Metformin protects against intestinal barrier dysfunction via AMPKα1-dependent inhibition of JNK signalling activation
Abstract
Disruption of the intestinal epithelial barrier, that involves the activation of C-Jun N-terminal kinase (JNK), contributes to initiate and accelerate inflammation in inflammatory bowel disease. Metformin has unexpected beneficial effects other than glucose-lowering effects. Here, we provided evidence that metformin can protect against intestinal barrier dysfunction in colitis. We showed that metformin alleviated dextran sodium sulphate (DSS)-induced decreases in transepithelial electrical resistance, FITC-dextran hyperpermeability, loss of the tight junction (TJ) proteins occludin and ZO-1 and bacterial translocation in Caco-2 cell monolayers or in colitis mice models. Metformin also improved TJ proteins expression in ulcerative colitis patients with type 2 diabetes mellitus. We found that metformin ameliorated the induction of colitis and reduced the levels of pro-inflammatory cytokines IL-6, TNF-a and IL-1β. In addition, metformin suppressed DSS-induced JNK activation, an effect dependent on AMP-activated protein kinase α1 (AMPKα1) activation. Consistent with this finding, metformin could not maintain the barrier function of AMPKα1-silenced cell monolayers after DSS administration. These findings highlight metformin protects against intestinal barrier dysfunction. The potential mechanism may involve in the inhibition of JNK activation via an AMPKα1-dependent signalling pathway.
Introduction
The gastrointestinal epithelium is a multilayered barrier that functions as a defence system against pathogens and other harmful compounds in the lumen. Increasing evidence indicates that the epithelial barrier dysfunction contributes to the onset and development of various diseases, including inflammatory bowel disease (IBD) 1. IBD, comprises ulcerative colitis (UC) and Crohn's disease (CD), is associated with a chronic and relapsing inflammation condition. One of the most typical feature of IBD is epithelial barrier disruption, which contributes to IBD pathogenesis 1, 2.
The epithelial barrier comprises epithelial cells and intercellular junctional complexes 3, including TJs. The transmembrane proteins of TJs, namely, occludin and claudins, interact with the central plague protein ZO-1, which associates with the cytoskeleton 4. This junction complex plays a critical role in forming and maintaining the epithelial barrier. Defective epithelial TJs cause paracellular barrier functional disturbances and increase the paracellular permeability of intestinal epithelium. Abnormally high paracellular permeability results in intestinal penetration by luminal bacteria and potentially harmful antigens, which appear to initiate and accelerate inflammation in IBD 1, 5-7. Therefore, improving intestinal barrier function can alleviate inflammation development or accelerate inflammation resolution 6, 8.
Metformin has been widely used for the treatment of type 2 diabetes mellitus (T2DM). Recently, researchers have uncovered increasing amounts of evidence that metformin has additional beneficial effects beyond facilitating improvements in glucose homoeostasis. For example, studies have reported that metformin plays a role in anti-ageing and increase lifespan 9, 10. Metformin has also been shown to partially attenuate gut dysbiosis caused by disease 11, 12. Furthermore, many studies have shown that metformin has anti-inflammatory effects in vitro and in vivo 13-16. Specifically, some studies have reported that metformin can attenuate intestinal inflammation in mice with colitis 17, 18.
Intestinal epithelial barrier function and inflammation are closely related, and metformin has anti-inflammatory property in the gut. However, there is little evidence that whether metformin can protect intestinal epithelial barrier function. In this study, we showed that metformin protects intestinal epithelial barrier function in vitro and in vivo and elucidated its possible mechanism via AMPKα1-dependent inhibition of JNK signalling pathway.
Materials and methods
Cell culture
Caco-2 cells were obtained from American Type Culture Collection and maintained in Dulbecco's modified Eagle medium (DMEM), supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin and 20% (v/v) foetal bovine serum (FBS). The medium was refreshed every 2–3 days, and cells were passaged using 0.05% trypsin.
Transepithelial electrical resistance (TEER) measurement
A total of 1 × 105 Caco2 cells were seeded in 24-well transwell chambers (6.5 mm diameter, 3 μm pore size; Corning, Tewksbury, MA, USA) and cultured for 15 days. The integrity of cellular monolayer was determined by measuring TEER values. When the TEER values were consistently above 300 Ω cm2, Caco-2 cells monolayers were considered to have been matched for the experiment 19; 3% DSS (MP Biochemical, Santa Ana, CA, USA) dissolved in DMEM was added to the upper chamber for 8 hrs after pre-treatment with metformin, and TEER values was measured.
Tissue samples
Paraffin-embedded colonic tissue samples from 22 UC patients with T2DM and 20 normal controls were randomly obtained on endoscopic examination from the Department of Gastroenterology, Nanfang Hospital during 2014–2016. Definitive diagnosis of UC was established by standard endoscopic, histological and clinical criteria. The partial Mayo score was determined previously as described 20. Patients with septic complications, short bowel syndrome or cancer, and pregnant women were excluded. The patients were divided into metformin group and insulin group according to basic treatment of T2DM. The study was carried out in accordance with the institutional ethical guidelines and had been approved by the medical ethics committee of Southern Medical University (number: NFEC-2014-035). The sociodemographic and clinical data are summarized in Table S1.
Animals
Wild-type male C57BL/6 mice (6–8 weeks, 20–23 g in weight) were housed in specific pathogen-free conditions. The mice were randomly divided into four groups: a control group, a DSS group, a DSS plus 100 mg/kg/day metformin group and a DSS plus 500 mg/kg/day metformin group. Acute colitis was induced by administrating 3% DSS for 7 days. Metformin were administered via gavage for 7 days before colitis induction and then administered in parallel with DSS. The disease activity index (DAI) was calculated as previously described 21. After 7 days, the animals were killed. Colon length was measured, and the colon, livers, spleens, mesenteric lymph nodes (MLNs) and serum samples were collected. Animal handling was approved by the Animal Experimental Ethics Committee of Southern Medical University (number: L2015059).
Histological analysis
The distal colon was fixed in 4% paraformaldehyde, and then, they were dehydrated with a graded ethanol series, cleared in dimethylbenzene, embedded in paraffin and stained with haematoxylin and eosin (H&E). The degree of intestinal inflammation was assessed according to the modified scoring system devised by Dieleman et al. 22. The scores were assessed by two pathologists in double-blinded manner.
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA from distal colon was isolated using RNAiso Plus (TaKaRa, Dalian, China). First-strand cDNA was synthesized, and qRT-PCR was performed with a Roche LightCycler® 480II using SYBR Premix Ex Taq (TaKaRa). The primer sequences are listed in Table S2. The expression level of each mRNA was calculated using the 2−ΔΔ Ct method after the corresponding sample was normalized to the expression level of β-actin level.
Enzyme-linked immunosorbent assay (ELISA) analysis
Serum samples were obtained from the different groups, and protein levels of the inflammatory factors IL-6, TNF-α, IL-1β were determined using commercially available ELISA kits (MultiSciences, Hangzhou, China), according to the manufacturer's protocol.
Western blot analysis
Total protein was extracted with cold radioimmunoprecipitation lysis buffer, protease inhibitor and phosphatase inhibitor cocktail. Equal amounts of proteins were separated by 8% or 10% SDS-polyacrylamide gels and then transferred to a PVDF membrane (Bio-Rad, Marnes-la-Coquette, France), which was blocked with 5% skim milk prepared in tris-buffered saline with Tween (TBST). Then, the membrane was incubated with primary antibodies against GAPDH (ZSGB-BIO, Beijing, China),ZO-1 (Invitrogen, Carlsbad, CA, USA), occludin (Protein Tech Group, Inc., Wuhan, China), JNK1/2, p-JNK1/2, AMPKα, p-AMPKα (Cell Signaling Technology Inc., Danvers, MA, USA), AMPKα1, AMPKα2 (Abcam, Cambridge, MA, USA) overnight at 4°C. Following washes in TBST, the membrane was subsequently incubated with the appropriate HRP-conjugated secondary antibodies for 1 hr and then visualized via enhanced chemiluminescence detection. Protein expression was quantified by densitometric analysis using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunohistochemical analysis
The immunohistochemical methods have been described previously 23. Sections were deparaffinized in xylene and rehydrated through a graded ethanol series. Then, they were antigen retrieved, quenched of endogenous peroxidase and incubated with primary antibodies against occludin and ZO-1 (dilution 1:100) overnight at 4°C. After washing with PBS three times, tissues were incubated with a second antibody (ZSGB-BIO), developed with the DAB reagent and counterstained with haematoxylin. Negative controls were incubated without antibody. The quantification of TJ proteins was assessed according to the staining intensity and the percentage of the stained epithelial cells. Briefly, the staining intensity was scored as negative (0), weak (1), moderate (2) or strong staining (3). The percentage of positive cells was graded on a scale of 0–4: 0, less than 5%; 5–25% scored 1; 26–50% scored 2; 51–75% scored 3 and more than 75% scored 4. The final score was calculated by multiplying intensity score and percentage score.
Intestinal permeability in vivo and in vitro
For the in vivo permeability assay, all mice were fasted for 8 hrs and then gavaged with FITC-dextran (4kD, FD4; Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 600 mg/kg bodyweight. After 2 hrs, they were anesthetized and their plasma was collected in the dark. FD4 permeability may be partially affected by change in gastrointestinal motility, although the influence of gastrointestinal motility is very limited. For the in vitro permeability assay, 1 mg/ml FD4 was added to upper chamber after Caco-2 cells monolayers being treated. FD4 flux was measured by placing 100 μl solutions from the basolateral chamber after 2 hrs. The fluorescence intensity was measured using a fluorescence spectrophotometer (485 nm excitation and 535 nm emission). FD4 concentration was obtained from standard curves generated by serial FD4 dilution.
Bacterial translocation in colitis
Total bacterial DNA from MLNs, livers and spleens were extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). DNA was amplified using universal 16s rDNA primers (5′-AGAGTTTGATCATGGCTCAG-3′, 5′-ACCGCGACTGCTGCTGGCAC-3′) for all eubacteria. The amplified product was purified. The plasmid was synthesized by Shanghai Biotechnology Company. To analyse the quantities of bacteria, we used 100 ng DNA to quantitate the bacteria by qRT-PCR analysis. The copies were quantitated using standard curves constructed with known plasmid DNA concentrations.
Immunofluorescence staining
Caco-2 monolayers were fixed in 4% paraformaldehyde. Following permeabilization in 0.1% Triton X-100, they were blocked in 1% bovine serum albumin. Then, the monolayers were incubated with a primary antibody against ZO-1 (1:50) and occludin (1:50) overnight at 4°C. The cells were subsequently incubated with the appropriate secondary fluorescence antibody for 1 hr. DAPI was used to stain the cell nuclei. The fluorescence was examined under a confocal laser scanning microscope (Fluoview FV10i; Olympus, Tokyo, Japan).
Transfection of small interfering RNA (siRNA)
AMPKα1 or AMPKα2 siRNA and negative control (NC) siRNA were obtained from GenePharma (Shanghai, China). The target sequences of siRNA are listed in Table S3. Caco2 cells were transfected with each siRNA (100 nM) using Lipofectamine 3000 Transfection Reagent (Invitrogen) and incubated in Opti-MEM® I reduced serum medium (Invitrogen) for 8 hrs, according to the manufacturer's instructions. The interfering efficiency of each target gene was determined by Western blot analysis.
Statistical analysis
All data were representative of at least three independent experiments and were expressed as the mean ± S.E.M. Student's t-test or one-way analysis of variance (anova) and an appropriate post hoc Dunnett's or Tukey's comparison were performed to determine the significance of the differences among the groups. P < 0.05 was considered statistically significant.
Results
Metformin alleviates DSS-induced barrier dysfunction in Caco-2 cell monolayers
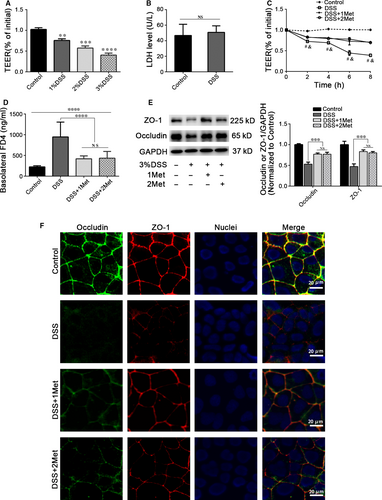
A ‘leaky’ barrier is attributable to TJ disruption, which alters TEER and paracellular permeability 1, 24. To confirm whether metformin can protect barrier function, we first used 1%~3% DSS to treat Caco-2 cell monolayers for 8 hrs and observed dose-dependent decrease in TEER (Fig. 1A). 3% DSS did not induce significant changes in cell viability (Fig. 1B), suggesting the barrier dysfunction was not caused by cell death. After DSS treatment,we noted a significant time-dependent decrease in TEER. Treatment with metformin (1 mM / 2 mM) could significantly alleviate the TEER decreases, but there is no significant difference between the two concentrations (Fig. 1C). The DSS-induced transepithelial high permeability of FD4 was significantly reduced by metformin treatment (Fig. 1D). The levels of TJ proteins ZO-1 and occludin was also significantly decreased after DSS exposure, and they were reversed when combined with metformin treatment (Fig. 1E). Immunofluorescence staining showed that DSS treatment led to both ZO-1 and occludin depletion and discontinuity. Metformin could attenuate these changes (Fig. 1F).
Metformin ameliorates the severity of DSS-induced acute colitis in mice
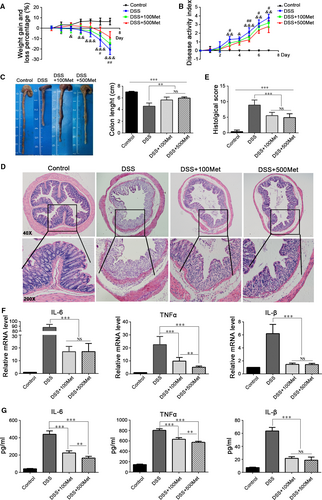
To determine whether metformin has beneficial effect in colitis, we treated mice with 3% DSS to induce acute colitis. Mice treated with metformin simultaneously experienced significantly less bodyweight loss than those treated with DSS only. Mice treated with 500 mg/kg/day metformin had less bodyweight loss than those with 100 mg/kg/day. (Fig. 2A). Consistent with these findings, metformin could significantly reduce the DAIs (Fig. 2B) as well as the colon length shortening of DSS-induced mice (Fig. 2C). Histological examination showed less intestinal mucosal morphological damage, less inflammatory cells infiltration and better histological scores in metformin-treated mice (Fig. 2D and E). Moreover, we assessed the mRNA and protein levels of pro-inflammatory cytokines IL-6, TNF-a and IL-1β in colon and in serum, and found that metformin could significantly inhibit the expression of those cytokines (Fig. 2F and G).
Metformin improves intestinal permeability and promotes TJ expression in colitis
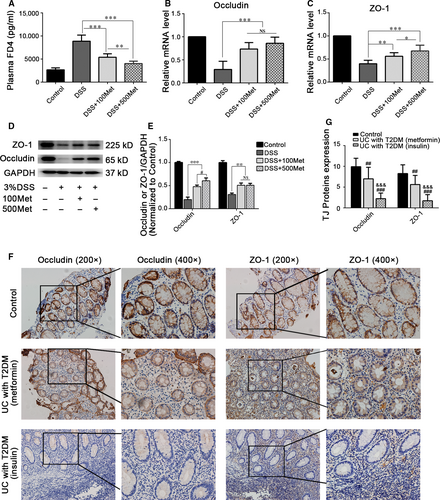
Intestinal barrier dysfunction leads to intestinal inflammation 25. We speculated that the anti-inflammation effect of metformin is probably facilitated by maintaining intestinal barrier function. To confirm this hypothesis, we first assessed intestinal permeability. Metformin could significantly alleviate increased FD4 flux caused by DSS, in a dose-dependent manner. (Fig. 3A). After treated with metformin, the mRNA expression levels of TJ proteins ZO-1 and occludin were significantly increased in colonic mucosa (Fig. 3B and C). Additionally, metformin treatment significantly improved TJ proteins expression (Fig. 3D and E). Furthermore, we collected colonic tissues from treated active UC patients with T2DM and assessed the expression levels of TJ proteins. We found that TJ proteins expression in patients with metformin treatment for T2DM were higher than those in patients with insulin treatment for T2DM (Fig. 3F and G), suggesting that metformin may participate in maintaining intestinal barrier function in UC patients.
Metformin reduces commensal bacterial translocation in DSS-induced colitis
Several studies have demonstrated that IBD patients and experimental colitis models have bacterial translocation, which can aggravate inflammation 26, 27. We extracted the bacterial DNA from MLNs, livers and spleens of the colitis models and performed quantitative PCR of bacterial 16S rDNA sequences. The average bacterial copies detected in the MLNs and livers, but not in the spleens, were significantly lower in metformin-treated groups than that in DSS-only group, although a significant difference was not observed between two metformin concentrations (Table 1).
| All eubacteria | Group | P | ||
|---|---|---|---|---|
| DSS | DSS+100Met | DSS+500Met | ||
| MLN | 4.29 ± 0.62*,† | 3.00 ± 0.30 | 3.26 ± 0.49 | 0.002 |
| Liver | 3.66 ± 0.17*,† | 2.98 ± 0.16 | 3.02 ± 0.41 | 0.005 |
| Spleen | 2.92 ± 0.02 | 2.80 ± 0.05 | 2.77 ± 0.09 | 0.08 |
- Bacterial translocation was determined by qRT-PCR and expressed as log10 16S rDNA gene copies. *P < 0.01, versus DSS+100Met; †P < 0.01, versus DSS+500Met.
Metformin improves DSS-induced barrier dysfunction via AMPKα1-dependent inhibition of JNK activation
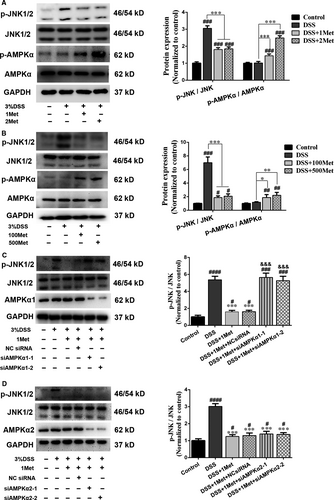
JNK activation is involved in epithelial barrier dysfunction 28, 29. DSS can disrupt TJs by inducing JNK activation. This disruption can be attenuated by JNK inhibition or silencing 29. Therefore, we investigated whether metformin improved barrier function by inhibiting JNK activation. DSS-induced JNK activation was significantly reduced by metformin treatment in Caco-2 cell monolayers (Fig. 4A) or in intestinal epithelium (Fig. 4B). Moreover, AMPKα phosphorylation levels were higher after metformin administration (Fig. 4A and B). To determine whether metformin inhibited JNK activation through AMPKα-dependent pathways, we used siRNA to silence AMPKα1 or AMPKα2 and found the inhibition of JNK activation by metformin was absent when AMPKα1, but not AMPKα2, was silenced (Fig. 4C and D), indicating that metformin suppresses DSS-induced JNK activation via an AMPKα1-dependent pathway.
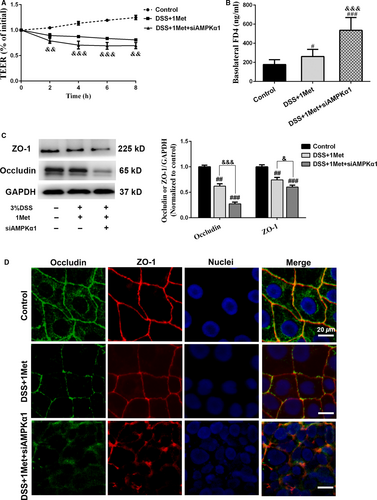
We also determined whether the barrier-protective ability of metformin was impaired after AMPKα1 knock-down. The data suggested that the DSS-induced TEER decreases and FD4 flux increases were more significant in AMPKα1-silenced cell monolayers after metformin treatment (Fig. 5A and B). We subsequently investigated the expression levels of ZO-1 and occludin, and found that the protective effects of metformin were eliminated when AMPKα1 was knocked down (Fig. 5C). Moreover, immunofluorescence staining demonstrated that the continuity of abovementioned TJ proteins was disturbed more severely in AMPKα1-silenced cell monolayers after DSS exposure (Fig. 5D). Taken together, these data confirmed that metformin improves DSS-induced barrier dysfunction by inhibiting JNK activation via an AMPKα1-dependent signalling pathway.
Discussion
Intestinal epithelial barrier dysfunction is common in IBD patients and is considered an underlying pathophysiological component of the disease. Epithelial barrier integrity and function are mainly regulated by TJs 3. Metformin is now considered to have multiple beneficial effects on human body except as an antidiabetic drug. In the current study, we provided evidence that metformin can protect against intestinal barrier dysfunction through its effects on intestinal permeability and TJ proteins expression in vitro and in vivo. The mechanism underlying this protective effect may involve inhibiting JNK activation via an AMPKα1-dependent signalling pathway.
The continuous physical barrier is composed of intestinal epithelial cells connected by TJs. TJs play an important role in the formation and maintenance of the intestinal epithelial barrier integrity. The disruption of TJ-associated barrier integrity contributes to increased intestinal permeability and aggravating inflammation. Previous studies have demonstrated that mice with DSS-induced colitis displayed intestinal barrier disruption and increased mucosal permeability 30, 31. We found that metformin protected against loss of the TJ proteins occludin and ZO-1, decreases in TEER, FD4 hyperpermeability and bacterial translocation in Caco-2 cell monolayers or in DSS-induced colitis mice. This findings indicated that metformin could protect intestinal barrier function. The pathogenesis of bacterial translocation is believed to be related to intestinal barrier disruption and microbial dysbiosis in the gut 32. Intestinal hyperpermeability can increase bacterial penetration in IBD 33, 34. We found that metformin treatment significantly reduced bacteria translocation in the MLNs and livers. We think that the protective effects of metformin on barrier function may be contributed to inhibition of bacteria translocation.
There is little evidence that metformin has protective effects on barrier function in the present. The evidence collected by some researchers suggests that metformin can improve blood–brain barrier function 35, 36. A recent study reported that metformin treatment increased goblet cell differentiation and improved barrier function in IL-10KO mice 37. Here, we showed that metformin protected against DSS-induced intestinal barrier disruption by ameliorating TJs depletion and discontinuity. In clinical practice, metformin has not yet been used in the treatment of IBD. Some studies indicated that the incidence of T2DM is only 2–3% in UC as a concomitant disease 38, 39. We found that the expression of TJ proteins in active UC patients complicated with T2DM is higher in metformin treatment group than in insulin treatment group. According to our cells and animal experiments, it is very likely that the barrier-protective effect of metformin is responsible for this phenomenon shown in UC patients with T2DM.
Studies have demonstrated that AMPK activation by metformin has anti-inflammatory effect 13, 14. In our study, we showed that metformin could significantly prevent DSS-induced colitis and inhibit production of the inflammatory factors IL-6, TNF-α and IL-1β, providing evidence that metformin can attenuate the development of inflammation in colitis, a finding consistent with those of two other studies about the colitis remission induced by metformin treatment 17, 18. As the improvement of epithelial barrier function can prevent the development of intestinal inflammation 6, 8, we believe that this anti-inflammatory effect of metformin is facilitated partly by maintaining intestinal barrier function.
The JNK family comprises three representative isoforms, JNK1, JNK2 and JNK3. JNK1 and JNK2 are expressed widely in many tissues 40. Studies have demonstrated that JNK is activated in the inflamed colonic epithelium of IBD patients 41, 42. Although the role of JNK activation in IBD is not clear, increasing amounts of evidence suggest that its activation plays a role in epithelial barrier dysfunction 28, 43. The epithelial junctions disrupted by JNK activation may be affected by multiple mechanisms, including impairment in adherens junction protein interactions, β-catenin phosphorylation and altered TJ protein expression 44, 45. Inhibition of JNK activation can promote assembly of epithelial junctions and improve epithelial barrier function 28, 29, 44. Samak et al. 29 reported that DSS-induced disruption of intestinal epithelial TJs was associated with JNK activation and the JNK inhibitor SP600125 could attenuate barrier dysfunction. In our study, we determined that metformin protects against DSS-induced intestinal barrier disruption. We observed that metformin treatment could significantly alleviate DSS-induced JNK activation and elevate AMPK phosphorylation. To determine the role of AMPK in metformin treatment-induced JNK inhibition, we knocked down AMPKα1 or AMPKα2 and observed that the suppressive effects of metformin on JNK activation were abrogated after AMPKα1 knock-down, as were its protective effects on intestinal barrier function. Some studies have reported that increased AMPK activation can suppress JNK signalling pathway phosphorylation levels 46, 47. In addition, some researchers suggested that AMPK activation can accelerate TJ assembly to enhance barrier functional integrity in MDCK cells with calcium depletion 48, 49. Taken together, these findings indicate that AMPKα1-dependent inhibition of JNK signalling activation plays an important role in barrier- protective effect of metformin.
In conclusion, our current study indicated that metformin has protective effects on intestinal epithelial barrier function and attenuates intestinal inflammation in colitis. We noted that metformin ameliorates intestinal epithelial barrier dysfunction by inhibiting JNK, whose suppression is dependent on AMPKα1 activation. The detailed mechanism underlying this phenomenon still needs to be investigated. The findings presented in this study indicate that metformin can be served as a potential therapeutic medicine for IBD patients.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Y.C., no. 81570480). The authors would like to thank Jing Tang, Xiaoying Luo and Liang Peng, Department of Gastroenterology, Nanfang Hospital, for general support.
Conflicts of interest
The authors declare no conflicts.




