A new in vitro method to predict in vivo photoprotection of skin hyperpigmentation induced by visible light
Conflicts of interest
LD, CQR and TP have received honoraria and grants from NAOS (France). BC, IG, FD, CG and AG are employees of NAOS (France).
Funding sources
The studies were sponsored by NAOS (France).
Abstract
Background
Ultraviolet radiation is the main cause of skin pigmentation, but more recently visible light has been shown to be an important contributor especially in melano-competent subjects. Photoprotection from visible light can improve several hyperpigmentation disorders. Recently, a visible light photoprotection assessment method has been proposed based on in vivo pigmentation; the visible light photoprotection factor (VL-PF) is determined by assessment of the change in colorimetry parameter ITA over several days measured using a chromameter. Although in vivo methods remain the most representative of real life, in vitro methods are more suited to screening sunscreen formulations.
Objective
The aim of this study was to evaluate the correlation between in vivo and in vitro methods in assessing protection against visible light induced pigmentation.
Methods
We first analysed the in vitro protective properties of the 10 commercially available sunscreens using transmission measurements in the visible spectrum. Then, we performed a monocentric, double-blind, randomized controlled study with intra-individual comparisons in 20 healthy subjects and measure the VL-PF in vivo of those sunscreens. The correlation between the VL-PF and the percentage of blocked light was evaluated using the coefficient of determination R2.
Results
A strong significant correlation was demonstrated between in vivo visible light protection factor and in vitro transmittance measurements, with the highest correlation factor at 420 nm and in the spectrum covering from 400 to 469 nm.
Conclusion
Transmittance measurements were found to be a good predictive tool to evaluate sunscreen visible light photoprotection efficacy and could be used to select formulations for final in vivo testing.
Introduction
Sunscreens are known to protect in the ultraviolet (UV) domain (UVB and UVA). However, until recently visible light (VL) (400–700 nm) was considered as devoid of any photobiological effects on cutaneous tissue. It has now been demonstrated that VL stimulates pigmentation, especially in melano-competent subjects.1-3 Protection from VL combined with UV photoprotection significantly prevents hyperpigmentation disorders such as melasma,4, 5 post-inflammatory hyperpigmentation6 and actinic lentigos.7 More specifically, the high energy visible light (blue and violet light) is responsible for skin pigmentation by inducing a dose-correlated hyperpigmentation, while red light does not induce pigmentation in vivo.1 Tinted sunscreens containing opaque pigments with large particle sizes, such as iron oxides, are the only currently available, efficient topical photoprotection products against visible light-induced hyperpigmentation. Several methods have been developed to evaluate the VL protection capability of sunscreens, but in vivo evaluation remains the most representative of real-life use. Recently, a group of experts agreed and proposed the calculation of a VL protection factor (VL-PF).8 This corresponds to the ratio of change of colorimetry measurements (individual typology angle, ITA°) between unprotected areas and protected areas after several days of visible light exposure using a solar simulator equipped with appropriate filters and measured using a chromameter.8 However, in vivo methods have their limitations due to ethical concerns over the photo-exposure of subjects, and related time and costs. Consequently, these methods are not ideal for screening multiple prototypes, whereas in vitro methods would be more ethically acceptable and flexible. However, no correlation has yet been demonstrated between in vitro and in vivo methods. The aim of this study was to evaluate the correlation between these two types of method.
Materials and methods
In vitro evaluation of VL protection
Ten commercially available sunscreens with very high photoprotection (≥ SPF50+) were evaluated: one non-tinted sunscreen SPF50+ as a control (sunscreen A), nine tinted sunscreens including eight claiming VL-induced hyperpigmentation protection (sunscreens C to J), all containing titanium dioxides and iron oxides (sunscreens B to J). The list of ingredients contained in each sunscreen is specified in Table S1, Supporting Information. For each sunscreen, a uniform layer of sunscreen was spread on the rough surface of three polymethylmethacrylate (PMMA) plates (Helioplate PMMA Molded HD6, HelioScreen, Creil, France) using a robot (HD-SPREADMASTER, HelioScreen, Creil, France) at a rate of 1.3 mg/cm2. After drying 15 min in the dark at room temperature, the PMMA plates were irradiated for 15 min at 600W/m² (1 DEM for a phototype III) using a UV-visible solar simulator (Suntest CPS+, Ametek, PA, USA). After irradiation, the plates were inserted into a UV-Visible Lambda 650S spectrophotometer (Perkin Elmer, MA, USA) equipped with an integrating sphere for post-irradiation reading from 400 to 700 nm in intervals of 1 nm. Each plate was measured nine times, the mean was calculated. To determine the background absorption, 15 µL of glycerin was spread in a uniform layer on a PMMA plate before measuring in the spectrophotometer.
In vivo evaluation of VL protection
The study was performed in accordance with the Good Clinical Practices and the principles of the Declaration of Helsinki. ISO standards for sun protection test methods (SPF and UVA PF) do not require ethics committee approval of the study protocol. Since VL is much less energetic than UV radiation and the fact that the VL dose used in this study corresponded to 4 days of a 1-h sun exposure, no EC approval was sought. Nevertheless, the procedures used in this study have been previously approved by a local Ethics Committee (Nice, France).
A monocentric, double-blind, randomized controlled study with intra-individual comparisons was performed using a previously described study design.3 Twenty healthy subjects of both sexes aged 18 to 50 years, with a skin type III to V according to Fitzpatrick scale were included in this study. The subjects received the necessary written and verbal information and gave their written informed consent. The same 10 sunscreens assessed in vitro were tested under in vivo conditions.
Sunscreens were applied (2 mg/cm²) once daily from Day 1 to Day 4 on the attributed areas (3x4 cm) in the middle section of the back according to the randomization list. After a resting period of 15 min, the subjects were exposed to VL (144 J/cm²). Standardized photographs were taken at Day 1 before the first exposure and at Day 5, 24 h after the last exposure. Pigmentation intensity was measured by chromametry (CR400, Konica-Minolta, Japan) using the colorimetric angle ITA° that allowed calculation of the VL-PF for each product.1 This reference VL-PF was also expressed as a percentage using the formula (1-(1/VL-PF)) ×100, called the pVL-PF, in order to facilitate comprehension by dermatologists and patients. The correlation between the pVL-PF and the percentage of blocked light was evaluated using the coefficient of determination R2. The statistical analysis was calculated with the Pearson correlation.
Results
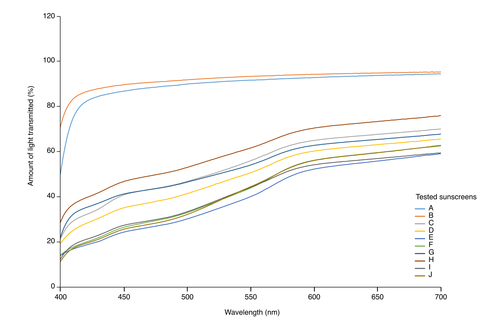
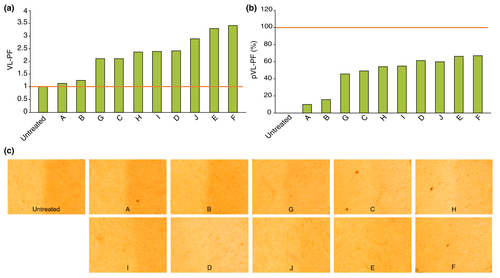
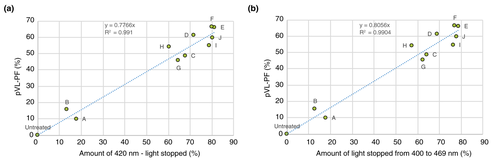
The transmission spectra of the tinted sunscreens showed that the most effective protection was against high energy visible light (Fig. 1). Twenty subjects (13 women and seven men) with a mean age of 34.9 years (range: 18–49 years) were included in the in vivo study; all completed the study. The calculated VL-PF was equal to 1 for the untreated zone and close to 1 for the zone treated with the non-tinted sunscreen A (Fig. 2a). For the non-tinted sunscreen A, a marked hyperpigmentation was observed on the exposed area compared to the unexposed (Fig. 2c). Despite the claim of protection against VL, we observed differences in the VL-PF between the tinted sunscreens. The sunscreens E and F presented the highest VL-PFs, 3.28 and 3.40, respectively, (Fig. 2a) with no visible hyperpigmentation compared to unexposed skin (Fig. 2c). The VL-PF was reinterpreted as a percentage using the formula (1-(1/VL-PF)) ×100 in order to facilitate comprehension by dermatologists and patients, and called pVL-PF. The pVL-PF was calculated for each sunscreen formulation; 0% corresponds to an untreated exposed area and 100% corresponds to complete protection from the VL (equivalent to an unexposed area). The lowest factor was for the non-tinted sunscreen A at 9.7%, and the highest were for sunscreens E and F with 65.7% and 66.4% respectively (Fig. 2b). The coefficient of determination, R², was then calculated between the percentage of blocked light and the pVL-PF of each test area, for every wavelength from 400 to 700 nm, and for every wavelength range starting from the wavelength 400 nm. The correlations were the highest at 420 nm (R² = 0.9910) for separate wavelengths, and between 400 and 469 nm (R2 = 0.9904) for wavelength ranges (Fig. 3). For statistical analysis, the Pearson correlation coefficient was used. Correlations were found to be statistically significant for every wavelength from 400 nm to 700 nm, and for every wavelength range (P < 0.001).
Discussion
Our results show a strong linear relationship between the sunscreen in vivo pVL-PF measurement and the in vitro percentage of blocked light. To our knowledge, only one other study has assessed both in vitro and human in vivo evaluations of sunscreen VL photoprotection but no correlation between these two approaches was demonstrated.9 Moreover, the in vivo assessment did not correspond to the standard method that is now proposed. We are proposing the pVL-PF calculation which is a new interpretation of the original VL-PF and allows simply evaluating from 0% to 100% the performance on VL-induced pigmentation in order to easily compare different formulations. It is also easier to understand for dermatologists and patients looking for high VL photoprotection. We previously demonstrated that melanocytes directly sense the light through direct stimulation of the opsin-3 receptor.10 Interestingly, the best correlation between in vivo pigmentation and the in vitro transmittance was observed from 400 to 469 nm, which corresponds to the absorption spectrum of opsin-3. These data support the activation of melanogenesis by the direct interaction of photons with the opsin-3 receptor and that so far, the best way to prevent VL-induced hyperpigmentation is to prevent the photons of the high energy VL to reach the opsin-3 receptor.
In conclusion, the transmittance measurements from 400 to 469 nm were found to be a reliable predictive tool to evaluate sunscreen VL photoprotection efficacy which could be used to screen products during formulation development.
Acknowledgements
We thank Fatoumata Ndiaye for her challenging discussion on the visible light protection factor, Marlène Chavagnac-Bonneville for her help writing and submitting the manuscript and Jane Murray for the English editing.
Open Research
Data availability statement
Raw data are available for scientific research upon reasonable request to Luc Duteil. ([email protected]).




