Pigment epithelium-derived factor peptide promotes limbal stem cell proliferation through hedgehog pathway
Funding information
This work was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 104-2314-B-195-006-MY3) (Y.P.T.), Mackay Memorial Hospital (MMH-E-105-006) (Y.P.T.) and Taipei Veterans General Hospital (V105-B-009; F.N.W.). All the funding sources have no involvement in this study.
Data Availability Statement: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abstract
Expansion of limbal epithelial stem cells (LSCs) is crucial for the success of limbal transplantation. Previous studies showed that pigment epithelium-derived peptide (PEDF) short peptide 44-mer could effectively expand LSCs and maintain them in a stem-cell state, but the mechanism remained unclear. In the current study, we found that pharmacological inhibition of Sonic Hedgehog (SHh) activity reduced the LSC holoclone number and suppressed LSC proliferation in response to 44-mer. In mice subjected to focal limbal injury, 44-mer facilitated the restoration of the LSC population in damaged limbus, and such effect was impeded by the SHh or ATGL (a PEDF receptor) inhibitor. Furthermore, we showed that 44-mer increased nuclear translocation of Gli1 and Gli3 in LSCs. Knockdown of Gli1 or Gli3 suppressed the ability of 44-mer to induce cyclin D1 expression and LSC proliferation. In addition, ATGL inhibitor suppressed the 44-mer-induced phosphorylation of STAT3 at Tyr705 in LSC. Both inhibitors for ATGL and STAT3 attenuated 44-mer-induced SHh activation and LSC proliferation. In conclusion, our data demonstrate that SHh-Gli pathway driven by ATGL/STAT3 signalling accounts for the 44-mer-mediated LSC proliferation.
1 INTRODUCTION
Corneal integrity and transparency are indispensable for normal vision. The corneal epithelium is the outmost layer of corneas and is constantly renewed from limbal epithelial stem cells (LSCs).1 Human LSCs have been identified at the basal epithelial layer of the limbus, anatomically located at the boundary of the cornea and the conjunctiva.2 Destruction of the limbus potentially leads to visual impairment because of conjunctivalization of the corneas, corneal neovascularization (NV), scaring, chronic inflammation or recurrent/persistent corneal epithelial defects.3
Transplantation of limbal tissue/stem cells is a prevalent therapeutic approach for patients with LSC deficiency (LSCD).4-7 Although combination with amnion membrane grafting has substantially improved the outcome, the long-term success rate for advanced LSCD remains low because of the lack of proper regeneration of LSCs.8-11 Therefore, promoting expansion of stem cell population can increase the success of ocular reconstruction.6, 11
Pigment epithelium-derived factor (PEDF) is a 50-kDa secreted glycoprotein with multiple biologic effects on various types of cells.12 The amino acid positions Val78-Thr121 of human PEDF (termed 44-mer) is responsible for neurotrophic and mitogenic activity.13-15 44-mer has been proven to be able to promote LSC proliferation and meanwhile maintain a stem-cell state.14 Further in vivo studies have confirmed that the 44-mer effectively repopulates LSCs in damaged limbus in rabbits.16, 17 These encouraging results suggest the potential role of 44-mer in treating LSCD or increasing the survival of limbal grafts. However, the mechanism of 44-mer-mediated LSC self-renewal has yet to be elucidated.
Sonic Hedgehog (SHh) signalling pathway is critical for maintaining and supporting stem cell properties in various tissues.18-21 Secreted SHh ligands act on responding cells by turning on an intracellular signalling pathway that induces Gli transcription factors18. The active stage of the SHh pathway is tightly controlled and regulated by the integrating pathways to keep cellular processes balanced. Corneal wounding induced transient up-regulation of SHh ligands and expression of Gli3 in the limbus, suggesting that LSC behaviour is regulated by the SHh pathway.22 In this study, we investigated whether SHh signalling pathway could be induced by 44-mer and promote the expansion of LSCs. We found that 44-mer-induced Gli1 and Gli3 expressions to promote LSC proliferation, and such effect was suppressed by ATGL and STAT3 inhibitor.
2 MATERIALS AND METHODS
2.1 Chemicals and antibodies
Antibodies used in this study were △Np63α (Biolegend, San Diego, CA.), Lrig-1 (ab36707, Abcam, Cambridge, MA), CK19 (ab15463; Abcam, Cambridge, MA) and CK12 (BS4625R; Bioss, Woburn, MA). Antibodies against Patched (Ptch), Smoothened (SMO), Gli1, Gli3, Gli3-R, histone H1 and cyclin D1 were purchased from GeneTex. Antibody against phospho-STAT3 (Tyr705) was purchased from Cell Signaling Technology (Beverly, MA). All the fluorescent dye-conjugated secondary antibodies were from BioLegend (San Diego, CA). Atglistatin (530151), STAT3 Inhibitor Peptide (s73096) and STAT3 Inhibitor V (573099) were from Calbiochem (La Jolla, CA). HPI4, cyclopamine, DAPT and 5-bromo-2’-deoxyuridine (BrdU), Hoechst 33258 dye and formalin were from Sigma-Aldrich (St. Louis, MO). Dispase II and epidermal growth factor were from Roche (Indianapolis, IN). Recombinant human SHh was from Peprotech (NJ). Dexamethasone (5 mg/1 mL) was from Taiwan Biotech Co. (Taoyuan, Taiwan). Haematoxylin and eosin (H&E) dyes were purchased from Merck (Rahway, NJ). The 44-mer (Val78-Thr121) and 18-mer (Glu97-Ser114; a control peptide) were synthesized, modified by acetylation of the NH2 termini and amidation of the COOH termini for stability, and characterized by mass spectrometry (>90% purity) at GenScript (Piscataway, NJ). PEDF were reconstituted in DMSO as a stock solution (5 mmol/L).
2.2 Animals
New Zealand albino rabbits (3.0-3.5 kg, 6 months old) and 3-5-month-old Balb/c mice were used. All procedures were approved by the Mackay Memorial Hospital Review Board for animal investigation and were conducted in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research.
2.3 Limbal stem cell culture
LSCs were isolated from rabbits and used for cell-suspension culture, colony-forming efficiency (CFE) and BrdU labelling assay as described previously.14 Briefly, the limbal rings were washed in phosphate-buffered saline containing 50 μg/mL gentamicin. After the iris and excessive sclera were removed, the limbal rings were exposed to dispase II (1.2 IU/mL in Hanks’ balanced salt solution free of Mg2+ and Ca2+) at 4°C for 16 hours. The loosened epithelial sheet was harvested with a cell scraper and separated into single cells by treating with 0.5 mL trypsin (0.25% and 0.01% EDTA) for 15 minutes at 37°C with gental shaking. Cells were transferred to 9 mL of 10% FBS/DMEM/F-12 medium and were then collected by centrifugation (400 g for 5 minutes). LSCs were cocultured with MMC-treated NIH-3T3 fibroblast feeder cells located within the transwell (0.4 μm pore, BD Biosciences, Bedford, MA). For passage, near confluent cells were harvested with 0.25% trypsin and then 1 105 subcultured cells were cultured in the respective medium described above.
2.4 Colony-forming efficiency
Approximately 1 x 103 LSCs were seeded in a 3.8-mm2 dish and cocultured with MMC-treated NIH-3T3 feeder cells located within the transwell. The medium was changed every 2-3 days. At 10 days, colonies were fixed by 4% paraformaldehyde (room temperature for 1 hour) for immunostaining and crystal violet staining. The CFE (%) was calculated using the following formula: number of colonies formed/number of cells plated × 100%.
2.5 Western blotting
Cell lysis and SDS PAGE were performed as described previously.23 NE-PER nuclear and cytoplasmic extraction kit (Pierce, Rockford, IL) was used to separate total cell lysate into cytoplasmic and nuclear fractions according to the manufacturer's instructions. Each cellular fraction was then resolved by SDS-polyacrylamide gel electrophoresis, electrotransferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA), and processed for immunoblot analysis. Antibodies used in the immunoblot study were cyclin D1, Ptch, SMO, Gli1, Gli3, Gli3-R, histone H1 and phospho-STAT3 (1:1000 dilution). Proteins of interest were detected using the appropriate IgG-HRP secondary antibody and ECL reagent. X-ray films were scanned on a Model GS-700 imaging densitometer (Bio-Rad Laboratories, Hercules, CA) and analysed with Labworks 4.0 software. Blots from at least three independent experiments were used for quantification.
2.6 Quantitative real-time PCR
Experiments were performed as described previously.23 The sequences of the PCR primers were rabbit Gli1 (accession number: XM_017350480.1) sense, 5′- CTTCAAGGCCCAGTACATGC-3′, anti-sense, 5′-TCGAGGCGTGAGTATGACTT-3′; rabbit Gli3 (XM_017344449.1) sense, 5′- ACAGGCGAGAAGCCTCATAA-3′, anti-sense, 5′- CAACCTTCGTGCTCACAGA C-3′; and rabbit GAPDH (NM_001082253;) sense, 5′-TCTGGCAAAGTGGATGTTGT-3′, anti-sense, 5′-GTGGGTGGAATCATACTGGA-3′. The cycle threshold (Ct) values of the PCR product and a GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) control mRNA were used to calculate relative quantities of mRNA.
2.7 siRNA transfection
LSCs were transfected with 10 nmol/L of siRNA targeting Gli1 or Gli3 genes (listed in Table 1; Stealth RNAi™ siRNA duplexes, Invitrogen) for 48 hours using the Lipofectamine RNAi MAX transfection kit according to the manufacturer's protocol (Invitrogen, USA). Scramble siRNA (sc-37007, Santa Cruz Biotechnology) that did not specifically target any gene were used as control.
| siRNA | Sequence (5′-3′) | Accession number |
|---|---|---|
| Gli1-1 |
F: CCA GUG UCC UCG ACU UGA ACA UUA U R: AUA AUG UUC AAG UCG AGG ACA CUG G |
XM_017350480.1 |
| Gli1-2 |
F: UAG AGU UGA GGA AUU GCG UCU CUC C R: GGA GAG ACG CAA UUC CUC AAC UCU A |
|
| Gli3-1 |
F: CAC GUG CCU UCU GCC UUA UCU AGU A R: UAC UAG AUA AGG CAG AAG GCA CGU G |
XM_017344450.1 |
| Gli3-2 |
F: GGA CCA AAU GGA UGG AGC ACG UAA A R: UUU ACG UGC UCC AUC CAU UUG GUC C |
To evaluate the influence of Gli1 and Gli3 knockdown on LSC proliferation, LSCs (2 × 103/well) were seeded in 96-well cell culture plate (Costar; cat. no. 3599) one day before transfection. Forty-eight hours after siRNA transfection, cells were maintained in serum-free DMEM/F12 for 2 hours and then stimulated by 44-mer (10 µmol/L) for 24 hours. The level of LSC proliferation was evaluated by Cell Proliferation Assay Kit based on the amounts of nuclear dye binding (BioVision; Catalog # K307-1000), according to the company's instruction. All assays were performed in triplicate, and the experiment was independently performed for three times.
2.8 Partial limbal injury
Mice were anaesthetized by intraperitoneal injection of a mixture of zoletil (6 mg/kg) and xylazine (3 mg/kg). One drop of 0.5% proparacaine hydrochloride (Alcaine; Alcon,Fort Worth, TX) was given before ocular procedures. The epithelium of the inferior 120 degree limbus was removed with a 0.5 mm metal burr (Rumex international Co, Clearwater, FL),24 0.75 mm into the cornea and 0.75 mm into the conjunctiva in the experimental eye.
The 44-mer was reconstituted in DMSO to a final concentration 100 μmol/L. At the end of limbal surgery, a separated dose of 10 μL of 44-mer (100 μmol/L) mixed with 90 μL of dexamethasone was injected into the upper and lower conjunctival fornix. 10 μL of DMSO mixed with 90 μL of dexamethasone served as a control. Mice were killed at 2 weeks.
To study the role of signalling pathways, subconjunctival injection of 10 μL 44-mer and 85 μL dexamethasone mixed with various inhibitors was performed: HPI4 (SHh inhibitor, 5 μL of 500 μmol/L) and Atglistatin (ATGL inhibitor, 5 μL of 500 μmol/L). 10 μL 44-mer and 85 μL dexamethasone with 5 μL DMSO served as a control. Mice were killed at 2 weeks and the eyeballs were harvested for immunofluorescence (IF) staining.
2.9 Immunofluorescence
Deparaffinized tissue sections (5 μm) or 4% paraformaldehyde-fixed LSCs were blocked with 10% goat serum and 5% BSA in PBS containing 0.1% Tween 20 for 1 hour. Staining was performed with primary antibodies against CK12 (1:200 dilution), CK19, △Np63α, Lrig1, Gli1, Gli3, BrdU (all 1:100 dilution) for 2 hours at 37°C, followed by incubation with appropriate rhodamine- or FITC-conjugated donkey IgG (1:500 dilution) for 1 hour at room temperature. Images were acquired with a Zeiss epifluorescence microscope and a charge-coupled device camera. Photographs were taken with the Zeiss Axiovision version 3.1 software (Carl Zeiss MicroImaging GmbH, Jena, Germany).
2.10 Organ culture
To evaluate the expressions of Gli1 and Gli3 in the nucleus of remaining LSCs after partial limbal injury, murine eyes were enucleated and placed in a 24-well culture plate containing 2 mL LSC culture medium14 supplemented with 10 µmol/L 44-mer for 2 hours at 37°C. LSC culture medium supplemented with DMSO served as the control. Each globe was fixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffer (PH 7.4) for 48 hours at 4°C and then embedded in paraffin.
2.11 Statistical analysis
Results were presented as mean ± SD. The statistic significances of the experimental results were assessed by Student's t test using SPSS version 18.0 (SPSS Inc, Chicago, IL). A two-tailed P < 0.05 was considered statistically significant. Statistical significance was as follows: P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***).
3 RESULTS
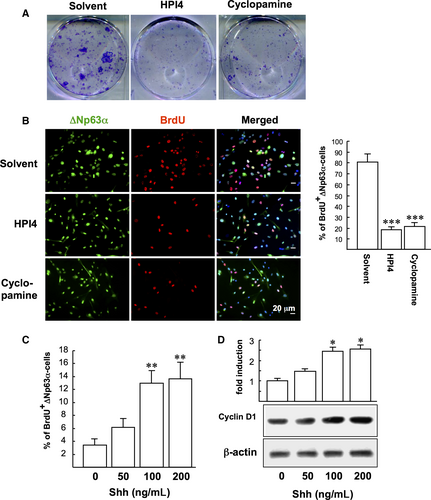
3.1 SHh signalling promotes LSC proliferation
We first examined the influence of SHh signalling on LSC clonogenicity by exposing LSCs to SHh inhibitors (HPI4 or cyclopamine) for 10 days. The clonogenicity of LSC was significantly suppressed by SHh inhibitors compared to control (Figure 1A). In addition the BrdU proliferation assay showed that HPI4 and cyclopamine significantly reduced the proliferation of ∆Np63α-positive LSCs compared to control (17.8 ± 2.7% and 21.3 ± 3.2% vs 80.5 ± 7.1%), suggesting that suppression of SHh signalling impaired LSC proliferation (Figure 1B). To confirm our findings, LSCs were further treated with recombinant human SHh at various concentrations (50, 100 and 200 ng/mL) for 24 hours before 2 hours of BrdU labelling. SHh was found to increase LSC proliferation (1.8-4.0-fold; Figure 1C) and cyclin D1 expression (1.4-2.6-fold; Figure 1D) in a dose-dependent manner.
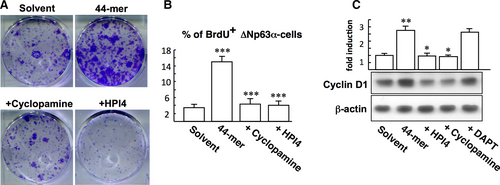
3.2 44-mer-induced LSC proliferation depends on Sonic Hedgehog (SHh) pathway
To study the role of SHh signalling in 44-mer-mediated LSC proliferation, CFE assays were performed with the treatment of 44-mer, in combination with HPI4 or cyclopamine. The result showed that 44-mer-promoted clonogenicity was decreased by HPI4 or cyclopamine (Figure 2A). Cyclopamine and HPI4 also reduced the number of BrdU-positive LSCs induced by 44-mer (4.3 ± 1.4% and 4.1 ± 1.1% vs 15.0 ± 1.3%; Figure 2B). In addition, exposure of LSCs to the 44-mer for 24 hours resulted in a 2.7-fold increase of the cyclin D1 protein compared to control (P = 0.008), and this effect was suppressed by the SHh inhibitors (P = 0.001) but not by Notch inhibitor (DAPT) (Figure 2C). These results indicate that 44-mer-induced LSC expansion requires activation of SHh signalling.
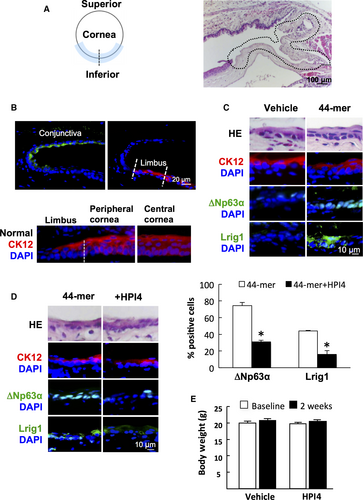
To explore the in vivo requirement of SHh signalling for 44-mer-induced LSC proliferation, we performed surgical removal of inferior limbal epithelium of mice and treated them with 44-mer or vehicle (Figure 3A). The presence of LSCs in wounded areas was evaluated by IF. In normal mice, conjunctival epithelium was stained positively with CK19, while corneal and limbal epithelia were stained positively with CK12. Of note, LSCs, which were at basal layer of limbus, were stained negatively with CK12, while whole layer of corneal epithelium was stained positively with CK12 (Figure 3B). The amount of △Np63α-positive or Lrig1-positive basal cells was calculated as percentage of the total limbal basal cells. The mice with inferior limbal epithelium removed were evaluated at 2 weeks after injury. The limbal epithelial basal cells in the 44-mer groups were stained positively with the LSC markers △Np63α (74.26 ± 3.94%) and Lrig1 (43.9 ± 0.87%), while none can be stained in control group (Figure 3C). Furthermore, subconjunctival injection of HPI4 along with 44-mer suppressed the 44-mer-induced ∆Np63α-positive LSCs by 2.4-fold decrease and Lrig1-positive LSCs by 2.8-fold decrease (Figure 3D). HPI4 injection did not show any severe adverse effect, such as weight loss (Figure 3E). These findings suggest that the 44-mer enhances restoration of LSCs in the damaged limbus through SHh signalling pathway.
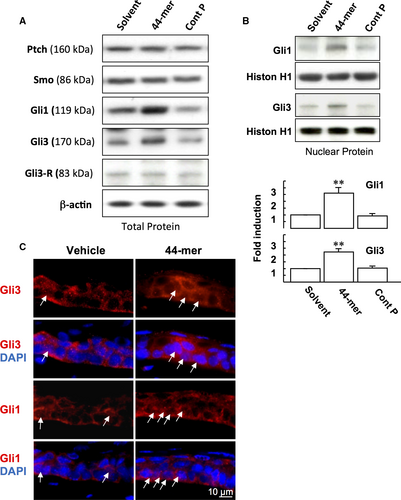
3.3 44-mer induces Gli transcription factors
Next, we examined the effect of 44-mer on the protein expressions of SHh receptors (Ptch and SMO), and Gli transcription factors. We found that after LSCs were stimulated by the 44-mer for 6 hours, the expressions of Gli1 and full-length activator of Gli3 (170 kDa) were increased by 3-fold and 2.7-fold, respectively, compared to vehicle or control peptide, while the expression levels of Ptch, SMO and Gli3 transcriptional repressor isoform (Gli3-R) remained unchanged (Figure 4A). The 44-mer-induced Gli1 and Gli3 expressions declined to basal levels at 24 hours (N.W.F., T.C.H., Y.P.T., unpublished data). Nuclear translocation of Gli proteins is an indicator of SHh signalling activation. After LSCs were treated with 44-mer for 6 hours, cell fractionation assays showed an increase of nuclear Gli1 and Gli3 levels compared to vehicle or control peptide-treated cells (Figure 4B). The mitogenic effect of recombinant SHh on promoting corneal epithelial healing has been reported using organ cultures.22 To investigate the activation of SHh signalling, organ cultures of eyeballs with removed inferior limbal epithelium were treated with the 44-mer. Consistently, nuclear Gli1 and Gli3 levels were increased in the LSCs of superior limbus with 44-mer treatment (Figure 4C). Taken together, the increase of nuclear Gli1 and Gli3 in LSC following stimulation by 44-mer suggests that 44-mer mediates its effect on LSC through activating SHh signalling pathway.
3.4 Knockdown of Gli3 impairs the mitogenic activity of 44-mer on LSCs
To examine whether Gli1 and Gli3 are required for the 44-mer-mediated LSC proliferation, we used siRNA to silence the two genes. Real-time PCR analysis confirmed that Gli-1 and -3 mRNA expressions were significantly inhibited by respective siRNAs. However, knockdown of Gli3 also inhibited 44-mer-induced Gli1 expression (Figure 5A, P < 0.05 vs control). Western blotting showed that si-Gli1 led to almost 80% Gli1 protein suppression, and si-Gli3 significantly suppressed both Gli1 and Gli3 protein levels, by 47% and 83%, respectively (Figure 5B), suggesting that Gli1 expression is partially mediated by Gli3.
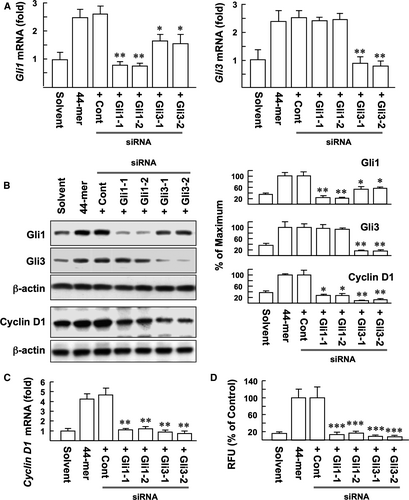
It has been demonstrated that cyclin D1 is a target gene of the Gli transcription factors.18 Real-time PCR and Western blotting showed that si-Gli1 or si-Gli3 significantly suppressed the ability of 44-mer to induce cyclin D1 expression, compared to control (Figure 5B,C). Most importantly, si-Gli1 or si-Gli3 blocked the 44-mer-induced LSC proliferation by 83%-92%, respectively, compared to control (Figure 5D). Our results indicate that 44-mer promotes LSC proliferation through Gli1- and 3-mediated cyclin D1 expression.
3.5 ATGL activates STAT3 signalling to promote Gli expression
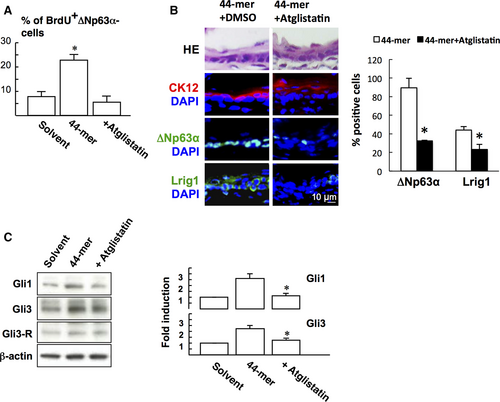
Given that 44-mer bound to its receptor ATGL to initiates signal transduction,23 the role of ATGL in LSC proliferation was investigated. BrdU labelling assay showed that Atglistatin blocked 44-mer-induced LSC proliferation in vitro (Figure 6A). In vivo experiment also showed that subconjunctival injection of Atglistatin suppressed the 44-mer-induced ∆Np63α- (P = 0.046) and Lrig1-positive (P = 0.046, Figure 6B) LSC expressions. We further examined the effect of pharmacological inhibition of ATGL signalling on 44-mer-induced Gli1 and Gli3 expressions. When LSCs were pretreated with Atglistatin, Gli1 and Gli3 proteins were suppressed to near basal levels (Figure 6C).
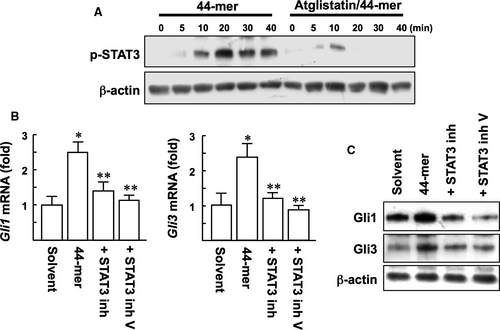
PEDF has been shown to induce phosphorylation of STAT3 in LSCs, and PEDF-ATGL signalling is relied on STAT3 signalling.14, 23 To determine whether the effect of 44-mer on SHh activation is dependent on ATGL/STAT3 signalling, pharmacological inhibitors for ATGL and STAT3 were evaluated. Our results showed that Atglistatin suppressed the phosphorylation of STAT3 at Tyr705 in response to 44-mer (Figure 7A). With the 44-mer treatment for 3 hours, the mRNA levels of Gli1 and Gli3 were significantly up-regulated by 2.5- and 2.4-fold, respectively, compared to control (Figure 7B). STAT3 inhibitors suppressed the 44-mer-induced Gli1 and Gli3 mRNA and protein expressions to near basal levels (Figure 7B,C). Collectively, pharmacological inhibition of ATGL/STAT3 signalling attenuated the ability of the 44-mer to up-regulate Gli1 and Gli3 expressions.
4 DISCUSSION
Damage to the human corneal limbus may lead to permanent dysfunction of the stem cells. Currently, there is no available therapeutics to slow down the progress of LSCD. We have previously demonstrated that LSCs can be expanded by 44-mer both in vitro and in vivo.14, 16, 17 In this present study, we have further shown that ATGL-STAT3-SHh signalling pathway is essentially involved in 44-mer-mediated LSC expansion.
The canonical SHh pathway is a stemness pathway which is largely inactive in adult tissue, except for tissue repair and maintenance.25 The SHh signal transduction involves binding of processed SHh proteins to their inhibitory receptor Ptch, which leads to activation of the pathway by phosphorylation of SMO.18 Subsequently, Gli transcription factors are activated and translocated into the nucleus and modulate the expression of downstream target genes, including cyclin D1, Myc, Bcl2, Bmi1 and Snail, which further regulate cell behaviour.18, 26 The role of SHh in the LSC population has remained unknown until our current study showing that intrinsic SHh is crucial for LSC self-renewal. In addition, 44-mer behaves as an activation signal to interact with SHh/Gli signalling, resulting in modulation of Gli protein expression to promote LSC proliferation. Our study proved that 44-mer up-regulates Gli1 and Gli3 in LSCs. We also found that knockdown of Gli3 reduced Gli1 mRNA and protein levels in 44-mer-treated LSCs, suggesting that Gli1 gene is probably a direct transcriptional target of Gli3.27 Other signalling molecules, which were reported to be positive regulators of Gli function, include EGF, PDGF, FGF and IGF.18, 21, 28 Although EGF, and FGF promote LSC proliferation,29 the interaction between EGF, FGF and SHh-Gli in LSCs is unknown.
STAT3 is a cytoplasmic protein with Src Homology-2 domains that act as signal messengers and transcription factors, participating in cellular responses to cytokines and growth factors. PEDF was found to cause a striking activation of STAT3 in myoblasts, hepatocytes and LSCs; pharmacological inhibition of STAT3 blocks PEDF function in these cells.14, 23, 30 Accordingly, in the present study, we found that SHh signalling activated by the 44-mer was regulated by STAT3 activity as well. These results suggest that cell signalling in response to 44-mer appears to rely essentially on STAT3 activity. Other well-studied signalling systems dependent on STAT3 include G-CSF receptor signalling, HGF and IL-10.31 Apart from promoting SHh signalling activation, the mechanism by which 44-mer increases LSC population may also involve the direct binding of STAT3 to the △Np63α promoter.32 Crosstalk between SHh and STAT3 has been studied in gastric metaplasia, lung adenocarcinoma and skin tumours. All these studies suggest that the interaction between STAT3 and SHh was indirectly mediated through up-regulation of third-party factors, such as IL-6, IL1β, TNFα, IL-11 and TIF1.26, 33, 34 The precise pathway by which the STAT3 and SHh pathway interacts in LSCs requires further studies.
Various membrane receptors for PEDF have been reported in different cells.35-37 It was found that ATGL is a putative receptor for PEDF in rabbit corneal epithelial cells.38 In our study, either direct inhibition of SMO by cyclopamine,26 or inhibition of downstream of SMO by HPI4,39 abolished the 44-mer-induced LSC expansion. A recent report indicated that SHh activates phospholipase A2 to release arachnoid acids, which promotes SMO ciliary accumulation and signalling.40 Because PEDF can regulate triglyceride metabolism in hepatocytes through ATGL,41 one may speculate that PEDF augments the function of SMO to enhance the expression of downstream Gli proteins.
The restoration of LSC population in the limbal wound promoted by the 44-mer is probably because of the migration of LSCs, either from lateral cell displacement or the central zone.16 The target genes of the SHh pathway responsible for epithelial-mesenchymal transition26 may contribute to LSC migration. On the other hand, a scenario of one-for-one replacement from neighbouring LSCs, stimulated by death of LSCs,42 is another possible mechanism in which symmetrical division could be promoted by the 44-mer. Our study progresses the LSC biology field by providing clues on the molecular programs that orchestrate LSC activity. Exogenous cues, such as PEDF, which modify the signals that determine stem-cell fate decisions, have the translational potential for the treatment of LSCD.
ACKNOWLEDGEMENTS
We thank Chu-Ping Ho for assistance with animal experiments, Dr. Erh-Hsuan Lin for technique support of immunostaining, and Dr. Tim J Harrison and Dr. Yihe Chen for polishing the English in this article.
CONFLICT OF INTEREST
Patent: Use of PEDF-derived polypeptides for promoting stem cells proliferation and wound healing (T.-C. Ho, Y.-P. Tsao). Nai-Wen Fan and Cheng-Wen Wu: none.
AUTHOR'S CONTRIBUTION
Nai-Wen Fan and Tsung-Chuan Ho performed the research and analysed the data. Nai-Wen Fan, Tsung-Chuan Ho, Cheng-Wen Wu and Yeou-Ping Tsao designed the research. Yeou-Ping Tsao contributed essential reagents or tools. Nai-Wen Fan and Tsung-Chuan Ho wrote the paper. Cheng-Wen Wu and Yeou-Ping Tsao revised the paper.
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.




