1,25(OH)2D3 blocks IFNβ production through regulating STING in epithelial layer of oral lichen planus
Funding information
This study was supported by both National Natural Science Foundation of China grant (81800499) and Stomatological hospital of Shanxi Medical University grant (RC2021-06).
Abstract
Stimulator of interferon genes (STING) is reported to exert vital functions in inflammatory responses and autoimmune diseases. Nevertheless, the status and roles of STING in oral lichen planus (OLP) remain elusive. Here, we state that STING and its downstream cytokine interferon-β (IFNβ) expression is boosted in the oral keratinocytes from patients suffering OLP in comparison with those from healthy participants. Mechanistically, transcription factor GATA-binding protein 1 (GATA1) which is highly increased in diseased samples specifically interacts with its element in the promoter of STING to enhance STING transcripts. 1,25(OH)2D3, the active form of vitamin D, is capable of restricting STING and IFNβ increases in oral keratinocyte models resembling OLP in vitro. Moreover, there is a negative correlation between vitamin D receptor (VDR) and STING or IFNβ in human samples. Using plasmids and small interfering RNA transfection technologies, we find 1,25(OH)2D3 regulates STING and IFNβ through a mechanism controlled by the hypoxia-inducible factor-1α (HIF-1α)-GATA1 axis. Collectively, our findings unveil that 1,25(OH)2D3 lowers STING and IFNβ overexpression in the context of OLP.
1 INTRODUCTION
Oral lichen planus (OLP) is identified to be an inflammatory-like mucocutaneous condition accompanied by recurrent lesions and symptoms.1 Recent systemic investigations have revealed that the prevalence of OLP in the globe is 1.01% and the risk for malignant transformation is approximately 1.2%.2, 3 OLP primarily affects the middle-aged population, and the prevalence ratio between females and males is approximately 2:1.2 Although the pathogenic mechanism of OLP remains unclear, infectious, genetic, neurological, pharmacological, immunological and psychological contributors are all considered to exert critical functions in the pathogenesis of OLP.4 Based on recent publications, OLP is clinically classified as seven forms, among which reticular subtype is the most common in clinic.1 Most of OLP patients claim the symptoms like pain and a burning sensation in the diseased oral mucosa.1 Due to the shortage of curative management for this disease, exploration on the contributing factors of this illness is urgent.
Stimulator of interferon genes (STING), an adapter bound by cyclic GMP-AMP (cGAMP), is located on the endoplasmic reticulum (ER) membrane of cells.5 STING expression is tightly modulated by transcriptional factors and posttranslational modifications as demonstrated.6 STING moves to the Golgi apparatus from the ER upon cGAMP binding, leading to IκB kinase (IKK) and TANK-binding kinase 1 (TBK1) activation.7 These activated kinases induce downstream factors to increase pro-inflammatory cytokine production, which mediates immune reactions.7, 8
Upregulation of STING is known to induce numerous autoimmunity and autoinflammatory diseases.9-11 In the sepsis animal model, compared to control wild-type mice, sepsis severity is ameliorated in STING-deficient mice.12, 13 Some investigations provide compelling evidence that STING insufficiency could decrease pro-inflammatory cytokine production and arthritis scores in a self-DNA-mediated autoimmunity animal model.14 What is more, overexpression of STING mutation results in an autoinflammatory disease which shows a severe lung inflammation and acral vasculopathy.15
Vitamin D is reported to have critical roles in regulating autoimmune dysfunctions.16-18 In our previous explorations, we found that vitamin D concentration in the serum derived from OLP patients is decreased relative to that from healthy controls.16 In addition, we provided robust evidence that vitamin D/vitamin D receptor (VDR) pathway curbs cell apoptosis and pro-inflammatory cytokine production in oral keratinocytes under inflammatory condition via regulating miRNAs, HIF-1α signalling and renin levels.19-24 However, the effect of vitamin D on STING and its downstream cytokines remains an enigma. Here, our data suggest 1,25(OH)2D3, the active form of vitamin D, decreases STING expression in oral keratinocyte to inhibit IFNβ production under OLP circumstance.
2 METHOD AND MATERIALS
2.1 Human biopsies
The diseased buccal tissues and blood samples were received from 14 OLP patients as described before.16 The normal mucosal and blood samples were obtained from 14 healthy individuals undergoing retained wisdom teeth extraction. Clinical OLP symptoms were identified in terms of the modified criteria. Clinical and function scores of OLP individuals were evaluated according to the previous investigation.25 Signed informed consents were received from participants. All human studies were approved by the Institutional Ethical Committee of Shanxi Medical University. Oral epithelial cells derived from human samples were isolated as previously described.19 Detailed information of human samples and statistical analysis between healthy controls and OLP groups were provided in Table S1–S3.
2.2 Cell culture
Cell line of human oral keratinocyte (HOK) was obtained and cultured in terms of previous investigation.19 Briefly, HOKs were cultured in oral keratinocyte medium (ScienCell) in the incubator under 37°C and 5% CO2 condition. Cell culture medium was replaced every 2 days. For mimicking OLP, 100 ng/ml lipopolysaccharide derived from Porphyromonas gingivalis (LPS-PG, MilliporeSigma) or the supernatant from activated CD4+ T cells was separately used to stimulate HOKs for 8 h as described before.19 Anti-human CD4 magnetic particles were applied for purifying CD4+ T cells from human peripheral blood samples. The enriched CD4+ T cells were further activated by anti-CD3 and anti-CD28 antibodies (BD Biosciences) and then cultured with RPMI 1640 medium. HOKs were pretreated with 1,25(OH)2D3 (20 nM) for 12 h prior to CD4+ T cells or LPS challenge. Plasmids or siRNAs were transfected into HOKs via vehicles for 36 h. 1,25(OH)2D3 was dissolved in ethanol, and the equivalent concentration of ethanol was used as negative control.
2.3 Western blot
Western blot was conducted as described before.17 Briefly, cell or tissue lysates were harvested with laemmli buffer with protease inhibitors. The same amounts of lysates were loaded and separated using SDS-PAGE and then transferred onto a PVDF membrane. The membranes with proteins were blocked by 5% milk buffer, followed by an overnight primary antibodies' incubation. On Day 2, after TBST buffer washes, membranes were treated with HRP-conjugated mouse or rabbit secondary antibodies at ambient temperature. Blots were visualized by using X-ray films in dark room. Anti-GATA-binding protein 1 (GATA1) antibody was purchased from ProteinTech (Cat: 60011-1-Ig), anti-STING antibody was purchased from ProteinTech (Cat:19851-1-AP), anti-IFNβ antibody was purchased from ThermoFisher (Cat: PA5-20390), and anti-β-actin antibody was purchased from Santa Cruz Biotechnology (Cat: sc-47778).
Due to space limitations, more details were provided in supplemental materials (Appendix S1 and S2).
3 RESULTS
3.1 STING levels are upregulated in oral epithelial cells of OLP
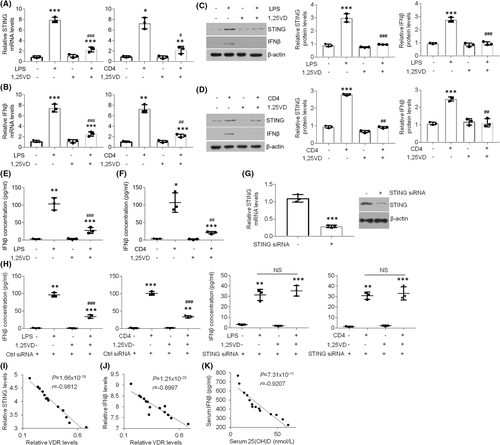
STING is reported to be closely associated with inflammatory diseases.9-11 To explore STING expression in OLP, we isolated oral mucosal epitheliums from healthy and OLP individuals for detection. As displayed, STING mRNA levels were remarkably elevated in oral epithelia derived from OLP patients compared to controls (Figure 1A). Accordantly, IFNβ, the downstream inflammatory factor of STING, was also increased in diseased human samples at the mRNA level (Figure 1B). Moreover, STING and IFNβ expression was also improved in lesion tissues at the protein level (Figure 1C). Likewise, serum IFNβ concentration showed a robust increase in OLP patients (Figure 1D). To test STING and IFNβ status in HOKs, we established two cell models for mimicking OLP as described before.19 As manifested, both mRNA and protein status of STING and IFNβ were enhanced in HOKs with challenges (Figure 1E-G). Moreover, forced expression of STING increased IFNβ levels in HOKs (Figure 1H). These data above demonstrate that STING and its downstream gene levels are increased in oral keratinocytes in the setting of OLP.

3.2 STING is induced by GATA1 in oral keratinocytes
To explain the mechanism by which STING expression is boosted in the oral keratinocytes derived from OLP, we analysed the promoter area of STING and searched a potential GATA1-binding site (Figure 2A). In addition, ChIP assays verified the binding action between transcript factor GATA1 and the element in the promoter of STING gene (Figure 2B). Luciferase report data confirmed transcript factor GATA1 promoted STING transcripts in HOKs transfected with PGL3-STING plasmids but not the mutant ones. (Figure 2C). Overexpression of GATA1 in HOKs increased STING and IFNβ protein levels (Figure 2D,E). Next, we applied siRNA transfection in HOKs prior to challenges (Figure 2F). As manifested, knockdown of GATA1 in HOKs hampered STING increases upon stimulation (Figure 2G,H). Since GATA1 is closely associated with inflammatory responses,26-28 we investigated GATA1 expression in human biopsies and found GATA1 was increased in OLP samples compared to control ones (Figure 2I). Consistently, a positive correlation between STING and GATA1 was also observed in human specimens (Figure 2J). In OLP cell models, GATA1 status was raised by LPS or activated CD4+ T cells challenge (Figure 2K). Collectively, our results reveal that increased GATA1 in OLP induces STING expression by promoting its transcription.

3.3 1,25(OH)2D3 treatment decreases STING and IFNβ levels in oral keratinocytes of OLP
Although vitamin D is capable of regulating inflammatory reactions, its role in STING expression in the context of OLP remains elusive. To this end, we sought to elucidate whether vitamin D could mediate STING status in oral keratinocytes. As shown in Figure 3, treatment-induced STING and IFNβ overexpression was suppressed by 1,25(OH)2D3 treatment in HOKs (Figure 3A-D). The increased IFNβ concentrations in the culture medium of HOKs with challenges were also inhibited by 1,25(OH)2D3 (Figure 3E,F). Next, we knocked down STING expression in HOKs and indicated that 1,25(OH)2D3 refused to regulate IFNβ concentrations in LPS/activated CD4+ T cells-treated HOKs with STING-siRNAs transfection (Figure 3G,H), indicating vitamin D mediates IFNβ production via STING signalling. Moreover, after analysing the data of this project and previous investigation,16 we found VDR had negative correlations with STING and IFNβ in human oral samples (Figure 3I,J). The IFNβ and 25(OH)D concentrations in human serum showed a negative correlation as well (Figure 3K). These findings note that 1,25(OH)2D3 is capable of suppressing increased STING and IFNβ expression in the epithelia of OLP.
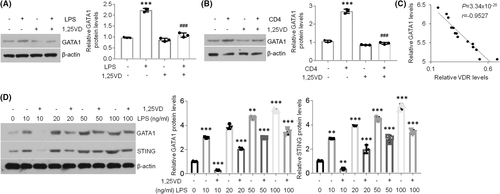
3.4 1,25(OH)2D3 is able to suppress GATA1 increases in oral keratinocytes
Given that GATA1 can induce STING expression in oral keratinocytes, we next sought to better explain the functions of 1,25(OH)2D3 in GATA1. As exhibited, 1,25(OH)2D3 treatment stopped GATA1 elevations in HOKs upon stimulations (Figure 4A,B). Moreover, GATA1 and VDR showed a negative correlation in oral epithelia derived from human oral mucosa (Figure 4C). Finally, the dose-dependent data indicated that 1,25(OH)2D3 failed to suppress STING expression when GATA1 levels were increasing in HOKs upon LPS treatment (Figure 4D), indicating 1,25(OH)2D3 blocks STING expression in a GATA1-dependent way. Together, these data imply 1,25(OH)2D3 represses STING overexpression in oral keratinocytes via regulating GATA1 signalling.
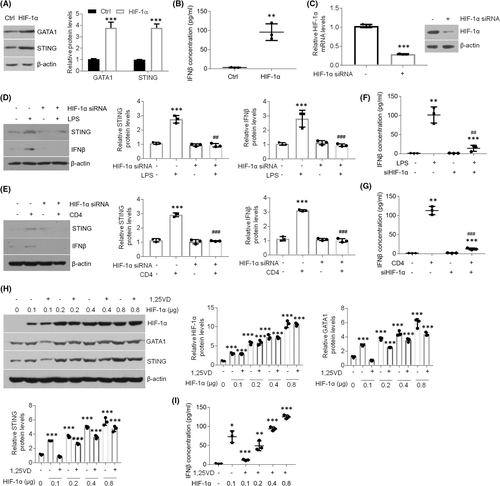
3.5 1,25(OH)2D3 inhibits GATA1 levels by regulating HIF-1α activation in oral keratinocytes
Some studies have suggested GATA1 is induced by HIF-1α signalling.29, 30 To confirm this in oral keratinocytes, we transfected HIF-1α plasmids into HOKs. Upon HIF-1α overexpression, GATA1 expression was enhanced (Figure 5A), suggesting HIF-1α signalling is able to induce GATA1 in HOKs. STING and IFNβ levels were also increased after HIF-1α overexpression (Figure 5A,B). Meanwhile, LPS or activated CD4+ T cells lost the ability to increase STING and IFNβ when HIF-1α was knocked down (Figure 5C-G). In the dose-dependent assay, 1,25(OH)2D3 suppressed HIF-1α-induced GATA1, STING and IFNβ upregulations when the dose of plasmids was 0.1 μg, but could not suppress them when HIF-1α levels were increasing (Figure 5H,I), noting the indispensable role of HIF-1α in vitamin D's mediation. These findings suggest 1,25(OH)2D3 suppresses GATA1 expression in oral keratinocytes dependent on HIF-1α.
4 DISCUSSION
In this investigation, we tested human oral samples and found STING levels are significantly enhanced in diseased biopsies from OLP patients compared with those from healthy individuals, consistent with other studies indicating STING activation is elevated in autoimmune diseases such as colitis.9 Some explorations suggest that STING activation worsens experimental colitis in animals, leading to robust pro-inflammatory cytokine production.9 Consistently, we confirmed that IFNβ and other inflammation-related cytokine expression is promoted in the diseased tissues of OLP, indicating suppression of STING might be an efficient strategy to inhibit inflammatory responses in the context of OLP.
Given OLP is considered to be driven by T cells in the lamina propria,1 we collected T cells from humans and treated HOKs with the supernatants of activated CD4+ T cells to resemble the microenvironment of this inflammatory condition in vitro. To better mimic OLP, we also treated HOKs with LPS since bacterial infection is also reported to be a contributing factor for the initiation of OLP.1 Importantly, our HOKs and human samples data in this study are consistent.
Next, we explored the mechanism by which STING expression is upregulated. In line with other findings,31 we observed that GATA1 promotes STING transcription to enhance its status. Forced expression of GATA1 increases both STING and IFNβ in oral keratinocytes. GATA1 is closely related to inflammatory reactions, and conditional GATA1 knockout in dendritic cells exhibits impaired migration towards lymph nodes.26 In addition, inflammasome of haematopoietic stem and progenitor cells mediates haematopoiesis via cleavage of GATA1.27 GATA1 also plays a critical role in inflammation-associated thrombocytosis.28 Given the upregulated levels of GATA1 in diseased oral epithelium, targeting GATA1 in clinic might be a new tool for OLP therapy.
Calcitriol, a form of vitamin D, is indicated to suppress the cGAS/STING/IFN signalling in the field of Hutchinson Gilford Progeria Syndrome.32 Moreover, vitamin D exhibits the ability to dampen HIF-1α expression through inactivating nuclear factor-κB (NF-κB) signalling pathway and enhancing von Hippel–Lindau (VHL) levels in oral keratinocytes.20 Other investigations suggest that vitamin D is able to suppress NF-κB activity and inflammatory responses in human keratinocyte.33, 34 Accordingly, we found that 1,25(OH)2D3 is capable of repressing activated STING and IFNβ expression in the lesion tissues of OLP, suggesting vitamin D might ameliorate cytokine storm in oral epithelial cells by regulating STING signalling. One limitation is that cGAS part is not involved in the current work. Because there are no publications concerning cGAS and OLP so far, the study of them might be really interesting. In addition, we claimed that 1,25(OH)2D3 is able to suppress GATA1 to decrease STING expression in oral keratinocytes. Consistent with these findings that HIF-1α induces GATA1 expression,29, 30 we provided compelling evidence that 1,25(OH)2D3 regulates GATA1 activation through HIF-1α signalling in oral keratinocytes in this investigation.
In conclusion, this study suggests 1,25(OH)2D3 suppresses STING and its downstream cytokine IFNβ production by mediating GATA1 in the context of OLP, providing a novel target for the treatment of OLP. However, in addition to 25 and 1alpah hydroxylation, alternative pathways also could regulate vitamin D activation. For example, CYP11A1 triggers lumisterol (L3) metabolism to synthesize 20(OH)L3, 22(OH)L3 and 20,22(OH)2L3 via the side chain hydroxylation. Moreover, CYP11A1 exerts important functions in 7-dehydrocholesterol (7DHC) which is closely associated with 7-dehydropregnenolone (7DHP), 22(OH)7DHC and 20,22(OH)27DHC production.35, 36 Based on recent investigations, AhR, liver X receptors (LXRs), RORα and RORγ are also found to be served as alternative receptors for vitamin D.37-39 Given the complicated biological network in the field of vitamin D, whether 1,25(OH)2D3 or targeting STING is qualified for OLP management requires more experimental and clinical trials.
AUTHOR CONTRIBUTIONS
Xuejun Ge: Data curation (equal); investigation (lead). Yaxian Wang: Investigation (supporting). Hanting Xie: Investigation (supporting). Ran Li: Investigation (supporting); methodology (supporting). Fang Zhang: Investigation (supporting). Bin Zhao: Investigation (supporting). Jie Du: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); methodology (lead); project administration (lead); writing – original draft (lead); writing – review and editing (lead).
ACKNOWLEDGEMENTS
We appreciate Juanjuan Xiang (Central South University, China) who provided the HIF-1α plasmids for this investigation. We thank Zifeng Deng (University of Chicago) for his statistical analysis work.
CONFLICT OF INTEREST
There are no conflicts of interest in this project.
Open Research
DATA AVAILABILITY STATEMENT
The data are available from corresponding author.




