). Theory suggests that mildly deleterious alleles contribute the most to heterosis (Whitlock et al., 2000). Frequencies of deleterious alleles are expected to vary among finite natural populations due to drift, and to be higher in small compared to large populations. Evidence of greater heterosis in crosses between populations with smaller expected effective population sizes (Lohr & Haag, 2015; Oakley & Winn, 2012; Oakley et al., 2015b; Spigler, Theodorou, & Chang, 2016; Willi, 2013) is consistent with heterozygous masking of deleterious partially recessive alleles in crosses between populations.
Estimates of inbreeding depression within individual populations can be used in combination with estimates of heterosis in crosses between populations to gain further insight into the effects of drift on the partitioning of deleterious alleles within and among populations. Inbreeding depression is the reduction in fitness of progeny resulting from self-fertilization relative to that of progeny resulting from outcrossing (Charlesworth & Charlesworth, 1987; Charlesworth & Willis, 2009; Goodwillie, Kalisz, & Eckert, 2005). Inbreeding depression is thought to be caused mainly by partially recessive deleterious alleles (Charlesworth & Willis, 2009), but unlike heterosis, alleles contributing to inbreeding depression are thought to have a bimodal distribution of selection and dominance coefficients (Charlesworth & Charlesworth, 1987; Charlesworth & Willis, 2009; Husband & Schemske, 1996; Lande & Schemske, 1985; Simmons & Crow, 1977). One category of alleles is very strongly deleterious and highly recessive, and is expected to segregate at low frequency within populations. Such alleles are expected to be selectively purged in selfing species (Husband & Schemske, 1996; Lande & Schemske, 1985; Porcher & Lande, 2005; Winn et al., 2011). Genetic drift (Bataillon & Kirkpatrick, 2000; Glémin, Ronfort, & Bataillon, 2003; Whitlock, 2002), or a combination of selection and drift (Glémin, 2003), could also reduce inbreeding depression within individual populations due to strongly deleterious alleles. The other category of alleles underlying inbreeding depression is more mildly deleterious and more nearly additive. These alleles may be unconditionally deleterious or may arise as the result of stabilizing selection on quantitative traits, resulting in weak dominance effects on fitness (Awad & Roze, 2018; Cheptou & Donohue, 2011; Lande & Porcher, 2015; Lande & Schemske, 1985; Porcher & Lande, 2005; Ronce, Shaw, Rousset, & Shaw, 2009). Mildly deleterious alleles are more difficult to selectively purge and may drift to high frequency within populations, particularly when these alleles are unconditionally deleterious and/or when the effects of drift are very strong.
A history of genetic drift should thus increase homozygosity and reduce the magnitude of inbreeding depression observed after crosses between closely related individuals within populations and after selfing, but should increase heterosis in between-population crosses. This predicted negative relationship between heterosis and inbreeding depression as a consequence of genetic drift has been observed in some studies (Lohr & Haag, 2015; Oakley & Winn, 2012), but may depend on whether the system is at equilibrium (Spigler et al., 2016).
Heterosis is expected to increase with increasing genetic distance between crossed populations because of greater opportunity for among-population differences in the identities and frequencies of deleterious recessive alleles (Charlesworth & Willis, 2009; Lynch, 1991). Indeed, there has been much interest in understanding the relationship between genetic distance and the fitness consequences of inter-population crosses (Armbruster et al., 1997; Demuth & Wade, 2007; Edmands, 2002; Fenster & Galloway, 2000). Increased fitness in inter-population crosses is not universal, and such crosses may result in outbreeding depression or decreased fitness relative to the parents. Outbreeding depression is often not manifested until the F2 or later recombinant generations (Edmands, 1999; Fenster & Galloway, 2000), but it has been observed in the first generation (i.e., F1) of some inter-population crosses (Bomblies et al., 2007; Gimond et al., 2013; Oakley, Ågren, & Schemske, 2015a).
The model plant Arabidopsis thaliana (hereafter Arabidopsis) is a good candidate for studying the consequences of inter-population crosses because it is highly selfing (Abbott & Gomes, 1989) and has a wide native geographic range (Beck, Schmuths, & Schaal, 2008; Durvasula et al., 2017; Koornneef, Alonso-Blanco, & Vreugdenhil, 2004), meaning that heterosis is likely and can be studied over a range of geographic/genetic distances. In comparison with outcrossing, selfing is expected to increase the magnitude of heterosis (Oakley et al., 2015b; Willi, 2013) because of the relative reduction in effective population size (Nordborg, 2000) and because it reduces gene flow among populations. In Arabidopsis, there is a pattern of decreased genetic variation in northern populations compared to southern populations, which has been attributed to a history of population bottlenecks and drift during post-Pleistocene range expansion (Beck et al., 2008). Even within the northern portion of the range in Fennoscandia, there is evidence of reduced genetic variation in northern compared to southern populations (Lewandowska-Sabat, Fjellheim, & Rognli, 2010; Long et al., 2013). Although this reduction in genetic variation may be attributed to selection as well as drift, the finding that nonsynonymous substitutions are mostly deleterious in Arabidopsis (Bustamante et al., 2002; Flowers, Hanzawa, Hall, Moore, & Purugganan, 2009) indicates that genetic drift can constrain adaptive evolution in this species. The recent finding of more maladaptive fitness QTL in northern Sweden compared to Italy (Ågren, Oakley, McKay, Lovell, & Schemske, 2013), as well as heterosis in crosses between these populations (Oakley et al., 2015a), raises the possibility that there is greater fixation of deleterious recessive mutations due to bottlenecks and/or drift in the northern portion of the range. Previous work in Arabidopsis has investigated the genetic basis of heterosis (Kusterer et al., 2007; Meyer et al., 2010, 2012) and hybrid necrosis (Bomblies et al., 2007; Chae et al., 2014; Smith, Bomblies, & Weigel, 2011; Świadek et al., 2016), a stunted F1 phenotype that has been interpreted as a type of outbreeding depression, but such studies were not designed to investigate the role of genetic drift in natural populations.
Few studies in any species have quantified fitness of inter-population crosses from a large collection of natural populations, and field estimates of fitness for such collections are rare (but see Fenster & Galloway, 2000). To understand the range of variation in fitness outcomes of inter-population crosses, fitness estimates are required from crosses between many populations grown under natural conditions. Combining estimates of inbreeding depression within populations with estimates of heterosis between populations is a particularly useful approach for interpreting the consequences of genetic drift for deleterious alleles. Furthermore, genetic information for the parental populations is necessary in order to investigate the relationship between heterosis and genetic diversity within populations and genetic distance between populations.
Here, we use progeny from crosses among 30 natural populations of Arabidopsis from Fennoscandia planted in an outdoor common garden in central Sweden to investigate the frequency and magnitude of heterosis and inbreeding depression. Crosses were done between independent population pairs chosen to maximize the range of geographic distances, and lines were subsequently genotyped using 2b-RAD (Wang, Meyer, McKay, & Matz, 2012) to examine correlations between relative fitness of crosses and estimates of genetic diversity and genetic distance. Specific questions we address are as follows: (a) What is the range of values of heterosis? (b) Is there inbreeding depression within populations, which would indicate that these populations harbour segregating variation in deleterious recessive alleles? (c) Is heterosis correlated with expected heterozygosity of parental populations, or genetic distance between parental populations?
2 MATERIALS AND METHODS
2.1 Seed collection and propagation of parental lines
Seeds from 12 to 48 replicate maternal plants from each of 30 populations from Sweden, Norway and Finland (Supporting Information Table S1, Figure S1) were collected in the field in 2012, 2010 and 2005, respectively. In total, we collected seeds from 615 maternal lines. Plants were grown in the laboratory for one generation to minimize maternal environmental effects including year of collection. Seeds were surface-sterilized, sown on nutrient agar in petri dishes and cold-stratified at 4°C in the dark for 5 days to break dormancy. Dishes were then moved to a growth chamber set at a constant temperature of 22°C with 16-hr days. Twelve-day-old seedlings were transplanted into potting soil in 5.1 cm square pots, and plants were grown for 3 weeks at the same conditions. Many native populations of Arabidopsis exhibit a winter annual life history (Ågren & Schemske, 2012; Burghardt, Edwards, & Donohue, 2016; Montesinos, Tonsor, Alonso-Blanco, & Pico, 2009): seeds germinate in the fall, and plants overwinter as rosettes and flower in the spring or summer. To promote synchronous flowering, we vernalized plants from all but one of our populations for at least 8 weeks at 4°C for 10-hr days. After vernalization, plants were moved to a growth chamber under the long-day conditions described above until they flowered. Plants from Lule (Supporting Information Table S1, Figure S1) in northern Sweden began flowering prior to vernalization, so for this one population, we used nonvernalized plants for controlled pollinations.
2.2 2b-RAD genotyping and genetic data analysis
For the subset of 180 lines involved in between-population outcrosses (see below), we collected tissue from 3-week-old seedlings, lyophilized the tissue and extracted genomic DNA using Qiagen DNeasy Plant Mini Kits following manufacturer's instructions. Details of the 2b-RAD (Fletcher et al., 2013; Wang et al., 2012) library preparation, sequencing and reference-based assembly are provided in the supplementary methods (Supporting Information Methods S1). DNA samples from 13 lines were excluded either because DNA extraction failed or because of low sample read coverage. In total, 167 lines were included in subsequent analyses of SNP genotypes, with an average of 5.6 samples (range = 4–7) per population.
From the reference-based assemblies, we ran the ref_map.pl and rx_stacks procedures in Stacks (Catchen, Hohenlohe, Bassham, Amores, & Cresko, 2013), followed by the populations procedure to call SNPs in each individual and produce population-level summary statistics. For the populations procedure, we employed the following filters: loci had to be called in all 30 of the populations and in at least 75% of the samples within a population, have a minimum read depth of 12× and have a minor allele frequency equivalent to at least three copies or ~25% of the alleles in the average population sample.
One of our main questions is whether heterosis increases with either decreased genetic variation within populations or increased genetic distance between populations. We use expected heterozygosity as our measure of within-population genetic variation because it is a robust proxy for relative effective population size even with small sample sizes per population (Nei, 1978), and it has been used in previous multi-population studies of heterosis and inbreeding depression (Escobar et al., 2008; Lohr & Haag, 2015; Oakley & Winn, 2012; Ouborg & Van Treuren, 1994; Spigler et al., 2016; Willi, Griffin, & Van Buskirk, 2013). Population-level estimates of expected heterozygosity were taken directly from the Stacks output, and for each population pair (see below), we used the mean of the two values. For genetic distance, we used pairwise FST values, calculated using Genepop on the Web (Rousset, 1997, 2008).
2.3 Controlled pollinations
From the 30 populations, we chose 15 unique population pairs to maximize the range of pairwise geographic distance. Within each population pair, one population was designated as the maternal population and the other as the paternal population. This designation was based on slight differences in flowering phenology, with the maternal population determined by which population had the most plants at the optimal stage of flowering to be ovule donors. If both populations in the pair had very similar flowering phenologies, maternal and paternal roles were assigned randomly. The large-scale crossing design made performing reciprocal pollinations infeasible. For each of the fifteen population pairs, we performed crosses between populations using 5–7 unique replicate line pairs (mean = 6). In each line pair, one plant was designated as the maternal parent and the other the paternal parent based on population as described above. In addition to between-population crosses, we also performed a set of within-population crosses using independent line pairs, in which one plant was designated as either the maternal or paternal plant. Within-population crosses were carried out in four populations (Lule, Tost, Vato and Roda) with a sufficient number of additional lines (9, 10, 10 and 12 line pairs, respectively). Crosses were repeated on multiple flowers until a sufficient number of seeds had been obtained. For all plants involved in crosses, we also collected seeds produced by autogamous selfing.
In total, we performed 3,478 controlled cross-pollinations. Success of controlled cross-pollinations was similar across population pairs in terms of both mean proportion fruit set (mean = 0.744, SD = 0.08, N = 15 population pairs) and mean seed number per fruit (mean = 19.89, SD = 3.28). Autogamous fruit and seed set was not quantified for these plants, but previous work has found mean fruit and seed set of ~93% and ~22 seeds per fruit respectively for autogamously selfed fruits (Unpublished data). Reduced fruit set, and to a lesser extent, reduced seed set, in experimental crosses relative to autogamously selfed fruits is likely due to damage during emasculation prior to controlled crossing. Previous work using molecular markers to confirm paternity on hundreds of individuals produced by crosses following the same protocol has demonstrated that fewer than 1% of seeds were the result of accidental self-pollination (Oakley et al., 2015a). Germination of seeds derived from outcrossing and selfing was not quantified directly, but overall germination was generally greater than ~80%.
2.4 Field transplant experiment
Seedlings were sown on agar in petri dishes and then stratified for 1 week at 4°C in the dark. Thereafter, petri dishes were placed in growth rooms for 2 weeks with 18-hr days with a light intensity of 150 μmol photons m−2 s−1 at 20°C day/15°C night. Transplanting took place at Uppsala University, Sweden, over three days in September 2013. Seedlings were distributed in 32 flats (hereafter blocks) and transferred to an experimental plot in the Botanical Garden of Uppsala University 1 day after transplanting was complete. Transplanting into the 32 blocks was done in a stratified random fashion. For each line pair, one individual each of the first-generation inter-population crosses (i.e., F1) and the selfed progeny from both the maternal and paternal parents was planted in a given block, and this was repeated across 24 different blocks. For the between-population crosses, an average of 4.5 (SD = 1.1) line pairs from each population pair was planted in each block, with line pairs containing the F1 and the selfed progeny from both the maternal and paternal parents. For the within-population crosses, an average of 7.7 (SD = 1.7) line pairs from each population was planted in each block. For each of these line pairs, we planted progeny resulting from outcrossing, as well as progeny resulting from selfing of the maternal plant.
In total, we planted 6,480 seedlings for the between-population comparisons, representing 90 line and 15 population pairs, respectively. We also planted a total of 1,968 seedlings for the within-population comparisons, representing 41 line pairs from four populations. Survival was scored after 1 week to check for transplant shock, and plants that had died during this first week were excluded from analysis. Plants were watered only during the initial week of the experiment and were not fertilized or weeded. Survival was scored periodically throughout the experiment, and total number of fruits produced per plant was counted at the end of the experiment in June 2014. Thus, our experimental garden replicates the natural life history of populations from central Sweden, though this site may represent a novel environment for more distant populations. The choice of conditions for assaying fitness under natural conditions in large multi-population studies of heterosis is difficult because for every measure of heterosis there are two ancestral environments. The large number of populations, level of technical difficulty to cross Arabidopsis and the massive scale of our common garden experiment limited us to a single site.
2.5 Statistical analyses and calculation of heterosis and outbreeding and inbreeding depression
Cumulative fitness, estimated as total fruit production per seedling planted, was analysed separately for the between- and within-population comparisons because they contained independent sets of lines, and the within-population comparison included a subset of four populations. The distribution of individual cumulative fitness was non-normal with an excess of zeros, so we used line pair–cross type mean fitness estimates in our analysis. For the between-population crosses, cumulative fitness was analysed with an ANOVA with the following fixed effects: population pair, cross type (F1, and maternal and paternal offspring derived from selfing) and their interaction. The results of the analysis on line pair–cross type mean fitness were very similar to results obtained from a zero-inflated Poisson generalized linear mixed model (using glmmTMB in R; Brooks et al., 2017), using individual-level fitness data and including block, line pair nested within population pair and the interaction between line pair and cross type nested within population pair as random effects (Supporting Information Table S2). We therefore proceed with the simpler approach of analysing line pair–cross type mean fitness.
A significant cross type effect might simply indicate significant differences between the two parental lines and is by itself insufficient to test for heterosis. Mid-parent heterosis (or outbreeding depression) was therefore tested with linear contrasts between the F1 and the mean of the parental values. Comparing the F1 to the mid-parent is a standard quantitative genetics approach to estimating heterosis (Lynch & Walsh, 1998) and is appropriate with highly inbred parental lines. Observed homozygosity was greater than 98.7% in all of our 30 populations, indicating that this approach to estimating heterosis is well suited to our study system. When the estimate of F1 fitness was greater than that of the parent with higher fitness, we also tested the significance of this “high-parent heterosis” using linear contrasts. Values of heterosis (or outbreeding depression) were calculated from population-level least-square means as a per cent increase/decrease in the fitness of the F1 relative to the mid-parent. High-parent heterosis was also calculated for population pairs where, on average, the F1 fitness exceeded that of both parents. Relationships between F1 fitness relative to the mid- or high parent in between-population crosses, mean expected heterozygosity of the population pair and pairwise FST were examined using a linear regression including mean distance (of the two parental populations) to the common garden site as a covariate to control for potential differences in fitness due to adaptation to local climatic conditions, which may influence estimates of heterosis and inbreeding depression (Ronce et al., 2009).
Fitness of the within-population crosses was analysed with an ANOVA model similar to that for the between-population crosses, including population in place of population pair as an independent factor. There are two cross types for this comparison, progeny derived from selfing of the maternal parent and progeny derived from within-population outcrossing. Inbreeding depression would be indicated by a significant cross type effect and a significant reduction in fitness of progeny derived from selfing compared to progeny derived from within-population outcrossing, as tested by linear contrasts. Values of inbreeding depression were calculated from population-level least-square means as a per cent decrease in the fitness of the progeny derived from selfing compared to progeny derived from within-population outcrossing.
3 RESULTS
3.1 Expected heterozygosity and population differentiation
After filtering, the 2b-RAD data yielded 869 total SNPs over all samples. However, the number of polymorphic loci varied considerably among individual populations (N = 30), ranging from just 2 to 265 with a median of 44. Expected heterozygosity was extremely low overall but varied by two orders of magnitude (0.001–0.105, median = 0.02; Supporting Information Table S1) among populations. Pairwise genetic differentiation (FST) was mostly high, ranging from 0.41 to 0.98 with a median of 0.82 (N = 15 population pairs; Supporting Information Table S3). Note that we only estimate differentiation between population pairs that were crossed in order to correlate these values with estimates of F1 fitness relative to the mid-parent (see below).
3.2 Field transplant experiment
Winter survival of seedlings in the field experiment averaged 78%, and average cumulative survival to the time of fruit collection was 54%. The overall mean number of fruits produced by surviving plants was 3.1, and the overall mean total fruit production, including zeros for plants that failed to survive or reproduce, was 1.7. While we have no direct comparison for this region, mean fitness of a locally adapted population from northern Sweden (Roda) is typically closer to 10 fruits per plant (Ågren & Schemske, 2012; Ågren et al., 2013), suggesting that the conditions in our garden may have been stressful in this year. We focus on the measure of total fruit production including zeros, because it represents our best estimate of lifetime fitness.
In the ANOVA of fitness for between-population crosses, there were significant effects of all model terms, including main effects of population pair and cross type (Table 1). The interaction between population pair and cross type is central to addressing our hypotheses and was significant, indicating that the effect of cross type (F1 and progeny derived from selfing of the maternal and paternal parent, respectively) on fitness varied among population pairs (Table 1; Figure 1). Linear contrasts comparing the F1 to the mid-parent value indicated that in 11 of 15 population pairs, the fitness of the F1 deviated significantly from the mid-parent (Figure 1; Supporting Information Table S3). In all but one of these significant contrasts, the F1 had increased fitness relative to the mid-parent value, thus exhibiting mid-parent heterosis. In the remaining contrast, the F1 exhibited reduced fitness or outbreeding depression (Figure 1; Supporting Information Table S3). On average, F1 fitness relative to the mid-parent ranged from −91% to +117%, with a median of +33% (Supporting Information Figure S2, Table S3). For five of the 10 population pairs that exhibited significant mid-parent heterosis, F1 fitness was also significantly greater than the most fit parent (Figure 1; Supporting Information Table S3).
| Effect | df | F | p |
|---|---|---|---|
| Population pair | 14 | 71.44 | <0.001 |
| Cross type | 2 | 34.88 | <0.001 |
| Population pair × cross type | 28 | 6.46 | <0.001 |
- The effects of population pair, cross type (F1 and progeny derived from selfing of the maternal and paternal parent, respectively) and their interaction were treated as fixed effects. This analysis was done using line pair–cross type means, and all denominator degrees of freedom = 269.
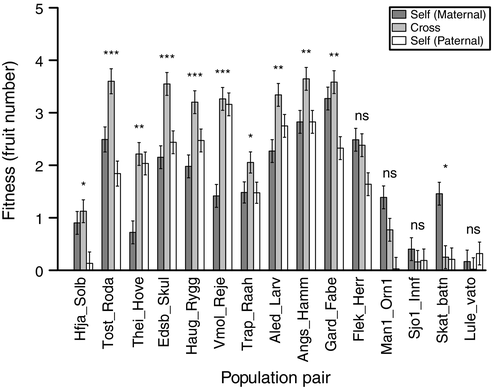
Despite significant deviations of the F1 from the mid-parent value in most cases, some of the interaction between population pair and cross type was attributable to differences between the parental means within a population pair. This could suggest differences among populations in mean fitness attributable to the fixation of partially recessive deleterious alleles. Alternatively, it could be that there are additive differences between populations in performance at the common garden site. We therefore examined the relationship between population mean fitness (N = 30, from the selfed progeny of either the maternal or paternal lines) and expected heterozygosity using a linear regression with distance to the planting garden as a covariate. There was a weak, but nonsignificant (F1,29 = 0.43, p = 0.52), positive relationship between expected heterozygosity and population mean fitness, and a strong and significant (F1,29 = 25.01, p < 0.001) negative relationship between distance to the common garden site and population mean fitness.
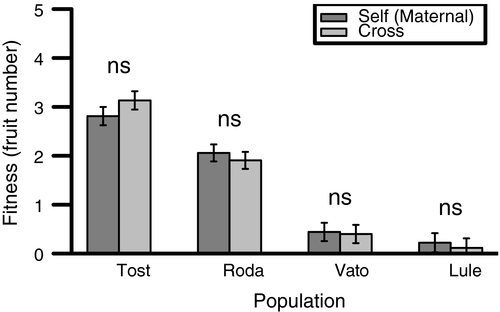
For the within-population crosses, population (Table 2; Figure 2) was the only significant effect. In other words, there was variation in mean fitness attributable to genetic differentiation among populations, but there was no evidence of segregating variation in deleterious recessive alleles and inbreeding depression within these populations. All estimates of inbreeding depression were nonsignificant, and all estimates were equal to or lower than 10%.
| Effect | df | F | p |
|---|---|---|---|
| Population | 3 | 117.05 | <0.001 |
| Cross type | 1 | 0.00 | 0.97 |
| Population × cross type | 3 | 0.80 | 0.50 |
- The effects of population (four populations), cross type (F1 and progeny derived from selfing of the maternal parent) and their interaction were treated as fixed effects. This analysis was done using line pair–cross type means, and all denominator degrees of freedom = 81.
3.3 Factors explaining variation in F1 fitness relative to the parental values
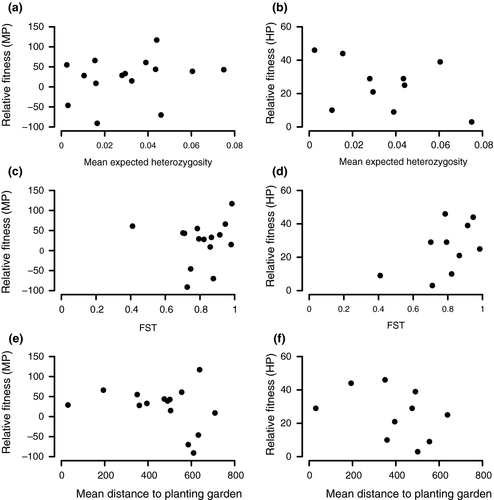
Among-population variation in F1 fitness relative to the mid-parental value was not well explained by any of the factors we examined. There was a positive, but nonsignificant (F1,14 = 1.13, p = 0.31) relationship between mean expected heterozygosity and F1 relative fitness (Figure 3a). The relationship between pairwise FST and F1 relative fitness was not significant (F1,14 = 0.06, p = 0.81) and had no clear pattern (Figure 3c). There was a negative, but nonsignificant relationship (F1,14 = 1.49, p = 0.25) between mean distance to the planting garden and F1 relative fitness (Figure 3e). For population pairs exhibiting high-parent heterosis (increased fitness of the F1 relative to the most fit parent), there was a negative, but nonsignificant (F1,14 = 0.54, p = 0.49) relationship between mean expected heterozygosity and high-parent heterosis (Figure 3b). There was a positive (Figure 3d), but nonsignificant relationship (F1,14 = 1.89, p = 0.22) between pairwise FST and high-parent heterosis. There was no clear relationship between high-parent heterosis and mean distance to the common garden site (F1,14 = 0.14, p = 0.72; Figure 3f). Relationships between relative fitness and both expected heterozygosity and genetic distance were qualitatively similar for analyses without distance to the common garden as a covariate (not shown).
4 DISCUSSION
Few studies have examined the fitness consequences of inter-population crosses for a large number of geographically distinct populations, and even fewer studies have estimated fitness of such crosses under field conditions. Consequently, we have a very limited understanding of the magnitude and geographic pattern of heterosis in crosses between natural populations, and the extent to which genetic variation within populations and genetic distance between populations can predict heterosis. Using crosses involving 30 Fennoscandian populations of Arabidopsis grown in a field common garden experiment in Sweden, we found significant heterosis in 2/3 of our population pairs, with estimates as high as 117%. Using a subset of populations, we also found no evidence of inbreeding depression, suggesting that mildly deleterious mutations that have been fixed by drift are largely responsible for heterosis in between-population crosses. Despite considerable variation in F1 fitness relative to the mid-parent, there were no significant relationships between F1 fitness relative to the mid-parent and either genetic diversity within the parental populations or genetic distance between populations.
4.1 Heterosis and outbreeding depression
Most of the inter-population crosses resulted in heterosis. Significant mid-parent heterosis was detected in 10 of the 15 population pairs, with an average value of ~50% for this subset. Five of these 10 population pairs also exhibited significant high-parent heterosis. The magnitude of heterosis observed in the present study is within the range of published estimates but is considerably lower than some estimates in plant species. A recent study on Daphnia reported estimates of heterosis for intrinsic growth rate under controlled conditions of up to 15% for populations with low genetic diversity (Lohr & Haag, 2015). In plants, estimates of heterosis have been reported ranging from about 15% to over 500% (Busch, 2006; Oakley & Winn, 2012; Oakley et al., 2015b; Paland & Schmid, 2003; Spigler et al., 2016; Willi, 2013). Some of the largest reported estimates of heterosis come from small populations of an endangered plant (Oakley & Winn, 2012) and self-compatible populations of Arabidopsis lyrata from the Great Lakes region (Oakley et al., 2015b; Willi, 2013). For each of these two species, populations exhibiting the strongest heterosis are those likely to have experienced the strongest effects of genetic drift, consistent with heterozygous masking of deleterious, partially recessive, alleles as a major genetic basis of heterosis.
Comparing estimates of heterosis in A. thaliana to its sister species A. lyrata is particularly interesting. A. lyrata ssp. lyrata is native to North America and most populations are self-incompatible (SI), but self-compatibility (SC) has evolved in some populations (Foxe et al., 2010; Mable & Adam, 2007; Mable, Robertson, Dart, Di Berardo, & Witham, 2005; Willi & Määttänen, 2010). Evidence suggests a history of population bottlenecks both in the establishment of A. lyrata in North America and during the evolution of SC in some populations in the Great Lakes region (Oakley et al., 2015b; Ross-Ibarra et al., 2008). Estimates of heterosis from a recent field study within the native range of A. lyrata ranged from 5% to 82% for SI populations to 221%–519% for SC populations (Oakley et al., 2015b). It is unclear why heterosis is so much stronger in SC populations of A. lyrata than in A. thaliana, as both are predominantly selfing and both have likely experienced a history of population bottlenecks and drift. One possibility is that, because the transition to SC in A. lyrata is very recent (Foxe et al., 2010; Hoebe, Stift, Tedder, & Mable, 2009), effective population sizes may have had somewhat less time to recover in A. lyrata compared to A. thaliana. Recent work has pointed out that the predicted relationship between expected heterozygosity and the magnitude of inbreeding depression and heterosis may not hold under demographic disequilibrium (Spigler et al., 2016). Indeed, inbreeding depression in SC populations of A. lyrata is strong (~66%) and comparable to that of SI populations (Oakley et al., 2015b), but see Willi (2013). It may also be that the larger genome and perennial life history of A. lyrata predispose it to greater heterosis because the larger genome size and greater complexity of cumulative fitness provide more opportunities to accumulate mildly deleterious partially recessive mutations. Comparative studies mapping the genetic basis of heterosis in these two species may shed light on this issue.
Outbreeding depression is not manifested until the F2 or later recombinant generations in many species (Edmands, 2002), but we found some outbreeding depression in the F1 generation. Three population pairs exhibited outbreeding depression, but this was only significant in one population pair (Figure 1). Outbreeding depression observed in the present study was not associated with a stunted or necrotic phenotype as with other F1 inter-population crosses in Arabidopsis (Bomblies et al., 2007; Oakley et al., 2015a; Smith et al., 2011; Świadek et al., 2016).
4.2 Inbreeding depression
Although our primary focus was the fitness consequences of between-population crosses, we also used a set of independent lines from four populations to examine the fitness of progeny from within-population crosses compared to progeny derived from selfing. We found no significant inbreeding depression, suggesting a low frequency of deleterious partially recessive alleles segregating within populations. The lack of strongly deleterious alleles is expected in a highly selfing species (Glémin, 2003; Husband & Schemske, 1996; Lande & Schemske, 1985; Porcher & Lande, 2005; Winn et al., 2011), as well as for populations that have a history of population bottlenecks and/or drift (Bataillon & Kirkpatrick, 2000; Glémin, 2003; Glémin et al., 2003; Whitlock, 2002). Omission of early life history stages from our estimates of inbreeding depression would also be expected to reduce the role of strongly deleterious variation in our study (Husband & Schemske, 1996). The remaining mildly deleterious alleles have either been lost (due to drift) or fixed within populations, and such fixed alleles can explain the heterosis we observed in between-population crosses.
4.3 Effect of environment on heterosis, outbreeding depression and inbreeding depression
Our experiment was not designed to test the effect of the environment on heterosis, outbreeding and inbreeding depression, but how the specific environmental conditions at our garden site might influence estimates of relative fitness warrants discussion. On the one hand, estimates from natural conditions may provide better estimates than those from controlled environments (cf. Prill, Bullock, van Dam, & Leimu, 2014). For example, heterosis of 10%–20% has been previously documented in Arabidopsis in a greenhouse study of a cross between an Italian (Castelnuovo di Porto) and a Swedish (Rödåsen) population (Oakley et al., 2015a). A pilot study conducted with progeny from the same cross, grown at the site of the present experiment, yielded estimates of heterosis of ~120% (Oakley, Unpublished data). On the other hand, the extent to which populations were maladapted to the common garden conditions could influence our estimates of relative fitness (Ronce et al., 2009).
There is a large literature on environmental dependent expression of inbreeding depression, and it has been argued that inbreeding depression should be stronger in more stressful environments (Armbruster & Reed, 2005; Cheptou & Donohue, 2011; Fox & Reed, 2010). Others have suggested that environmental novelty per se may be important for the environmental dependent expression of inbreeding depression (Agrawal & Whitlock, 2010; Cheptou & Donohue, 2011) particularly if inbreeding depression is caused in part by additive genetic variation for quantitative traits under stabilizing selection (Awad & Roze, 2018; Cheptou & Donohue, 2011; Lande & Porcher, 2015; Lande & Schemske, 1985; Porcher & Lande, 2005; Ronce et al., 2009). For example, under conditions where a population is strongly maladapted, it is predicted that there should be little inbreeding depression (Ronce et al., 2009). Such a scenario might also influence estimates of heterosis, but there are not presently clear predictions for large multi-population studies of heterosis where each population is presumably adapted to its own environment.
Our results are inconsistent with an effect of maladaptation on estimates of heterosis or inbreeding depression. Population mean fitness significantly declined with geographic distance from the common garden site, but there was no relationship between mean distance from the common garden site and F1 relative fitness suggesting that maladaptation did not strongly effect estimates of heterosis. Furthermore, high-parent heterosis was common and cannot be explained by additive differences due to maladaptation. For inbreeding depression, the population that originated nearest to the common garden site and that had the highest parental fitness, Tost, had an average inbreeding depression of 10.2%. So, while inbreeding depression is predicted be lower in more maladapted populations (c.f. Ronce et al., 2009), the extremely low inbreeding depression in the population most likely to be adapted to the common garden conditions would make it very difficult or impossible to observe such a pattern here. Future studies estimating heterosis and inbreeding depression from large collections of populations at multiple sites would be needed to directly address the question of environmental dependence of heterosis and inbreeding depression, though such experiments would be logistically challenging.
4.4 Effect of genetic diversity and genetic distance on heterosis
The lack of a relationship between F1 fitness relative to the mid-parent, or population mean fitness, and expected heterozygosity may appear surprising given evidence from other species that small and/or less genetically variable populations tend to exhibit greater heterosis (Lohr & Haag, 2015; Oakley & Winn, 2012; Spigler et al., 2016). On the other hand, the crosses conducted here were not done within groups defined a priori based on their levels of genetic diversity. While we expect greater heterosis in a cross between two populations with very low gene diversities compared to a cross between two populations with high gene diversities, it is less clear what to expect for crosses between populations at opposite ends of the gene diversity continuum. Additionally, the limited range of expected heterozygosity in these northern populations of this highly selfing species may limit our power to detect the expected relationship.
We found no relationship between F1 fitness relative to the mid-parent and genetic distance. These results add to a body of literature that suggests that genetic distance between parents is not necessarily a good predictor of the outcome of inter-population crosses (Armbruster et al., 1997; Demuth & Wade, 2007; Edmands, 2002; Fenster & Galloway, 2000). The extremely strong differentiation and limited range of FST values in these populations may have contributed to limited power to detect a relationship between F1 relative fitness and genetic distance. The lack of pattern may also reflect in part effects of outbreeding depression. The net effect on F1 relative fitness might represent the balance between positive effects of heterosis at some loci and negative effects of outbreeding depression at other loci. Finally, it must be pointed out that our crossing design, while allowing for as many independent population pairs as possible to maximize the range of genetic distances examined, cannot control for and/or systematically vary other important factors such as genetic diversity and latitude of origin. Thus, studies such as this represent a first step in understanding the patterns of heterosis in nature and require follow-up studies to examine the effects of individual variables on the magnitude of heterosis.
5 DATA SHARING
Both phenotypic and 2b-RAD Illumina data are available from the Dryad Digital Repository: DOI: https://doi.org/10.5061/dryad.2fb36bc
ACKNOWLEDGMENTS
The authors would like to thank the many assistants and undergraduate students who helped with this project. In particular, we thank Jaclyn Schlang for assistance with the hand pollinations, Jon Spoelhof and Jacob Snelling for assistance with the 2b-RAD genotyping and Jenny Glans, Mattias Vass and Linus Vikström for assistance with the outdoor common garden experiment. We thank Sylvain Glémin, Emmanuelle Porcher, Jennifer Schleberger and an anonymous reviewer for helpful comments on versions of the manuscript. This work was funded by NSF grants to D.W.S. (DEB 1022202), and to C.G.O. and D.W.S. (DEB 1743273), and a grant from the Swedish Research Council to JÅ.




