A novel open-source software-based high-precision workflow for target definition in cardiac radioablation
Disclosure: Stephan Hohmann has in the past participated in a training program by the German Cardiac Society supported by an unrestricted educational grant from Boston Scientific. David Duncker has received lecture honorary and/or travel grants from Abbott, Astra Zeneca, Biotronik, Boehringer Ingelheim, Boston Scientific, Medtronic, Pfizer, and Zoll. Christian Veltmann has received honorary for lectures or consulting from Abbott, Bayer, Biotronik, BMS, Boehringer Ingelheim, Boston Scientific, CVRx, Daiichi Sankyo, Medtronic, and Zoll. J Bauersachs reports honoraria for lectures and/or consulting: Novartis, BMS, Pfizer, Vifor, Bayer, Servier, Daichii Sankyo, CVRx, MSD, Boehringer Ingelheim, AstraZeneca, Abiomed, Abbott, Medtronic; Research support: Zoll, CVRx, Vifor, Abiomed. Other authors: No disclosures.
[Correction added on 13 January 2021, after first online publication: Projekt Deal funding statement has been added.]
Abstract
Introduction
Noninvasive ablative radiotherapy of cardiac arrhythmias (stereotactic ablative body radiation) has shown promising initial results. Precise targeting of the arrhythmogenic substrate is paramount to limit adverse effects to healthy myocardium, organs at risk, and cardiac implantable electronic devices. Using electroanatomic maps for treatment planning is technically challenging.
Methods and Results
Using the free open-source 3D Slicer software platform we established a workflow for high-precision target definition based on electroanatomic maps. An import plug-in for 3D Slicer has been designed that reads electroanatomic maps generated with three mapping systems in widespread clinical use. Using our proposed workflow in a real-world patient case we were able to align the map to the computed tomography (CT) with a mean distance of 3.1 mm. Thus, points defined on the map were translated into CT space with high accuracy and a radiotherapy treatment volume was defined in CT space based on these map-derived points.
Conclusion
We describe a novel high-precision target definition method for stereotactic ablation of cardiac arrhythmias. Multimodal integration of the electroanatomic map with the planning CT allows for highly accurate localization of previously identified electrophysiological features in CT space. It remains to be shown whether this novel planning workflow leads to superior ablation outcomes when compared with other approaches.
1 INTRODUCTION
Noninvasive ablative radiotherapy of cardiac arrhythmias (stereotactic ablative body radiation [SABR]) has shown promising initial results.1-3 Precise targeting of the arrhythmogenic substrate is paramount to limit adverse effects to healthy myocardium, surrounding organs, and cardiac implantable electronic devices (CIEDs) at risk.4 Radiotherapy planning is usually performed on a computed tomography (CT) scan. As opposed to tumors, which are readily identifiable on imaging modalities such as CT or magnetic resonance tomography, the target for stereotactic arrhythmia ablation is defined by its electrical properties. Patients who are candidates for SABR have typically undergone at least one prior unsuccessful invasive catheter ablation attempt.2 Endocardial or epicardial electroanatomic maps are, therefore, available in most of these patients. However, the use of these data for treatment planning has been fraught with technical difficulties. In the present report, we describe the methods for a high-precision target definition workflow based on multimodal integration of cardiac three-dimensional (3D) mapping data and the planning CT.
2 METHODS
2.1 Software
3D Slicer version 4.11 (3D Slicer contributors; www.slicer.org) was used for multimodal data integration. 3D Slicer is an open-source software application for medical image computing widely used for quantitative imaging research and image-guided therapies.5 The core application can be extended with custom plug-ins. We used the SlicerRT extension which adds features for the import and export of radiotherapy-related Digital Imaging and Communications in Medicine (DICOM) datasets to 3D Slicer.6 The registration accuracy metrics reported were measured using the segment comparison feature in SlicerRT. The GNU Octave numerical computation suite7 was used for rapid prototyping and testing of algorithms as the map file formats were decoded.
2.2 Electroanatomic mapping
A 3D endocardial electroanatomic map of the left ventricle was acquired using the CARTO 3 version 6 mapping system (Biosense Webster, Diamond Bar, CA, USA) with the Pentaray mapping catheter and the Thermocool Smarttouch force-sensing ablation catheter (Biosense Webster). Anatomy of the thoracic aorta and the coronary sinus was acquired as a separate map in the study to enhance spatial registration to the CT.
2.3 Radiotherapy planning CT
With the patient in an immobilization device (Bodyfix; Elekta AB, Stockholm, Sweden) a noncontrast, nongated chest CT with a skin-to-skin field of view was acquired for radiotherapy planning (Somatom AS; Siemens, Forchheim, Germany). In addition, a contrast-enhanced cardiac CT was performed with ECG-gating to 70% of the RR interval (diastolic phase).
2.4 Import and 3D registration of electroanatomic maps
Radiotherapy planning is performed in the space of the planning CT. Spatial registration of the electroanatomic map into the CT coordinates is, therefore, a crucial step in the treatment planning workflow based on electrophysiological data. Data export in different cardiac mapping systems differs and export formats are sparsely documented. We, thus, analyzed data files exported or archived from the mapping workstations. Through manual inspection of binary raw data, comparison of the file structure to established standard formats, and meticulous correlation of data displayed on the workstation to the exported data stream we were able to reverse engineer significant parts of the undocumented export/archive file formats.
CARTO 3 (Biosense Webster) exports the anatomy model in a “.mesh” file which contains the triangulated mesh of the map geometry as well as (interpolated) electric data such as voltage and local activation time (LAT) for every vertex of the mesh. In addition, a point cloud of the original electroanatomic points acquired during mapping is exported for every single map of the study. Since point numbers are consistent with the point list on the CARTO workstation, specific points labeled as a good pace map or fractionated signal can later be identified by their point number in 3D Slicer.
EnSite Velocity/Precision (Abbott, St. Paul, MN, USA) exports a single standard Extensible Markup Language (XML) file with the triangulated mesh of the map as well as one electric scalar data point per mesh vertex. The electric information displayed (bipolar voltage, unipolar voltage, or LAT) can be defined at the time of export from the mapping workstation.
RHYTHMIA HDx (Boston Scientific, St. Paul, MN, USA) currently lacks dedicated export functionality, but studies can be archived on external storage media for backup and later review. The archive file format is undocumented but seems to be nonstandard XML files containing binary data. Fields holding the triangulated mesh and electric information have been identified in the file format and this information can thus be extracted.
A plug-in for 3D Slicer, EAMapReader, has been written which reads maps exported from these three mapping systems. We have made EAMapReader (including its source code) available at https://github.com/stephan1312/SlicerEAMapReader.
2.4.1 Software architecture
Upon installation of EAMapReader through the 3D Slicer application, the plug-in registers as a so-called scripted module which is seamlessly integrated into the existing user interface. The plug-in has been written in the interpreted Python programming language and, therefore, runs on all platforms supported by 3D Slicer (Windows, Mac OS, and various Linux distributions). EAMapReader parses the data exported or archived from the mapping workstation and recreates the map as a triangulated mesh with overlaid scalar information (voltage and LAT). This allows 3D Slicer's powerful built-in visualization capabilities and analysis tools to be used with the map.
3 RESULTS
3.1 Description of the high-precision target definition workflow
3.1.1 Patient vignette
A 72-year-old male patient was admitted with recurrent therapies of his CRT-D device (Intica Neo 5 HF-T; Biotronik, Berlin, Germany) for monomorphic ventricular tachycardia (VT). The patient had received his first CRT-D in 2012 for primary prevention in nonischemic cardiomyopathy and was followed via remote monitoring (Home Monitoring; Biotronik, Berlin, Germany). In 2016, he underwent replacement of the aortic valve and aortic root with a bioprosthesis for severe aortic regurgitation and aneurysm of the ascending aorta. Because of an asymptomatic significant stenosis of the distal left anterior descending (LAD) coronary artery an arterial coronary bypass (LIMA to LAD) was performed at the same time. Approximately 2 months before the current admission the patient started experiencing recurrent VT. A total of 16 VT episodes were treated by the device over the course of 6 weeks, 9 of these with high voltage shocks. The patient was started on amiodarone and eventually mexiletine was added.
An electrophysiological study was performed where monomorphic VT could be induced (one VT, cycle length: 320 ms [matching the episodes recorded on the CRT-D], inferior axis, negative in I, positive in V1–V5, qR in V6) which was poorly tolerated by the patient and had to be terminated. Pace mapping showed the best correlation in the basal posterolateral left ventricle (LV) in an area with normal endocardial bipolar voltage (>1.5 mV). Unipolar voltage was slightly reduced, approximately 7.0 mV. Endocardial radiofrequency (RF) ablation was performed here (40 W, irrigated, ablation index: 600), but the VT remained inducible. An epicardial or midventricular origin was suspected based on the voltage and the pace mapping results, as well as the VT morphology.8 Since epicardial access was limited after prior cardiac surgery the patient was offered cardiac SABR as an individual treatment trial and informed consent was obtained. Following planning and treatment as described below the CIED showed unchanged sensing amplitudes, pacing thresholds, and impedances. No acute toxicity was observed. However, a different VT with a tachycardia cycle length of 380 ms recurred in the first weeks after treatment and the patient underwent another invasive study 2 months after radiation treatment. The treated VT morphology was no longer inducible, but a different VT (inferior axis, left bundle branch block configuration) was successfully ablated from the right ventricular outflow tract using irrigated RF ablation. No recurrences were observed since then with a total follow-up of 5 months.
3.1.2 Target definition workflow
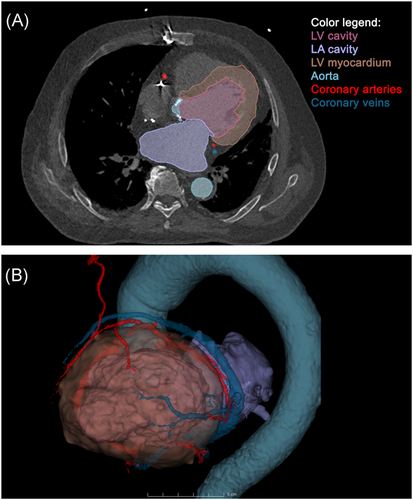
An overview flowchart of the multimodal image integration and target definition workflow is shown in Figure 1. A contrast-enhanced cardiac CT was acquired and loaded into 3D Slicer using the built-in DICOM importer. The following structures were segmented on the CT: LV blood pool, left atrium blood pool, LV myocardium, aorta, coronary sinus, coronary arteries, and left internal mammary artery/coronary bypass graft (Figure 2).
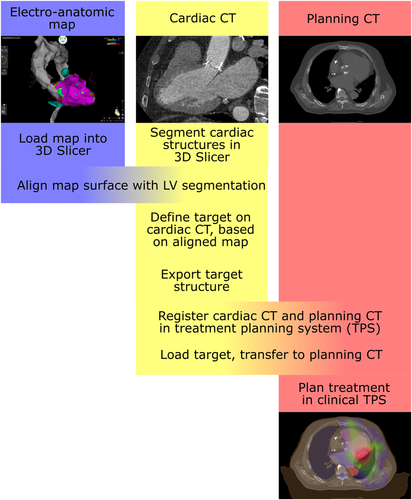
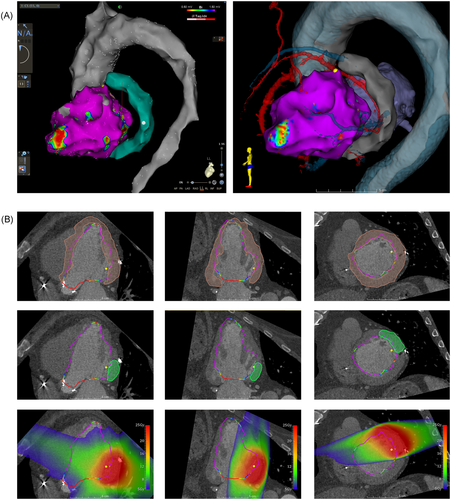
The electroanatomic map was exported from the CARTO workstation and imported into 3D Slicer using EAMapReader. The left ventricle and the thoracic aorta were spatially registered (linear transformation, 6 degrees of freedom) to the segmentation of the LV blood pool and the aorta on the cardiac CT. A manual alignment was performed first, followed by an automated registration using an iterative closest point (ICP) algorithm. The CARTO points labeled as good pace-map locations were displayed in the CT views and were projected onto the myocardial segmentation. Using multiplanar reformations of the cardiac CT, a transmural target structure was defined containing these points and at least 2 mm of margin beyond the outermost points (Figure 3).
The target structure was then exported from 3D Slicer as a DICOM radiotherapy structure dataset and imported into the clinical radiotherapy planning system (Monaco; Elekta AB) where it was used as an internal treatment volume to plan a prescription dose of 25 Gy with planning constraints to avoid the epicardial coronary arteries and the CIED hardware. Delivery was performed as volumetric arc therapy.
3.2 Accuracy metrics
We calculated various accuracy metrics to estimate errors introduced by the registration of the electroanatomic map to the CT. The mean pointwise distance of the CARTO map to the LV endocardial surface on the CT was 3.1 mm after ICP registration, with the 95th percentile of 8.3 mm. The Dice similarity coefficient (DSC) is a metric for the spatial similarity of two 3D structures, with values ranging from 0 for non-overlapping structures to 1 for identical, fully overlapping structures.9 We obtained a DSC of 0.83 between the CARTO map of the LV and the corresponding CT endocardial surface, indicating very good agreement.
4 DISCUSSION
We propose a high-precision targeting workflow for stereotactic ablation of ventricular arrhythmias based on invasively obtained electroanatomic information. We created software that enables the import and analysis of electroanatomic maps from three widely used clinical mapping systems and validated it with real-world clinical data from the CARTO system.
Previously reported case series and cohorts of stereotactic ablative radiotherapy of ventricular arrhythmias relied on electrocardiographic imaging (ECGi) to determine the origin of arrhythmias.2, 10 ECGi uses CT volumes to delineate the cardiac anatomy and to determine the relative location of 252 body surface electrodes. Virtual electrograms are then calculated on an epicardial surface segmented from the CT. Thus, electroanatomic information obtained through an ECGi system is natively in the CT coordinate system, and the transfer of ECGi-derived target locations to the planning CT is trivial.11 Spatial accuracy of ECGi, however, is limited and depends on a number of factors. Septal activation, for example, is inherently difficult to represent on the epicardial surface.11 Furthermore, epicardial exit sites as delineated by ECGi have been shown to be spatially distant from the diastolic isthmus in ischemic VT in humans.12
Invasive electroanatomic mapping has been shown to achieve high spatial resolution and accuracy, and high-definition acquisition of electrical signals facilitates the detection of conducting channels in areas of the scar.13 The proposed workflow unlocks this high-precision data to be used for planning SABR treatments of cardiac arrhythmias. We were able to align the CARTO map to the planning CT within a few millimeters, thus enabling an accurate transfer of map-derived locations to the planning CT.
Quality of alignment (registration distance) between different modalities is affected by cardiac and respiratory motion. The respiratory motion moves the whole heart in the chest and can be addressed by rigid transformations of the electroanatomic map. Differences in the cardiac cycle are more problematic but can be overcome by the acquisition of a diastolic phase cardiac CT. However, depending on the quality of the imaging and the map, resulting registration distances might be larger than in the present data.
All components specific to this workflow are freely available, open-source, and platform-independent. 3D Slicer has been created over the last two decades by a worldwide community of developers mainly through support from the US National Institutes of Health. The software has been used for image analysis and visualization in hundreds of scientific applications, from automated image segmentation to surgical navigation.5 Likewise, the SlicerRT extension has been validated extensively for radiotherapy research.6, 14 Our module, EAMapReader, is freely available for download and its streamlined design can easily be extended further if the need for new features arises.
Just a few weeks ago a similar approach has been published, using MATLAB and 3D Slicer.15 While providing similar capabilities in reading CARTO maps, our workflow is seamlessly integrated into 3D Slicer as a plug-in and does not depend on proprietary MATLAB software. Also, EAMapReader reads Ensite and RHYTHMIA maps in addition to CARTO.16
4.1 Limitations
Stereotactic radioablation of cardiac arrhythmias is still a novel technique and patient numbers are limited. In the present paper, we propose a novel high-precision target definition workflow and report a patient case as proof of concept, but no larger-scale clinical trial has been performed. Thus, it is unclear whether high-precision planning based on electroanatomic maps is superior to ECGi-based target identification or “eyeballing” with regard to ablation outcomes and adverse events. Although the import of map data exported from the EnSite and RHYTHMIA systems is feasible with our software and has been tested with sample data, no clinical validation has been performed yet.
Furthermore, a limitation in radioablation of VT is that easy inducibility of one VT might mask other VT substrate at the time of the diagnostic study. This is different from RF ablation with sequential VT induction, ablation, and induction of the next VT followed by ablation in a single session. In the clinical case presented as an illustration of our workflow, a second VT was never seen at the initial invasive study since the index VT was so easily inducible. Only after radioablation of the first VT this second morphology became clinically relevant and was successfully ablated in a second RF ablation session. This underscores that that radiation target choice is a complex process and further studies are needed to establish robust criteria to target cardiac tissue for radiation therapy.
5 CONCLUSION
We proposed a novel high-precision target definition workflow for stereotactic ablation of cardiac arrhythmias and demonstrated it in a patient case. Multimodal integration of the electroanatomic map with the planning CT allows for highly accurate localization of previously identified electrophysiological features in CT space. The alignment of the electroanatomic map with corresponding structures on the CT was within a few millimeters when used with real-world clinical data. In the present study, we developed the workflow and the necessary software tool and made it freely available. It remains to be shown whether this novel planning workflow leads to superior ablation outcomes when compared with other approaches.
ACKNOWLEDGMENTS
The authors would like to thank Anna Malek (Biosense Webster, Germany) for help with re-enabling map export in CARTO 3 v6. This study was funded in part by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) — Project No. 380200397 (to Stephan Hohmann). Open access funding enabled and organized by Projekt DEAL.
AUTHOR CONTRIBUTIONS
Stephan Hohmann designed the workflow and wrote the necessary software tool. Stephan Hohmann, Christoph Henkenberens, Christos Zormpas, David Duncker, and Christian Veltmann performed the clinical treatment and collected data. Stephan Hohmann analyzed the data and wrote the first draft of the manuscript. All authors reviewed and edited the manuscript for important intellectual content.