Racial differences in nocturnal dipping status in diabetic kidney disease: Results from the STOP-DKD (Simultaneous Risk Factor Control Using Telehealth to Slow Progression of Diabetic Kidney Disease) study
Abstract
While racial variation in ambulatory blood pressure (BP) is known, patterns of diurnal dipping in the context of diabetic kidney disease have not been well defined. The authors sought to determine the association of race with nocturnal dipping status among participants with diabetic kidney disease enrolled in the STOP-DKD (Simultaneous Risk Factor Control Using Telehealth to Slow Progression of Diabetic Kidney Disease) trial. The primary outcome was nocturnal dipping—percent decrease in average systolic BP from wake to sleep—with categories defined as reverse dippers (decrease <0%), nondippers (0%–<10%), and dippers (≥10%). Twenty-four-hour ambulatory BP monitoring was completed by 108 participants (54% were nondippers, 24% were dippers, and 22% were reverse dippers). In adjusted models, the common odds of reverse dippers vs nondippers/dippers and reverse dippers/nondippers vs dippers was 2.6 (95% confidence interval, 1.2–5.8) times higher in blacks than in whites. Without ambulatory BP monitoring data, interventions that target BP in black patients may be unable to improve outcomes in this high-risk group.
1 INTRODUCTION
Persons with diabetic kidney disease (DKD) benefit significantly from hypertension control.1-3 Evaluating blood pressure (BP) based on clinic-based values alone results in an inaccurate understanding of BP control because BP naturally varies throughout a 24-hour period.4 There are three distinct BP phenomena that can be missed when relying exclusively on clinic-based values: white-coat hypertension, masked hypertension, and nocturnal dipping status. White-coat hypertension (ie, BP readings collected in a clinical setting that are higher than other settings, such as the patient's home) and masked hypertension (ie, clinical BP measurements that are in the controlled range, but home-measured and/or ambulatory BP that are not) cannot be easily detectable using traditional in-office BP monitoring techniques.4 Among patients with DKD, masked hypertension is associated with a higher risk of end-stage renal disease.5 Thus, relying on in-office BP measurements alone misses important opportunities to identify BP control problems and intervene. Ambulatory BP monitoring (ABPM), which provides information about BP throughout a 24-hour period, may be advantageous because ABPM provides a more comprehensive, longitudinal understanding of BP variation and control.
Our goal in this study was to elucidate racial differences in the third BP control phenomena—nocturnal dipping status. For most healthy individuals, systolic BP (SBP) decreases by at least 10% while sleeping. Individuals who experience a ≥10% reduction from average day to nocturnal systolic SBP are termed “dippers,” those who experience a 0% to <10% decrease from average day to nocturnal SBP are termed “nondippers,” and those who experience an increase in average SBP from day to night are termed “reverse dippers.” Nondipping and reverse dipping are associated with elevated cardiovascular risk including increased incidence of chronic kidney disease and higher risk of developing end-stage renal disease, diabetes mellitus, hypertension, inflammation, and cardiovascular-related morbidity and mortality.6-8 Thus, 24-hour ambulatory BP, which considers variations in BP throughout daytime activities and sleep, is an important factor to consider when evaluating masked hypertension or subclinical cardiovascular disease risk among individuals with DKD.8-10
Ambulatory BP is affected by various characteristics including a patient's age and sex,11-13 social support and/or marital status,14 employment status and job strain,15, 16 and health literacy level.17 While racial variation in ambulatory BP is known,18 the patterns of diurnal dipping among high-risk populations, specifically in the context of DKD, have not been well defined. The objective of this article was to identify factors independently associated with nocturnal dipper status among a cohort with DKD.
2 METHODS
Our analyses used baseline data from the STOP-DKD (Simultaneous Risk Factor Control Using Telehealth to Slow Progression of Diabetic Kidney Disease) trial (ClinicalTrials.gov identifier: NCT01829256). The overarching study, STOP-DKD, is a 3-year randomized controlled trial evaluating whether a multifactorial clinical pharmacist-administered telehealth intervention reduces progression of DKD compared with an education control. The purpose of the STOP-DKD trial is to help patients with diabetes mellitus and hypertension better understand their risk for DKD and provide specific feedback via a telephone intervention on how to reduce that risk through medication management and behavioral changes.
2.1 Participants
In 2014–2015, STOP-DKD trial participants were recruited from seven Duke-affiliated primary care clinics. To be eligible for the STOP-DKD trial, patients met all of the following inclusion criteria: age of at least 18 years and no older than 75 years; a regular user of Duke primary care services (≥2 primary care visits in the 3 years prior to baseline); diagnosis of type 2 diabetes mellitus (International Classification of Diseases, Ninth Revision codes 250×0, 250×2); two or more serum creatinine values available in the past 3 years; relatively preserved kidney function (estimated glomerular filtration rate [eGFR] >45 mL/min per 1.73 m2); evidence of diabetic nephropathy; and poorly controlled hypertension (mean clinic SBP ≥140 mm Hg in the year before baseline and/or diastolic BP (DBP) ≥90 mm Hg or two elevated values). Patients were excluded for a variety of reasons including, but not limited to: not being proficient in English; not having access to a telephone; receiving home health care; participating in another pharmaceutical or behavioral trial; evidence of current drug or alcohol abuse; being pregnant or breastfeeding; diagnosis of non-DKD; active malignancy; dementia; renal transplant; and/or class III or IV heart failure.
Participants were identified in Duke's electronic health record. Study staff conducted chart reviews to screen for eligibility. Potentially eligible participants received a letter from their primary care provider describing the study. Approximately 1 week later, study staff telephoned potential participants for follow-up screening, to assess interest in participation, and schedule an in-person baseline assessment. At baseline, participants were either randomized to receive the intervention or to an educational control group. Prior to receipt of the intervention, as an ancillary part of the study, participants were given the opportunity to monitor their BP using a single cycle of 24-hour ABPM device.
2.2 Ambulatory BP Monitoring
ABPM was measured with a Spacelabs 90207 Ambulatory Blood Pressure Monitor every 20 minutes during waking hours and no more than 60 minutes during sleeping hours.19-21 Study staff measured participants’ upper arm circumference for appropriate cuff size and provided instructions for monitor use as well as an appropriately sized cuff. Participants were instructed to wear the monitor continuously for 24 hours and to log notable activities—specifically, exercise, sleep/wake times, and any time the monitor was removed (eg, during bathing). When participants returned the monitor, they were provided a $10 financial incentive in the form of a check. Because the completeness of participants’ activity logs varied, we used standard wake and sleep times of 6 am and 10 pm, respectively, where the percent of nocturnal decrease was computed as the percent of the average day SBP value minus the average night SBP value over the average day SBP value. These wake and sleep times have been used in previous research.22 The percent of nocturnal decrease was further categorized into three levels: reverse dipping (if the percent of decrease was <0%), nondipping (if the percent of decrease was 0 to <10%), and dipping (if the percent of decrease was ≥10%).23, 24 ABPM data were cleaned by removing artificial readings (eg, caused by artificial sources such as muscle movement or environmental noises) and/or other error readings (eg, overpressure, kinked hose, cuff applied too loosely).
2.3 Race
Race has been associated with BP control and dipping status.18, 25, 26 Thus, we classified participants per their self-reported race. Because of small sample sizes for participants of nonblack minority race, this analysis included only patients reporting white or black race. Participants of other races were excluded.
2.4 Demographic and psychosocial measures
Existing evidence suggests that people of advanced age and men are more likely to have elevated ABPM.11-13 Thus, we collected baseline data regarding participants’ age and sex. Because these factors are associated with BP control, we also collected information about patients’ marital status (married or living with partner or not partnered), highest level of education (completed high school or advanced education vs less than a high school education), employment status (working full- or part-time vs not working), health literacy level, and chaotic lifestyle (eg, having a routine, predictability of schedule).14-17, 27, 28 Health literacy was evaluated using the Rapid Estimate of Adult Literacy in Medicine (REALM).29 Participants were considered to have low health literacy if their REALM score was below 60, which generally equates to less than a ninth-grade reading level. A chaotic lifestyle was measured using a modified Confusion, Hubbub, and Order Scale (CHAOS) measure.30
2.5 Medication adherence
Suboptimal medication adherence is associated with worse hypertension control31 and chronic kidney disease progression.32 Medication adherence was evaluated using a modified eight-item self-report measure.33 Individuals were classified as nonadherent if they endorsed any or all of the statements, or indicated “don't know” or “refused” to any of the statements. Patients refuting the statements were considered adherent. Participants with missing data on any of the adherence items were excluded from multivariable regression analysis.
2.6 Estimated glomerular filtration rate
BP control directly affects kidney function and kidney function directly influences systemic BP control.34 Using participants’ blood collection at baseline visit and laboratory values determined by LabCorp, we included eGFR as a measure of kidney function estimated using the Chronic Kidney Disease Epidemiology Collaboration equation.35 Among included participants, we dichotomized between participants with adequate kidney function (eGFR ≥60) and those with worse kidney function (eGFR <60).
2.7 Microalbuminuria
Microalbuminuria is an early sign of vascular disease and is associated with BP.36 Among included participants, we dichotomized between participants with or without microalbuminuria if urine albumin was 30 ≥μg/mL or <30 μg/mL, respectively.
2.8 Statistical analyses
To ensure an accurate reflection of diurnal dipping patterns, participants were excluded from analysis if they had fewer than 10 ABPM readings or had no BP measurements during the day or sleep. The sample included for analyses was developed after consideration of these exclusions. We compared the characteristics of the included vs excluded groups to assess selection bias. Participant characteristics and ABPM measurements were described by the nocturnal dipping status, where Wilcoxon rank sum test for continuous variables and chi-square test for categorical variables (or Fisher exact test for small samples) were used for comparisons among the three categories of nocturnal dipping status. This multinomial proportional odds model is an extension of a logistic regression model allowing three outcome categories rather than only two. It defines two (cumulative) logits to be modeled simultaneously as a function of the predictors: a comparison of reverse dippers (decrease <0%) to others and a comparison of nondippers (decrease ≥10%) to dippers (>10%). A stepwise process was used to choose the final model, focusing both on which predictors to retain and whether the two regression coefficients for the same predictor were different across the two logits (test of the proportional odds assumption). Candidate models were compared using the score test for nested models and Akaike's Information Criterion for non-nested models. These approaches balance the trade-off between the goodness of fit of the model and overfitting the data by adding unnecessary complexity. The goodness fit of the model was evaluated using Pearson's goodness-of-fit test for models with only categorical covariates and the generalized Hosmer-Lemeshow goodness-of-fit test for models with at least one continuous covariate.37 Using the same basic process to identify covariates, we also used linear regression to model the continuous outcome of percent decrease in average SBP from wake to sleep as a function of baseline predictors. A P value ≤.05 was considered statistically significant. All analyses were performed with SAS software version 9.4 (SAS Institute Inc).
3 RESULTS
Of 281 participants, 127 (45%) participated in the 24-hour ABPM. After exclusions for insufficient BP measurements (ie, <10 BP measurements or no nighttime readings) or nonblack/white race, 108 participants were included for analysis (Figure). To evaluate for potential selection bias, we compared characteristics between 108 participants included versus 173 participants excluded from the aforementioned analyses. Participants did not differ significantly in terms of sex, age, marital status, educational attainment, employment status, health literacy, self-reported medication adherence, chaotic lifestyle, or kidney function.
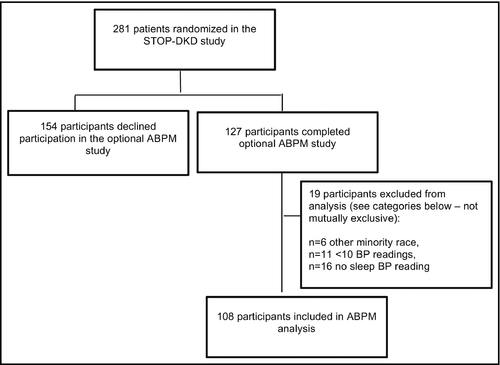
Among participants included in this analysis (n=108), there was a mean of 45.4 BP readings (SD, 10.61) per participant. Participants had a mean of 36.6 (SD, 9.33) waking BP readings and 8.9 (SD, 2.78) sleeping BP readings. About half of the participants who completed the 24-hour ABPM were black (n=53, 49%). Participants were predominantly men (n=63, 58%), married or partnered (n=72, 67%), and had completed high school (n=98, 91%; Table 1).
| All (N=108) | Reverse dipper (<0%) (n=24) | Nondipper (0–<10%) (n=58) | Dipper (≥10%) (n=26) | P value | |
|---|---|---|---|---|---|
| Sex, No. (%) | |||||
| Female | 45 | 4 (8.9) | 26 (57.8) | 15 (33.3) | .01 |
| Male | 63 | 20 (31.7) | 32 (50.8) | 11 (17.5) | |
| Race, No. (%) | |||||
| White | 55 | 12 (21.8) | 25 (45.5) | 18 (32.7) | .09 |
| Black | 53 | 12 (22.6) | 33 (62.3) | 8 (15.1) | |
| Age, mean (SD), y | 62.2 (8.35) | 63.4 (7.07) | 62.7 (9.03) | 60.2 (7.80) | .26 |
| Married or partnered, No. (%) | |||||
| No partner | 36 | 7 (19.4) | 21 (58.3) | 8 (22.2) | .79 |
| Married or partnered | 72 | 17 (23.6) | 37 (51.4) | 18 (25.0) | |
| Education, No. (%) | |||||
| Less than 12th grade | 10 | 2 (20.0) | 5 (50.0) | 3 (30.0) | .90 |
| High school graduate | 98 | 22 (22.4) | 53 (54.1) | 23 (23.5) | |
| Employment status, No. (%) | |||||
| Not working | 60 | 16 (26.7) | 33 (55.0) | 11 (18.3) | .21 |
| Working | 48 | 8 (16.7) | 25 (52.1) | 15 (31.3) | |
| Health literacy, mean (SD), N=107 | 61.4 (8.33) | 57.7 (13.66) | 62.2 (6.13) | 62.8 (4.63) | .11 |
| Medication adherence, mean (SD) | 1.8 (1.64) | 1.8 (1.69) | 1.7 (1.66) | 2.1 (1.60) | .54 |
| Chaotic lifestyle, mean (SD), N=107 | 13.4 (4.02) | 13.2 (3.41) | 13.7 (3.90) | 13.1 (4.85) | .77 |
| eGFR, mean (SD), mL/min per 1.73 m2 | 80.7 (20.53) | 74.6 (21.48) | 82.0 (21.39) | 83.6 (17.01) | .29 |
| Poor kidney function (eGFR <60), No. (%) | |||||
| Yes | 17 | 7 (41.2) | 8 (47.1) | 2 (11.8) | .10 |
| No | 91 | 17 (18.7) | 50 (54.9) | 24 (26.4) | |
| Clinic SBP, mean (SD) | 131.1 (17.96) | 129.0 (21.00) | 135.0 (15.91) | 124.4 (17.65) | .02 |
| Clinic DBP, mean (SD) | 74.5 (11.96) | 73.3 (16.42) | 76.3 (10.80) | 71.5 (8.94) | .11 |
| Microalbuminuria (urine albumin ≥30 µg/mL), No. (%) | |||||
| Yes | 53 | 13 (24.5) | 31 (58.5) | 9 (17.0) | .25 |
| No | 52 | 10/52 (19.2), 10 (19.2.0), 16/52 (30.8) | 10/52 (19.2), 26 (50.0), 16/52 (30.8) | 10/52 (19.2), 16 (30.0), 16/2 (30.8) | |
| Body mass index, mean (SD) | 34.3 (5.96) | 34.0 (6.69) | 34.4 (5.83) | 34.3 (5.77) | .90 |
| Heart rate, mean (SD), beats per min | 70.0 (12.59) | 70.8 (13.47) | 70.9 (11.31) | 67.1 (14.45) | .47 |
| Serum glucose, mean (SD), mg/dL | 157.4 (65.05) | 161.4 (65.03) | 158.5 (61.98) | 151.3 (73.50) | .47 |
| Cholesterol, mean (SD), mg/dL | 172.4 (46.57) | 174.9 (43.59) | 175.1 (51.33) | 164.1 (37.83) | .71 |
| Taking antihypertensive medication, No. (%) | 94/98 (95.9) | 24/24 (100.0) | 46/50 (92.0) | 24/24 (100.0) | .17 |
| No. of ABPM readings, mean (SD) | 45.4 (10.61) | 42.7 (11.40) | 45.5 (10.71) | 47.7 (9.38) | .40 |
- a Row percentages are presented for categorical characteristics to compare distribution of nocturnal dipping status between covariate patterns.
- Abbreviations: ABPM, ambulatory blood pressure monitoring; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Overall, 22% (n=24) of participants had reverse nocturnal dipping defined as an SBP decrease <0%. Approximately half (54%, n=58) were nondippers and 24% (n=26) of participants experienced a >10% dip in average SBP (Table 1). Average daytime DBP was higher in black than white participants (76.8 [SD, 7.61] vs 73.8 [SD, 11.20], respectively; P=.04). Similarly, average nocturnal DBPs were higher in black than white participants (71.7 [SD, 9.20] vs 66.6 [SD, 12.27], respectively; P<.01). However, average SBPs did not significantly differ by participants’ race. Men or blacks or participants with low kidney function (eGFR <60 mL/min per 1.73 m2) were less likely to be dippers and more likely to be reverse dippers than women, whites, or those with normal kidney function (P=.01, P=.09, P=.10, respectively; Table 1). Specifically, among men, 31.7% were reverse dippers and 17.5% were dippers. Among women, 8.9% were reverse dippers and 33.3% were dippers. Among black participants, 22.6% were reverse dippers and 15.1% were dippers. Among white participants, 21.8% were reverse dippers and 32.7% were dippers. Among participants with eGFR <60 mL/min per 1.73 m2, 41.2% were reverse dippers and 11.8% were dippers, while participants with eGFR ≥60 mL/min per 1.73 m2, were 18.7% reverse dippers and 26.4% dippers. The mean percent decrease in average SBP (DBP) from wake to sleep was −6.2% (−3.1%), 4.6% (8.3%), and 14.4% (18.1%) (P<.01 for both SBP and DBP; Table 2) for reverse dippers, nondippers, and dippers, respectively.
| All (N=108) | Reverse dipper (<0%) (n=24) | Nondipper (0–<10%) (n=58) | Dipper (≥10%) (n=26) | P value | |
|---|---|---|---|---|---|
| ABPM duration, mean (SD), h | 25.7 (8.39) | 26.0 (10.61) | 25.8 (8.19) | 25.1 (6.63) | .17 |
| No. of ABPM readings, mean (SD) | 45.4 (10.61) | 42.7 (11.40) | 45.5 (10.71) | 47.7 (9.38) | .40 |
| No. of ABPM readings per h, mean (SD) | 1.86 (0.486) | 1.76 (0.529) | 1.85 (0.490) | 1.96 (0.432) | .54 |
| Overall SBP, mean (SD) | 133.9 (12.33) | 135.2 (14.81) | 135.8 (10.63) | 128.5 (12.30) | .04 |
| Waking SBP, mean (SD) | 135.2 (12.43) | 133.6 (15.19) | 137.1 (10.66) | 132.3 (13.09) | .13 |
| Sleeping SBP, mean (SD) | 129.0 (15.39) | 141.7 (14.73) | 130.8 (11.10) | 113.1 (10.46) | <.01 |
| Percent decrease in SBP waking to sleeping, mean (SD) | 4.5 (7.89) | –6.2 (4.52) | 4.6 (3.01) | 14.4 (3.81) | <.01 |
| Overall DBP, mean (SD) | 74.0 (9.57) | 74.9 (13.78) | 74.6 (8.32) | 72.0 (7.37) | .38 |
| Waking DBP, mean (SD) | 75.3 (9.68) | 74.4 (14.06) | 75.9 (8.34) | 74.7 (7.66) | .43 |
| Sleeping DBP, mean (SD) | 69.1 (11.12) | 76.4 (13.10) | 69.7 (9.34) | 61.2 (7.42) | <.01 |
| Percent decrease in DBP waking to sleeping, mean (SD) | 8.1 (9.59) | −3.1 (6.73) | 8.3 (6.21) | 18.1 (6.30) | <.01 |
- Abbreviations: ABPM ambulatory blood pressure monitoring; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table 3 shows the results of the full proportional odds model with all considered factors as main effects. Table 4 shows the final proportional odds model for the evaluation of the predictors associated with nocturnal dipping status. Age, marital status, educational attainment, employment status, health literacy level, self-reported medication adherence, chaotic lifestyle, and kidney function were not selected for inclusion in the final multivariable models predicting dipper status. However, sex, race, eGFR, and the interaction of race and eGFR were chosen. The adjusted common cumulative odds of being in a less favorable dipping category were more than two times higher for blacks than whites (OR, 2.63; 95% CI, 1.19–5.83 [P=.02]) (Table 4). Overall, men had higher odds of being in a higher risk dipping category than women, where the magnitude of the odds depended on eGFR. Among participants with better kidney function (eGFR ≥60 mL/min per 1.73 m2), the estimated odds of being in a higher risk dipping category were higher for men than for women (OR, 2.37; 95% CI, 1.03–5.46). When kidney function was lower (eGFR <60 mL/min per 1.73 m2), the disadvantage of being male was estimated to be much stronger (OR, 24.2; 95% CI, 3.05–192.4), but the precision of this estimate was low. Worse kidney function (eGFR <60 mL/min per 1.73 m2) was also associated with a higher risk dipping category, but only significantly so in men (OR, 9.80; 95% CI, 2.34–40.96].
| Effect | Odds ratioa | 95% CI |
|---|---|---|
| Male vs female sex | 3.85 | 1.47–10.06 |
| Age | 1.01 | 0.96–1.06 |
| Black vs white race | 1.89 | 0.80–4.47 |
| eGFR <60 vs ≥60 mL/min per 1.73 m2 | 3.31 | 0.97–11.25 |
| Married or partnered vs not partnered | 0.51 | 0.18–1.42 |
| High school graduate vs less than a high school education | 3.00 | 0.53–17.02 |
| Working employment status vs not working or retired | 0.49 | 0.21–1.18 |
| Health literacy score | 0.95 | 0.88–1.03 |
| Self-reported medication adherence | 1.10 | 0.46–2.65 |
| Chaotic lifestyle score | 0.90 | 0.89–1.09 |
| Microalbuminuria | 1.32 | 0.56–3.12 |
- a Common odds ratio for reverse dipping vs nondipping/dipping and for reverse dipping/nondipping vs dipping.
- The score test for proportional odds assumption resulted in a P value of .78 and C-statistic of .74.
- Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate.
| Odds ratioa | 95% CI | |
|---|---|---|
| Black vs white race | 2.63 | 1.19–5.83 |
| Interaction of sex and eGFR (P=.04) | ||
| Male vs female sex for eGFR <60 mL/min per 1.73 m2 | 24.21 | 3.05–192.43 |
| Male vs female sex for eGFR ≥60 mL/min per 1.73 m2 | 2.37 | 1.03–5.46 |
| eGFR <60 vs ≥60 mL/min per 1.73 m2 for women | 0.96 | 0.18–5.20 |
| eGFR <60 vs ≥60 mL/min per 1.73 m2 for men | 9.80 | 2.34–40.96 |
- a Odds ratio for reverse dipping vs nondipping/dipping and for reverse dipping/nondipping vs dipping.
- The score test for proportional odds assumption resulted in a P value of .45; Pearson's goodness-of-fit test resulted in a P value of .80 and C-statistic of .70.
- Abbreviation: eGFR, estimated glomerular filtration rate.
Because incremental reduction in BP has health benefits, we also assessed the percent decrease in SBP and DBP from wake to sleep using linear regression. For both average SBP and DBP, participants who were male and/or black had smaller decreases in BP from wake to sleep (Table 5). Similar to the above findings on nocturnal dipping status, blacks, males or lower kidney function had a lower percent of decrease in SBP from wake to sleep than whites, women, or participants with better kidney function. Specifically, on average and compared with white patients, the decrease in SBP from wake to sleep in black patients was three percentage points lower (regression coefficient=−3.32, P=.02). Compared with women, on average, the decrease men experienced in SBP was nearly 4 percentage points lower in SBP from wake to sleep (regression coefficient=−3.98, P<.01). Participants with lower kidney function (eGFR <60 mL/min per 1.73 m2) had a 5 percentage point decrease in SBP from wake to sleep than those with normal kidney function (eGFR ≥60 mL/min per 1.73 m2) (regression coefficient=−5.39, P<.01). These findings are similar to the analysis results for the nocturnal dipping status, except that the interaction between sex and eGFR was not found for the continuous outcome.
| Regression coefficient | Standard error | P value | |
|---|---|---|---|
| Black race vs white race | −3.32 | 1.44 | .02 |
| Male sex vs female sex | −3.98 | 1.42 | <.01 |
| eGFR <60 vs eGFR ≥60 mL/min per 1.73 m2 | −5.39 | 1.98 | <.01 |
| Working employment status vs not working or retired | 2.84 | 1.41 | .05 |
- R2=0.18.
- Abbreviation: eGFR, estimated glomerular filtration rate.
4 DISCUSSION
Among patients with DKD, we observed that black participants were more likely to be reverse dippers and less likely to be dippers than whites. In addition to our primary observations about racial differences in dipping, we noted that there were differences by sex. Men were more likely to be reverse dippers and less likely to be dippers than women. Among complex patients with diabetes mellitus and chronic kidney disease, inadequate nocturnal dipping is associated with a higher risk of organ damage and development of end-stage renal disease.38-41 Our findings suggest that certain subpopulations of patients with DKD, particularly men and black patients, may be more likely to have elevated ambulatory BP that could be overlooked when relying solely on traditional in-office BP measurement. This suggests that ABPM can contribute to more optimal risk stratification during routine clinical care for patients with DKD and elevated cardiovascular disease risk.
Identifying and managing subclinical cardiovascular disease risk is critical to reduce cardiovascular-related morbidity and mortality. The participants in this study were already at elevated cardiovascular risk because they had comorbid hypertension and diabetes mellitus. While our study population was distinctive (eg, patients with diagnosed DKD), our findings support several existing studies demonstrating that black patients are less likely to experience a nocturnal BP dip, thus placing them at increased likelihood of having subclinical cardiovascular disease risk.8, 25, 26, 42, 43 There have been several existing interventions that have successfully improved BP control among minority patients.44, 45 Interventions targeting BP control in black patients may minimize differences in nocturnal BP, and, in turn, improve outcomes in this high-risk group. Our findings also suggest that men with DKD are less likely to experience a nocturnal dip than their female counterparts. We purport that optimally designed interventions should be tailored based on patients’ characteristics including ethnic background, sex, and kidney function, among others.
5 STUDY LIMITATIONS
Our analysis has a few noteworthy limitations. First, our sample size (n=108) was relatively small, which may limit the generalizability of our findings. Second, we used estimated, standard sleep and wake times as opposed to patients’ actual reported sleep and wake times. This leads to less precise interpretation of the data. To be eligible for the STOP-DKD parent trial, patients had to have a diagnosis of hypertension and most were prescribed medication for BP control. We did not have information about dosing generally or during the ABPM period. We also lacked information about related diagnoses, such as orthostatic hypotension or sleep apnea. Third, while we found no association between marital status or employment and dipping status, other studies have suggested that these factors are associated with dipping status and that social support could mediate the association between race and BP dipping.46, 47 Our lack of evidence of social support in our study may be because we measured marital status, as opposed to marital quality. Marital quality and marital satisfaction have been demonstrated to impact ambulatory BP.14, 48 Similarly, we measured employment status as opposed to job strain and job strain is known to increase daytime ambulatory BP.15, 16 Third, enrolling individuals from the same medical center may limit generalizability to nonacademic primary care settings. While race and sex interactions are possible, the study was insufficiently powered to evaluate these potential effects. Despite these limitations, our analysis makes an important contribution to the literature regarding BP dipping among patients with DKD.
6 CONCLUSIONS
Our study is one of the first to assess ABPM among patients with DKD and suggests that solely relying on clinic BP measures misses a subgroup of individuals with diurnal BP patterns that put them at increased risk of cardiovascular disease events.
ACKNOWLEDGMENTS
The research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award No. P30DK096493. Dr Zullig was supported by a VA Health Service Research and Development (HSR&D) Career Development Award (CDA 13-025). Dr Diamantidis was supported by grants funded by the National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK099385& R01-DK093938). Dr Bosworth was supported by a Research Career Scientist award from VA HSR&D (VA HSR&D 08-027). Dr Patel was supported by R01DK093938, R34DK102166, and P30DK096493 prior to joining Gilead Sciences in 2016. The content is solely the responsibility of the authors and does not necessarily reflect the position or policy of Duke University, the US Department of Veterans Affairs, or the US government.
CONFLICTS OF INTEREST
Drs Zullig, Diamantidis, Bosworth, Barnhart, and Pendergast, and Ms Bhapkar and Ms Miller have no conflicts of interest to disclose. Dr Patel is employed by Gilead Sciences.




