Convergent ablation for persistent atrial fibrillation: A UK multicentre perspective
Disclosures: Outside of the submitted work: Nilanka N. Mannakkara is funded by a research grant from Heart Research UK (grant no. RG2701). Felicity De Vere and Nadeev Wijesuriya are in receipt of research funding from British Heart Foundation. Sandra Howell has received educational fellowship funding from EBR systems Inc. Vishal S. Mehta has received educational fellowship funding from Siemens Ltd; Christopher A. Rinaldi has received research funding and/or consultation fees from Abbott, Medtronic, Boston Scientific, Spectranetics and Microport; Christopher Blauth has consulted for New Cardioplegia Solutions and as a proctor for Atricure; Jaswinder S. Gill has received research funding from Abbott and lecture honoraria from Atricure. Other authors: No disclosures.
Abstract
Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide and remains a major cause of morbidity and mortality. Unfortunately, a significant proportion of patients have persistent AF, for which conventional catheter ablation is less effective. However, convergent ablation has emerged in recent years as a hybrid treatment targeting both the epicardium and endocardium in a multidisciplinary joint cardiothoracic and electrophysiology procedure, with promising efficacy outcomes in recent studies. This treatment is increasingly being performed in the United Kingdom. This review article discusses the rationale and evidence behind convergent ablation, along with factors that need to be considered when setting up a successful ablation service.
Abbreviations
-
- AA
-
- atrial arrhythmia
-
- AADs
-
- antiarrhythmic drugs
-
- AF
-
- atrial fibrillation
-
- AOF
-
- atrio-oesophageal fistula
-
- CFAEs
-
- complex fractionated atrial electrograms
-
- CONVERGE
-
- Convergence of Epicardial and Endocardial Ablation for the Treatment of Symptomatic Persistent AF
-
- CTI
-
- cavo-tricuspid isthmus
-
- ECG
-
- electrocardiogram
-
- HF
-
- heart failure
-
- LA
-
- left atrium
-
- LAA
-
- left atrial appendage
-
- LsPsAF
-
- longstanding persistent atrial fibrillation
-
- LVEF
-
- left ventricular ejection fraction
-
- MAE
-
- major adverse event
-
- OAC
-
- oral anticoagulation
-
- PFA
-
- pulsed-field ablation
-
- PsAF
-
- persistent AF
-
- PVI
-
- pulmonary vein isolation
-
- PVs
-
- pulmonary veins
-
- PW
-
- posterior Wall
-
- RCT
-
- randomised controlled trial
-
- SR
-
- sinus rhythm
-
- UK
-
- United Kingdom
-
- US/USA
-
- United States (of America)
1 INTRODUCTION
Atrial fibrillation (AF) is the most common sustained arrhythmia, affecting over 37 million individuals worldwide, and is the subject of an ever-growing epidemic.1-5 United Kingdom (UK) AF prevalence is estimated to be at least 2% in the general population and is greater in high-risk groups such as the elderly.6, 7 It can cause debilitating symptoms, worsen quality of life (QOL),8 and increase the risk of morbidity and mortality.4 AF is a common cause of hospital attendances and places a significant resource burden on healthcare systems.4
Catheter ablation is an established treatment to improve AF-related symptoms. Whilst it is very effective for paroxysmal AF (pAF),9 success rates are worse for persistent AF (PsAF; AF lasting continuously for 7 days or more) and longstanding PsAF (LsPsAF; PsAF lasting 1 year or more), even with multiple procedures.4, 10, 11 Over 70% of those with AF are estimated to have PsAF according to epidemiological data.12 Non-pAF has been associated with a higher risk of stroke and mortality than pAF.13 PsAF is therefore a significant issue but patients who are highly symptomatic often have insufficient or ineffective treatment options. The limited success of conventional catheter ablation in this setting has resulted in the pursuit of newer and alternative approaches.
Convergent ablation integrates aspects of surgical and catheter ablation in a hybrid procedure, aimed at achieving a complete treatment of the posterior wall (PW) of the left atrium (LA) with pulmonary vein isolation (PVI). Initial outcomes were reported in 201014, 15 and the procedure has been performed in the UK since 2012.16 It has emerged as a promising option for the treatment of PsAF.17-19 The aim of the following article is to provide an overview of convergent ablation and to discuss evidence and service considerations as part of a strategy to treat PsAF in the UK.
2 PSAF: CURRENT TREATMENT CHALLENGES
First-line treatment for AF involves pharmacological therapy targeted at rate or rhythm control. Oral anticoagulation (OAC) guided by individual thromboembolic risk, and modification of AF risk factors are also crucial early management steps.4, 20-22
Antiarrhythmic drugs (AADs) may be an unsuitable long-term option for many, either due to intolerability or concerns over long-term side-effects, and some experience persistent symptoms or left ventricular (LV) dysfunction despite adequate rate control.23 Furthermore, given the ongoing persistence of arrhythmia precipitates AF progression, early restoration of sinus rhythm (SR) is often desirable.24-28 AF ablation can be an excellent treatment option and over 9000 AF ablation procedures per year are performed in the UK, with numbers generally increasing year-on-year.29
PVI via catheter ablation has been shown to be highly effective for pAF in multiple randomised controlled trials (RCTs), with single procedure success rates above 70% in preventing arrhythmia recurrence 1-year postprocedure, and further improvements possible with the addition of a repeat procedure.26, 30-32
However, though still superior to AADs,33 catheter ablation is less successful in PsAF than in pAF, often necessitating continuation of medical therapy or repeat ablation.31 In the Hamburg sequential ablation study, 202 patients with PsAF underwent PVI, with ablation of additional targets performed during this index procedure if there was acute non-response to PVI. At 5-years, success rate was only 20% with a single procedure and 45% with multiple procedures (including AADs). Success rates were better in patients with PsAF for less than 2 years compared to those with longer PsAF duration (76.5% vs. 42.2%).34 The STOP PsAF Trial was a single-arm multicentre trial of 165 patients with PsAF for less than 6 months, who underwent PVI using cryoablation. At 12 months, freedom from atrial arrhythmia (AA) was 54.8% and the adverse event rate was 0.6%.35 A meta-analysis of 113 studies involving over 18 000 patients with PsAF receiving catheter ablation for PsAF found efficacy to be 43% with a single procedure, and 69% with multiple procedures.11 Therefore, whilst PsAF ablation has had some success in these studies, results have been highly variable and generally disappointing. They suggest that though PsAF catheter ablation may be a good option for some, likely those with less advanced PsAF, it cannot be recommended universally.
The pathophysiology and arrhythmogenic substrate are more complex in PsAF. AF drivers often exist outside the pulmonary veins (PVs) and so are not addressed by PVI. Chronic persistence of arrhythmia over time induces structural and electrophysiological atrial remodelling, priming the atria for arrhythmia.36 The LA PW is the site for many AF drivers, which include rotors, re-entry circuits, focal ectopic triggers and complex fractionated atrial electrograms (CFAEs). PVI alone is therefore often insufficient to treat PsAF.
However, performing additional substrate modification via catheter ablation does not clearly provide an incremental benefit above PVI alone. Despite some encouraging results, there is a tendency for efficacy to wane over time and other studies have been negative.37-39 The STAR-AF-II trial randomised 589 PsAF patients to PVI alone, PVI with CFAE ablation or PVI and additional roof and mitral linear ablation. Eighteen-month freedom from AF recurrence was 59% but there was no benefit when additional CFAE ablation (49%) or linear ablation (46%) was added.37 1 incidence of AOF was noted resulting in mortality in the PVI with CFAE ablation arm.
In the recent CAPLA trial, 338 patients undergoing first-time ablation for PsAF were randomised to receive either PVI alone or PVI with the addition of empiric PW isolation.40 Patients with a baseline AF duration over 3 years were excluded. Ablation involved wide antral circumferential PVI, with the addition of a roof and floor ablation line in those receiving PW isolation. Freedom from AA at 12 months was not significantly different whether receiving PVI alone (58.2%) or with the addition of PW isolation (60.1%, HR 1.10, p = .57). AF burden was also similar. A strategy of empirical endocardial PW isolation is therefore not supported by these findings.
The success of additional endocardial ablation lines is limited as it is difficult to produce durable PW lesions, and incomplete lesions may provide further iatrogenic substrate for re-entrant arrhythmia.41 Even in the case of effective endocardial PW isolation, transmural ablation may be difficult to achieve resulting in epi-endo breakthrough, which may explain the lack of efficacy in PsAF catheter ablation studies.40 Furthermore, performing extensive endocardial PW ablation increases the risk of complications and damage to nearby organs.41
Surgical ablation, via the Cox-Maze procedure, involves creating a series of ablation lesions in both atria, including the posterior LA, PVs and left atrial appendage (LAA) to block macro re-entrant circuits and prevent the propagation of AF.42 Though effective, it is more invasive than catheter ablation, often requiring median sternotomy or thoracotomy incision, cardiopulmonary bypass, and single-lung ventilation. It is therefore usually reserved for selected cases or those undergoing concomitant cardiac surgery.4, 43, 44 Minimally invasive alternatives have been developed, including purely thoracoscopic approaches,45-47 and hybrid epicardial-endocardial approaches,48, 49 including convergent ablation which is the least invasive of these hybrid techniques.14, 17, 50
3 THE CONVERGENT ABLATION PROCEDURE
Convergent ablation is a hybrid procedure that integrates minimally invasive surgical epicardial ablation with catheter ablation to harness the advantages and minimise the limitations of each approach when performed on their own.
When initially conceived, Kiser et al. designed a transdiaphragmatic pericardioscopic cannula to provide completely endoscopic access to the epicardial surface of the posterior heart, allowing direct visualisation of cardiac structures and ablation on the beating heart without the need to open the chest.51, 52 Originally, this was combined with right thoracoscopy to access the epicardium and perform bi-atrial ablation in a strategy derived from a maze lesion set. This was developed into a combined multidisciplinary epicardial-endocardial procedure, in which transdiaphragmatic epicardial ablation to perform near complete PVI and posterior box ablation was followed by percutaneous endocardial catheter ablation which included PVI, coronary sinus and cavo-tricuspid isthmus (CTI) ablation. The procedure has evolved through several iterations over the years with refinements to the access, strategy, equipment, and lesion set.15
Full procedure steps for the current iteration of the procedure have been described extensively elsewhere.53 However, in brief, in the first stage (epicardial ablation), the cardiac surgeon makes a 2-3 cm incision in the subxiphoid area, through which a cannula is inserted into the pericardial space (see Figure 1), allowing a closed-chest endoscopic (“pericardioscopic”) approach. The Epi-Sense coagulation catheter (AtriCure Inc.) is inserted via this cannula and allows direct visualisation and epicardial sensing of the posterior LA. It is used to deliver sequential parallel vertical ablation lines to homogenise the posterior LA wall and perform partial PVI. This is performed under general anaesthetic with no cardiopulmonary bypass, taking approximately 2 h. Access to the anterior PVs and the most upper aspect of the atrium can be somewhat limited by the pericardial attachments and these areas can therefore be addressed during the endocardial stage. The second stage (endocardial ablation) takes place either immediately afterwards or is staged approximately 6−12 weeks later. The electrophysiologist completes PVI and usually undertakes electro-anatomical mapping to assess completeness of PW isolation and perform any additional ablation lesions deemed necessary (see Figure 1), though a cryoablation strategy has also been used successfully.54 Patients can usually be discharged 24−48 h postprocedure.
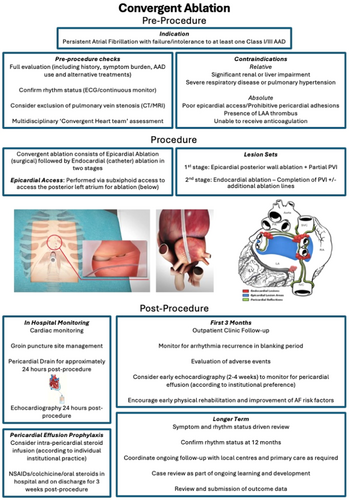
Convergent ablation has many potential advantages over catheter ablation. It facilitates durable transmural PW ablation, which can be difficult to achieve with a solely endocardial approach that can be limited by the proximity of the PW to the oesophagus and risk of atrio-oesophageal fistula (AOF). In convergent ablation, epicardial ablation radiofrequency (RF) energy is directed away from the oesophagus, allowing extensive PW ablation whilst minimising risk of oesophageal or phrenic nerve injury. The addition of epicardial ablation means that less extensive endocardial PW ablation is required during the catheter ablation stage. Usually only PVI and sometimes a PW touch-up, with or without a CTI line, are required rather than extensive endocardial PW ablation.16 Epicardial ablation also disrupts epicardial fat and ganglionic plexi that may be important contributors to sustaining PsAF.55, 56
Compared to surgical ablation, convergent ablation is less invasive, and allows detailed electro-anatomical mapping to help validate surgical ablation lesions. It is though more invasive than catheter ablation, requires additional preoperative risk assessment, a potentially longer hospital stay and additional logistical arrangements to coordinate two procedure stages.
Convergent ablation can be performed in a single-sitting or staged approach. In a single-sitting or “simultaneous” procedure, epicardial ablation is immediately followed by endocardial ablation under the same general anaesthetic. Alternatively, in a staged approach, the patient is woken and recovered after the epicardial stage, and then is re-admitted approximately 6−12 weeks later for catheter ablation. The merits and disadvantages of each approach are shown in Table 1. There has not been a definitive comparison to determine if either is better. In a meta-analysis of 16 hybrid AF ablation studies, including over 1200 patients undergoing convergent or other forms of hybrid ablation, there was a trend towards higher freedom from arrhythmia with a staged approach (81.6% vs. 68.2%, p = .19), which tallies with the general consensus that this may be the favourable approach, however this was not statistically significant.57
| Same-sitting | Staged | |
|---|---|---|
| Details |
|
|
| Advantages |
|
|
| Disadvantages |
|
|
4 STUDIES OF CONVERGENT ABLATION
Kiser et al. reported the results of an initial cohort of 28 patients in 2010, with 76% free from AF and off AADs at 6 months. There were no deaths, but two patients required percutaneous drainage for symptomatic pericardial effusion and one experience phrenic nerve injury.14 A follow-up of this analysis with an expanded cohort of 65 patients found 88% of the 42 patients with available rhythm data (Holter or electrocardiogram [ECG]) at 12 months to be in SR and 83% in SR and off AADs.50 This was compared to cohorts of patients undergoing either concomitant open chest maze (80% in SR) or pericardioscopic/thoracoscopic maze (57% in SR) only and found to have superior success rates. Three patients (4.6%) died post-convergent procedure, one attributed to torsades de pointes potentially related to use of dofelitide, and 2 (3.1%) following the development of AOFs.
Several subsequent observational and single-centre analyses have evaluated convergent ablation (see Table 2). An early study of 27 patients in Europe who had a mean AF duration of 3.5 years found that 6 months after convergent ablation, 72% were in SR and 67% in SR and off AADs, with four patients in AF and one patient in atrial flutter. Of those returning to follow up to 2 years, success rates remained good though numbers were small (6 out of 6 patients in SR), and those with reduced LVEF (≤35%) experienced improvement in LVEF postablation.66
| Study | Study design | Population | Mean AF duration | Mean LA diameter | Single-sitting or staged | Follow-up outcome | Free from AF | Free from AA | Complications | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| Kiser et al.50 | Observational | n = 65 | 4.8 years | 5.2 cm | Single-sitting | 12 months | - | 82% | Major: 4.6% Overall: 7.7% | 4.6% |
| pAF (8%), PsAF (18%), LsPsAF (74%) | ||||||||||
| Gehi et al.58 | Observational | n = 101 | 5.9 years | 5.1 cm | Single-sitting | 12 months | - | 66.3% | Major: 5.9% Overall: 5.9% | 2% |
| pAF (17%), PsAF (47%), LsPsAF (37%) | ||||||||||
| Civello et al.59 | Observational | n = 104 | 5.2 years | 4.1 cm | Single-sitting | 12 months | - | 87.5% | Major: 2.9% Overall: 2.9% | 0% |
| pAF (27%), PsAF/LsPsAF (73%) | ||||||||||
| Gersak et al.60 | Observational | n = 76 | 5.2 years | 4.7 cm | Single-sitting or staged | 48 months | - | 72% | Major: 5.2% Overall: 12% | 2.6% |
| pAF (5%), PsAF (16%), LsPsAF (79%) | ||||||||||
| Zembala et al.61 | Prospective | n = 90 | 4.5 years | 4.5 cm | Staged | 12 months | - | 86% | Major: 5.6% Overall:8.9% | 1.1% |
| PsAF (43%), LsPsAF (57%) | ||||||||||
| Kress et al.62 | Propensity-matched Cohort Study* | n = 64 | 1 year | 4.8 cm | Single-sitting | 16 months** | 72% | 63.5% | Major: 6.3% Overall: 7.8% | 1.6% |
| PsAF/LsPsAF (100%) | ||||||||||
| Gulkarov et al.63 | Observational | n = 31 | 2 years | 4.3 cm | Single-sitting | 24 months | 71% | 52% | Major: 12.9% Overall: 12.9% | 0% |
| PsAF (52%), LsPsAF (48%) | ||||||||||
| Tonks et al.64 | Observational | n = 36 | 3.9 years | LA size > 4 cm in 58% | Single-sitting or Staged | 12 months | - | 77.3% | Major: 2.8% Overall: 16.7% | 0% |
| pAF (11%), PsAF/LsPsAF (89%) | ||||||||||
| Maclean et al.18 | Propensity-matched Cohort Study* | n = 43 | 3 years | 4.7 cm | Staged | 31 months** | - | 58.1%*** | Major: 7.0% Overall: 11.6% | 0% |
| LsPsAF (100%) | ||||||||||
| Larson et al.19 | Observational | n = 113 | 5.1 years | 4.8 cm | Single-sitting | 12 months | - | 53% | Major: 5.3% Overall: 14% | 0% |
| pAF (12%), PsAF/LsPsAF (88%) | ||||||||||
| DeLurgio et al.17 | RCT* | n = 102 | 4.4 years | 4.4 cm | Single-sitting | 12 months | 71% | 67.7% | Major: 2% Overall: 7.8% | 0% |
| PsAF/LsPsAF (100%) | ||||||||||
| Mannakkara et al.16 | Observational | n = 67 | 3.2 years | 4.6 cm | Single-sitting or Staged | 28 months** | 61.5% | 44.6% | Major: 3% Overall: 14.9% | 0% |
| PsAF (19%), LsPsAF (81%) | ||||||||||
| Carpenter et al.65 | Observational | n = 24 | AF duration 1-2 years (25%)/ > 2 years (75%) | LA vol ≥ 79 mls (severe) in 71%) | Staged | 36 months | 61% | - | Major: 0% Overall: 20.8% | 0% |
- Note: *Data refers to those in study undergoing convergent ablation (excludes comparator group who underwent endocardial catheter ablation only); **Denotes mean/median follow-up duration; *** Allowing for multiple procedures. In general, complications and mortality defined as that within 30 days of procedure. Overall complications include minor complications such as wound infection, mild acute kidney injury and pericardial effusion without tamponade. Major complications in this table defined as stroke/transient ischaemic attack, atrio-oesophageal fistula, pericardial tamponade, major bleeding, pulmonary vein stenosis, groin complication requiring surgery, emergency surgery and mortality. Freedom from AF/AA in this table refers to freedom from arrhythmia irrespective of antiarrhythmic drug use. Follow-up duration is that pertaining to outcomes listed in table.
- Abbreviations: AA, atrial arrhythmia; AF, atrial fibrillation, LA, left atrium, LsPsAF, longstanding persistent AF; PsAF, persistent AF; RCT, randomised controlled trial; UK, United Kingdom; USA, United States of America.
Gersak et al. reported longer-term outcomes in 76 patients, the majority of whom had LsPsAF (79%).60 At 1 year, 85% were in SR, allowing for repeat procedures (77% in SR with no interventions). 30-day complication rate was 11.8%, which included two AOFs, one thromboembolic event, one pericardial effusion requiring pericardiocentesis and one exploratory laparotomy for investigation of abdominal bleeding. Two AOFs occurred during the first 20 cases and safety measures were then implemented with no significant oesophageal injury noted thereon. At 4 years, 81% of 36 evaluable patients were in SR, allowing for repeat procedures, and 72% in SR without repeat interventions. Implanted loop recorder analysis in 28 patients at 4 years, 79% had an AF burden less than 10%, and 61% had AF burden ≤ 0.5%.
Another study retrospectively compared 133 patients who had been treated with either convergent ablation or catheter ablation, finding that the convergent ablation group were less likely to have arrhythmia recurrence (37% vs. 58%, p = .01) or repeat ablation (9% vs 26%, p = .01) after a median follow-up of 16 months.62
A study of 43 LsPsAF patients treated by convergent ablation in a UK centre between 2013 and 2018 found AF-free survival at 12-months to be 60.5% on AADs and 37.2% off AADs.18 Patients were propensity matched to a group undergoing catheter ablation alone and had significantly higher AF-free survival on and off AADs. Over entire follow-up (mean 30.5 months), convergent ablation was associated with significantly higher arrhythmia-free survival (58.1% vs. 30.2% on AADs; 32.5% vs. 11.6% off AADs), allowing for multiple procedures, and was a predictor of freedom from arrhythmia. Patients undergoing convergent ablation had a numerically higher incidence of new atrial tachycardia postprocedure (32.6% vs. 13.9%) and of complications (11.6% vs. 2.3%) though these did not reach statistical significance.
A further UK single-centre study of 67 patients undergoing convergent ablation between 2012 and 2019 found freedom from AF on/off AADs to be 81.3% and freedom from any AA to be 69.2% after 12 months.16 Over a mean follow-up of 2.8 years, overall freedom from AF was 61.5% and freedom from any AA was 44.6%. 30.8% experienced an atrial tachycardia/flutter post-blanking period.
In a study of 113 patients undergoing convergent ablation at a US centre, 81% of patients underwent continuous rhythm monitoring with loop recorder or implanted cardiac device.19 This contrasts with most studies that have used less extensive rhythm monitoring. 88% had PsAF or LsPsAF, 31% had impaired LVEF, 62% had moderate or severe LA enlargement and mean AF duration was 5.1 years. Overall arrhythmia-free recurrence was 53% at 12 months. Amongst those with AA recurrence the mean arrhythmia burden was low (<5%) and 94% of patients were free from an arrhythmia burden of >5%. These results are encouraging as due to comprehensive rhythm monitoring, potential under-detection of arrhythmia recurrence was minimised. Most patients with arrhythmia post-convergent ablation had a low burden, suggesting they still may benefit from treatment.
The LAA contributes to arrhythmogenesis in recurrent AF and to thrombogenic risk.67, 68 Epicardial LAA closure may reduce stroke risk.69 The LAAOS-III study found that in 4770 patients with AF undergoing open heart surgery randomised to LAA exclusion or no LAA exclusion (most patients in both groups remained on OAC), exclusion was associated with a reduction in ischaemic stroke or systemic embolism.70 Addition of LAA exclusion may also reduce recurrent arrhythmias,71 however results are conflicting, and its exact utility for this purpose is unclear. A recent RCT found that addition of LAA exclusion to PVI in nonparoysmal AF did not improve arrhythmia outcomes.72
LAA exclusion can be added on to the first stage of convergent ablation, through use of the Atriclip device (AtriCure Inc.), utilising additional thoracoscopic access, or Lariat device (SentreHeart) that can be performed via the same subxiphoid access.73, 74 Gegechkori et al. reported a retrospective cohort of 139 patients who underwent convergent ablation for PsAF from 2014 to 2019, with those treated from 2016 (64 patients) undergoing concurrent LAA exclusion using the Atriclip device.73 There was a trend towards numerically higher freedom from AA in those with LAA exclusion compared to those without (88% vs. 76%), and freedom from AA off AADs was significantly higher in those undergoing LAA exclusion (77% vs. 58%). Augmentation with LAA exclusion may provide further benefits over and above Convergent ablation alone and is performed in some centres. The benefits of an additional procedure stage must be balanced against the potential risks. Larger, longer-term studies are required to explore this, especially in the convergent ablation setting.
Over time the procedure has been adapted since its initial iteration to improve efficacy and safety.15 The earlier epicardial ablation technique has since been simplified by changes to the catheter configuration and a switch to linear sequential lesions to homogenise the PW and only posterior PVI, rather than the previous strategy of creating a posterior box akin to a modified maze procedure alongside more extensive PVI.15 Measures to reduce oesophageal complications have also been widely instituted in response to early incidences of AOF.15, 60 These include the routine use of oesophageal temperature monitoring, saline-irrigation during epicardial ablation, directing epicardial ablation only towards the PW and minimising endocardial PW ablation. In 2016, there was a transition from transabdominal access to a subxiphoid approach to further enhance safety.15 The Larson et al. study of 113 patients undergoing convergent ablation between 2015 and 2018 found the switch to subxiphoid access dramatically reduced the rate of peri-procedural complications from 23% to 3.8%.19
The subsequent CONVERGE trial was an international multicentre RCT of 153 patients with PsAF randomised 2:1 to convergent ablation or endocardial catheter ablation alone. The primary endpoints were freedom from AA at 12 months minus new or increased dosage of AADs and 30-day major adverse event (MAE) rate. The trial had wide inclusion criteria, including patients with a left atrial diameter up to 6 cm and without limitation on baseline AF duration. 42% had LsPsAF. Exclusion criteria included previous catheter ablation, LVEF < 40% and implantable cardioverter defibrillator devices.53
During the catheter ablation stage, all patients had PVI and 96% had CTI ablation. Additional ablation lesions such as rooflines or CFAE were permitted in the catheter ablation arm at operator discretion, but patients did not undergo comprehensive PW ablation. In the convergent ablation group, 12.7% required additional touch-ups above PVI during the endocardial ablation stage to treat gaps in isolation due to the limitations of the pericardial reflections. LAA exclusion was not performed.
Freedom from AA at 12 months was significantly higher in the convergent ablation group (67.7% vs. 50% on/off AADs and 53.5% vs. 32% off AADs).17 Additionally, at 18 months assessment of AF burden using 7-day ECG monitoring found AF burden reduction greater than 90% in 74% of convergent ablation patients and 55% of catheter ablation patients. There were no deaths or AOFs, however 30-day MAE rate was 7.8% in the convergent ablation arm, and 0% in the catheter ablation arm. MAEs included one stroke, one transient ischaemic attack, one excessive bleeding event, one phrenic nerve injury and three pericardial effusions requiring nonemergency pericardiocentesis; all but one of these were reported to have subsequently resolved. A post hoc analysis found that specifically in LsPsAF patients, achievement of the 12-month primary effectiveness outcome was significantly greater with convergent ablation than catheter ablation (65.8% vs. 37.0%, p = .02).75
This was the first multicentre RCT to compare convergent ablation for PsAF to catheter ablation and the procedure was performed using the latest iteration of the technique. The results are especially useful given the inclusion of a challenging cohort with prolonged AF durations and large LA diameters, as this is reflective of the complex patients considered for PsAF ablation with unfavourable factors for procedural success. Though it is important to consider the risks of the procedure, the trial indicated that convergent ablation is a safe and effective treatment, especially in LsPsAF.
In summary, initial studies of convergent ablation, which were predominantly single-centre, single-treatment observational studies, found good short and medium-term efficacy outcomes after convergent ablation, though some showed a high risk of complications (8−12%), especially early on18, 50, 66 (see Table 2). Studies that involved cohort matching suggested that convergent ablation was superior to either epicardial ablation or catheter ablation alone 18, 50 and a meta-analysis of four studies comparing convergent to catheter ablation found convergent ablation was associated with a significantly higher freedom from AF but an increased rate of complications.76 Given that this is a novel procedure, some of these earlier analyses spanned a period in which the procedure has evolved over time and therefore there is some heterogeneity within the cohorts. The procedure has been adapted based on early experiences with results suggesting that these improvements have simplified the procedure and improved its safety profile.15
Later studies, especially the CONVERGE RCT have found good success rates in effectively reducing arrhythmia recurrence, reducing arrhythmia burden, and improving QOL compared to catheter ablation with acceptable safety outcomes especially in those with LsPsAF.17, 19, 75 Some studies have suggested that patients undergoing convergent ablation may have a high requirement for post-procedural blanking period DCCV and notable incidence of atrial tachycardias, which may though be more amenable to repeat catheter ablation than AF recurrences.16, 18 Observational data has included a wide range of patients in a real-world setting, however certain comorbidities such as a history of previous ablation or heart failure were excluded from the CONVERGE study. Therefore, further evaluation of such patients, in addition to further longer-term outcomes would be of much value.
5 PATIENT SELECTION
Convergent ablation is a multidisciplinary procedure and therefore decisions on patient selection should be undertaken as such, involving electrophysiologists, surgeons, anaesthetists, specialist nurses and physiologists in the assessment, work-up and strategy. This will usually take the form of a specialist ‘Convergent team’ or ‘Heart team’, which will review referrals. This team encompasses the necessary expertise to evaluate efficacy and safety considerations to determine whether patients would be suitable candidates for convergent ablation or should consider alternative management such as conventional catheter ablation or medical management.4, 77, 78 A comprehensive review of the patient's arrhythmia history, procedure history, comorbidities, current symptoms, limitations and QOL are essential.
Symptomatic patients with PsAF who have failed or are intolerant to AADs, and who are estimated to have a low probability of success from catheter ablation treatment should be considered for convergent ablation (see Figure 1). European Society of Cardiology (ESC) guidelines provide a Class II recommendation for hybrid ablation to be considered in the case of symptomatic AF refractory to medical therapy and with failed catheter ablation and/or risk factors for catheter failure.4 However, these predate the results of the CONVERGE trial and there is no recommendation specific to convergent ablation. Those with prolonged baseline AF duration, large LA or raised body mass index who may be considered to have unfavourable factors for catheter ablation may benefit from convergent ablation. The CONVERGE trial permitted patients with LA diameter up to 6.0 cm and did not place limits on baseline AF duration, with an average of 4.4 years and 42% patients with LsPsAF. Average BMI was 33.
Data on patients with LVEF < 40% is limited and whilst this area requires further study, reduced LVEF is not a specific contraindication for treatment. Such patients have been included in observational studies and treatment has been associated with subsequent improvement of LVEF.16, 66 Additionally, patients with previous ablation were not included in CONVERGE, but there is no specific reason they should be unsuitable. In an observational study of 108 patients undergoing convergent ablation, those who had undergone previous ablation had a lower requirement for PVI during the endocardial stage, and similar freedom from arrhythmia compared to those with no previous ablation.79
Certain characteristics will represent relative or absolute contraindications53, 80 (see Figure 1). Patients who have a high risk of significant pericardial adhesions or who may have difficult epicardial access may not be suitable for the procedure, which particularly may affect those who have undergone previous cardiac surgery. Patients with severe renal or liver impairment may be unsuitable due to increased surgical risk and have a relative contraindication. The presence of severe respiratory disease or pulmonary hypertension should prompt specialised assessment in conjunction with the anaesthetist to determine suitability. Those with recent myocardial infarction, stroke or active infection should be deferred until recovered. Patients who are unable to receive anticoagulation, or who have LAA thrombus will have an absolute contraindication for the procedure.
6 SETTING UP A CONVERGENT ABLATION SERVICE
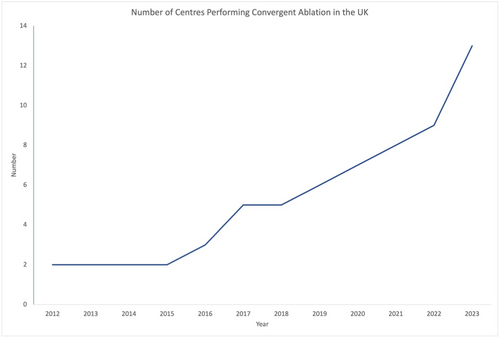
Since 2012 over 500 convergent ablation procedures have now been performed in the UK. Over time more centres have begun to perform convergent ablation, with a marked increase in recent years (see Figure 2). The adoption of any new procedure comes with challenges, and it is important that adequate training, clinical pathways, and collaboration networks are implemented to facilitate the uptake of this procedure safely and effectively. Some lessons from the UK experience are described below.
6.1 Referral pathways
Convergent ablation centres in the UK are regional tertiary referral centres with on-site specialist cardiac electrophysiology catheter lab and cardiothoracic surgery facilities. Patients with AF may be referred via primary or secondary care for further management in an arrhythmia clinic, which will usually be located at a local or regional hospital. Patients suitable for consideration of convergent ablation will be referred on by their cardiologist for review at a tertiary centre performing convergent ablation. This utilises the existing structure that operates for complex electrophysiology procedures such as AF ablation, and cardiac surgery.
The referral model is arranged regionally, in which specialist regional centres are affiliated with surrounding local and secondary care hospitals to provide extensive coverage and access to specialist procedures.78, 81 This is managed via regional cardiac networks with many clinicians working cross-site, spending time at both local and tertiary hospitals, providing a link for local patients and facilitating collaborative care across the network.78
6.2 Staffing and infrastructure
Setting up a convergent ablation service requires detailed planning. Considerations include staff, infrastructure, adequate referral pathways, barriers to treatment, education, and follow-up. Staff requirements include a dedicated cardiac electrophysiologist, cardiac surgeon, anaesthetist, and multidisciplinary teams (MDT) including cardiac surgery teams, arrhythmia specialist nurses and cardiac physiologists as part of a specialist “Convergent Heart Team” or “Complex AF Team.” The MDT should together determine a local protocol to co-ordinate the organisation of procedure stages, pre-assessment, and periprocedural medication management. Patient education with respect to risks, benefits, procedure details, periprocedural instructions and postprocedural management is crucial. This collaborative model leverages the individual expertise of the cardiac electrophysiology and cardiac surgery teams to enhance arrhythmia awareness and management, and postoperative safety. Developing a close working relationship between electrophysiologists and surgeons is not only essential to the successful establishment of a hybrid AF programme but also helps to emphasise the importance of AF more generally throughout the multidisciplinary team. Our experience suggests that this collaborative approach further strengthens the Complex AF team and helps to increase awareness and develop structured assessment pathways to guide the use of concomitant surgical AF ablations during non-AF cardiac surgery where indicated.
The required infrastructure includes a dedicated cardiac catheter lab set up for specialist electrophysiology procedures, including the use of electro-anatomical mapping, and the facility to perform cardiac surgical procedures either in theatre, a hybrid lab, or the cardiac catheter lab. Whilst the procedure is performed with closed chest and off-pump, it is necessary to have facilities available in case of need for emergency sternotomy, including an available perfusionist. Postprocedure monitoring will require a ward and staff set up to manage pericardial drains and the ability to step up to a level 1 or 2 bed if needed. Logistical arrangements need to be considered so that there is adequate coordination between theatres and catheter lab to perform the procedure on the same day when desired, or to coordinate the waiting lists for the appropriate timing of each stage of the procedure when performed separately.
Specialist centres performing cardiac surgery or complex electrophysiology procedures will likely already have much of the necessary infrastructure and regional clinical networks to receive referrals from local centres.78 It is important to disseminate information on convergent ablation to local centres and within the specialist centre, including on appropriate selection criteria to aid those who are less familiar with the treatment to identify those who may and may not be suitable for referral. This is supplemented by a case review of referrals by the Convergent Heart Team in the specialist centre to determine suitability, and clear communication with the referring centre.
Good communication is essential to the success of a programme. This enables high quality care of patients from disparate geographical locations, who may have different aspects of their overall healthcare fragmented between different sites. Another lesson from UK experience is the importance of ongoing communication between the electrophysiologist and surgeon throughout the patient journey. Resource considerations and capacity pressures have often necessitated performing this procedure in a staged approach, but that this can work well especially when the electrophysiologist has a clear idea of the prior surgical ablation to help plan the endocardial ablation approach, and the surgeon receives feedback on electro-anatomical mapping.
6.3 Postprocedure follow-up
It is important to set up appropriate postprocedure follow-up pathways so that any problems can be detected early (see Figure 1). Incidences of postprocedure pericardial effusions in early experiences have prompted many institutions to adopt policies to protect against this, combining routine post-surgical pericardial drain and prophylactic use of steroids or colchicine with enhanced monitoring such as early outpatient echocardiography (within 1 month).15, 16 Some patients may require arrhythmia management or early cardioversions in the blanking period16 and therefore close liaison with electrophysiology follow-up, via specialist arrhythmia nurses, can help greatly and facilitate advice, troubleshooting, assessments and expedited review in the case of any issues. These checks usually take place at the specialist centre, but it is important that local hospitals are aware of these needs and the potential issues that may arise in the case of patients not being able to reach their specialist centre when in difficulty. Follow-up will usually continue at the specialist centre for at least the first year before handing back to the local centre, though this will vary according to the patient and area.
6.4 Challenges and economic considerations
Potential barriers to setting up a service include the initial lack of awareness and scepticism of newer treatment modalities amongst physicians, surgeons, and other staff. Additionally, the funding, resources and business case for this service needs to be determined on a local basis. In some cases, this will not be suitable for the individual centre.
When considering this treatment in a UK-specific context, cost-effectiveness is particularly important given that most healthcare is delivered via the National Health Service (NHS), a large-scale, publicly funded universal healthcare system. This is centrally funded, however many strategic management decisions on resource allocation are made at a regional and localised level. This means that overall resources need to be balanced and prioritised according to the needs of the population on a local, regional and nationwide scale.
Despite the potential downstream long-term benefits and savings that reduction of AF burden can bring such as reduced hospital attendances, hospitalisations and reduced need for AADs, the higher upfront cost of convergent ablation compared to conventional ablation and medications needs to be weighed up when determining the use of periodic budgets. Optimal patient selection is therefore especially pertinent in the UK-healthcare system, not only for the patient's benefit, but also to allocate resources for this treatment towards those who would most benefit. NHS England take an equivocal stance on the utility and cost-effectiveness of multiple repeat AF ablation procedures.82 Convergent ablation as a repeat procedure after a previous failed attempt may potentially be more successful than conventional ablation and shift this balance, however conversely there is a higher upfront cost to factor in and more specific data to address this scenario would help to assess the cost-effectiveness of repeat ablations in such a healthcare system.
When setting up a service, deploying new treatments requires costs of equipment, training, infrastructure, facilities, staff and time and this can potentially divert staff and facilities such as high-level care beds away from other cardiac and non-cardiac conditions, which may limit expansion in some regions according to the local population needs and the circumstances of an individual centre. Further, an uneven expansion of a novel treatment can potentially exacerbate geographical disparities in access to treatment, however the establishment of regional cardiac networks helps to mitigate against this and improve UK-wide coverage.
A US cost-effectiveness analysis based on a Markov simulation model has compared convergent ablation to catheter ablation and medical therapy in three cohorts categorised according to risk of AF-related complications including stroke, cardiovascular hospitalisation, heart failure and bleeding.83 Though limited by the use of only observational data and simplifying assumptions required for the model, it found that a 5-year period convergent ablation was cost-effective compared to catheter ablation, especially in medium and high-risk patients. Despite a higher upfront cost, this cost-effectiveness was driven by the reduced need for repeat procedures, reduced AF-related complications and improved QOL scores compared to catheter ablation. To the authors' knowledge,a formal cost-effectiveness analysis of convergent ablation specifically within the UK has not been published. However, based on these findings, such cost-effectiveness benefits are likely to also apply elsewhere.
6.5 Training and quality assurance
Any new procedure requires appropriate training and monitoring. There is an inevitable learning curve with new techniques or treatments. Collaboration with other teams and centres experienced in this approach and with industry, including to share ideas and best practices, is beneficial. This helps to set up the appropriate training, proctoring, and mentoring for the initial cases. It also helps with anticipation of potential issues and dealing with any postprocedural complications. Patients may not be familiar with this new treatment. It is important to communicate clearly with patients throughout when they are considering the procedure so that they are aware of the benefits and risks, evidence, likelihood of efficacy and complications, procedure details, relative experience of the centre and available alternatives to help them to make an informed choice that is in their best interests.
Engagement with research, audit and regular multidisciplinary case review is essential to learn from experience and continually improve processes and outcomes. Outcome data is routinely reviewed both internally and submitted to national bodies, such as National Institute for Cardiovascular Outcomes Research (NICOR) 29 as per well-established practices in cardiac surgery and interventional electrophysiology.
7 THE FUTURE OF CONVERGENT ABLATION
There is now over a decade of experience behind Convergent ablation internationally and in the United Kingdom. The studies performed to date have shown its potential for use in the management of PsAF. Since the initial analyses, success and safety have improved as the procedure has evolved,15 and it is hoped that outcomes may improve further as techniques are refined and as experience grows. The results of the CONVERGE trial17 provide an encouraging foundation for this treatment and the ongoing CONVERGE-PAS (post-approval study) will provide longer-term efficacy and safety outcomes in a wider variety of centres.84
There remain further questions to be addressed with future studies. Studies to date have broadly reported short or medium-term follow-up. Some studies have reported follow-up durations up to 3−4 years.60, 65 However longer-term follow-up to determine the ongoing freedom from arrhythmia and burden reduction would aid decision making when considering this procedure. Further data on specific patient groups such as those with reduced LVEF and previous ablation would help to inform patient selection, risk stratification and planning ablation strategy. To supplement existing clinical data and single-centre analyses, a large-scale multicentre registry akin to that used in other highly specialised electrophysiology procedures may also be of benefit.
Catheter ablation is also continually evolving. The development of techniques such as pulsed-field ablation (PFA), high power short duration (HPSD) ablation and oesophageal cooling devices offer promise in improving outcomes of catheter ablation alone for PsAF. Dedicated oesophageal cooling devices appear to reduce oesophageal injury and therefore improve the safety of PW ablation.85, 86 PFA has had promising early findings, however its use in non-PVI settings, such as to perform PW ablation, has not yet been fully developed or extensively evaluated.87 HPSD may reduce procedural and fluoroscopy times in PsAF ablation,88 yet further studies are required to determine if efficacy is superior to standard ablation. Whilst PFA and HPSD may be useful, their efficacy in achieving transmural PW ablation and improving arrhythmia outcomes in PsAF and LsPsAF is yet to be determined. They are exciting techniques, which could potentially be utilised as part of the catheter ablation stage of convergent ablation in the future. Further experience incorporating cryoablation or PFA during the endocardial stage would demonstrate the impact of incorporating these modalities in place of RF energy on procedure logistics and outcomes. Given the rapidly advancing nature of interventional electrophysiology, as with all procedural treatments it is important to continue to evaluate convergent ablation alongside the latest developing technologies.
As with any ablation technology, further studies to add to the evidence base and to assess ongoing, long-term efficacy and safety over time would be of much value and help patients to weigh up benefits and risks when making decisions about their treatment or undergoing an intervention. The ongoing HALT-AF study will evaluate the impact of convergent ablation combined with LAA exclusion (using the Atriclip device, [AtriCure Inc.]) on efficacy and safety outcomes compared to catheter ablation in patients with PsAF or LsPsAF.89
8 CONCLUSIONS
Convergent ablation is a promising emerging hybrid treatment for the treatment of PsAF, now with RCT evidence supporting its utility in this patient group, especially for patients with LsPsAF. As uptake of this procedure increases, it is crucial to set up appropriate pathways and clinical networks to facilitate patient selection, optimise efficacy and safety, and monitor outcomes postprocedure. The results of ongoing clinical studies will provide further information on outcomes and longer-term durability.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no data sets were generated or analysed during the current study.




