Disentangling the Evolutionary History of the Woody Species in Earth's Most Diverse Tropical Savanna
Funding: This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul.
ABSTRACT
Aim
We investigate the evolutionary structure of vegetation assemblages in the Cerrado Domain, the main extent of savanna in the American Tropics, a biodiversity hotspot, to understand the role of ecological and geographical factors in constraining plant diversification and shaping biogeographic patterns across this complex mosaic of environments. We test the following predictions: (1) savanna assemblages form a distinct evolutionary group, rather than grouping with forest assemblages from adjacent biogeographic regions; (2) moist forests contain the highest phylogenetic diversity, followed by dry forests and savannas and (3) edaphic variables are stronger predictors of evolutionary group differentiation than climatic variables.
Location
Cerrado, Brazil.
Methods
Our data set comprises 3072 tree species, belonging to 656 genera and 151 families, found across 1165 assemblages. We used a phylogenetically informed ordination analysis to place assemblages in a multivariate space, followed by K-means clustering to identify the main evolutionary groups of tree assemblages. To determine which environmental variables were associated with the evolutionary groups found, we implemented classification tree approaches. We quantified both the unique and shared phylogenetic diversity among evolutionary groups and identified the lineages most strongly associated with each evolutionary group using an indicator analysis.
Results
We find a clear evolutionary differentiation between savanna and forest assemblages, pointing to the importance of fire and water availability in driving turnover in evolutionary lineage composition of tree communities in the Cerrado Domain. When dividing assemblages into three evolutionary groups, the forest group splits into deciduous versus evergreen/semideciduous subgroups. The evergreen and semideciduous forests harbour the highest overall and unique phylogenetic diversity, and deciduous forests the second highest, but the savanna group also contains a significant portion of unique woody angiosperm evolutionary diversity.
Conclusions
We identified that savannas assemblages constitute a distinct evolutionary group. Tree species that can inhabit fire-prone areas belong to a restricted set of phylogenetic lineages, giving the savannas in the Cerrado Domain a unique evolutionary identity. Dry forests also constitute a unique evolutionary group. Given the marked evolutionary variation of tree assemblages across the Cerrado Domain, it is imperative to recognise and address the specific conservation challenges faced by each group (savanna, evergreen and semideciduous forest and tropical dry forest) to ensure the preservation of this biodiversity hotspot.
1 Introduction
Understanding how ecological and geographic factors influence species distribution patterns and their evolutionary history has been one of the main goals of macroecological and macroevolutionary research. This research is essential for effective conservation strategies and managing biodiversity (Hendry et al. 2010). Phylogenetic measures can estimate how much evolutionary history is represented in a particular region, making it possible to infer the relative influence of various biogeographical events on the evolutionary assembly of floras (Liu et al. 2017). Evolutionary assembly can be shaped by neutral processes (stochastic events such as dispersal limitation, speciation, and local extinction; Hubbell 2001) and deterministic, niche-related processes (e.g., ecological interactions and environmental filters; Webb et al. 2002; Keck and Kahlert 2019).
The concept of biome conservatism (the tendency of organisms to remain in the same broad environmental conditions over evolutionary time, with low biome shift rates) and phylogenetic niche conservatism (the tendency of species to retain their ecological requirements and related functional traits) have emerged as consensus explanations for understanding patterns of phylogenetic clustering within regions in global analyses (Fine and Lohmann 2018; Segovia et al. 2020). Recent findings suggest that environmental associations of lineages may be the primary force organising the course of diversification, but a key knowledge gap is in studies comparing the degree of evolutionary similarity among species assemblages at large geographic scales (Luize et al. 2024; Rezende et al. 2020a).
The main extent of savanna in the American Tropics is largely found within Brazil and known as the Cerrado (Ab'Saber 2003; Bueno et al. 2018; Gottsberger and Silberbauer-Gottsberger 2006; Ribeiro and Walter 2008). It is one of the most diverse and endangered regions on earth, a global biodiversity hotspot (Myers et al. 2000), with more than 12,000 plant species, of which 44% are endemic (REFLORA).
In Brazil, large-scale phytogeographic regions are formally categorised as ‘Domains’, a biogeographic framework that encompasses interconnected ecosystems based on geomorphological, climatic and vegetation characteristics (Ab'Saber 2003). One of these, the Cerrado Domain, corresponds to the extensive tropical savanna region in central Brazil, where savanna vegetation predominates, which also contains a highly heterogeneous mosaic of vegetation types, including many different grassland and savanna formations as well as different types of forest (e.g., gallery forests, dry forests; Bueno et al. 2013, 2018; Dexter et al. 2018; DRYFLOR et al. 2016; Eiten 1978; Haidar et al. 2013; Olson et al. 2001).
The Cerrado Domain includes six main woody vegetation types that have a substantive arboreal component, namely cerrado sensu stricto, cerradão (dystrophic cerradão on poor soils and mesotrophic cerradão on richer soils) (Ribeiro and Walter 2008), deciduous forest (a.k.a. tropical dry forest sensu DRYFLOR et al. 2016), semideciduous forest, and evergreen forest (Bueno et al. 2018; Ribeiro and Walter 2008). Cerrado sensu stricto is classic savanna with an open tree canopy and grassy layer. Cerradão is a closed canopy formation with little grass in the understorey. It is structurally more akin to a forest but dominated by savanna tree species.
Tree species representative of cerrado sensu stricto vegetation showed expansions and contractions during the climatic fluctuations of the Quaternary, related to a warmer and more seasonal climate (Bueno et al. 2016). In fact, climatic fluctuation may have influenced species distributions, permitting the delineation of biogeographic regions that are delimited based on species composition (Amaral et al. 2017; Francoso et al. 2020), but phylogenetic lineages in angiosperms are millions of years old and other processes operating on deeper time scales (e.g., niche conservatism, diversification) may have a larger influence on where units fall in geographic and environmental space (Rezende et al. 2020a).
Previous analyses of the biogeography and composition of Cerrado tree communities were performed based on species-level floristics, without considering their evolutionary or phylogenetic relatedness. Bueno et al. (2018) demonstrated that tree species composition in the Cerrado is strongly structured by edaphic factors, with savannas and forests showing distinct species assemblages. These authors also found that the lack of variation in tree species composition attributed to climatic variables indicates that within homogeneous macroclimatic zones, many types of forest and savanna co-exist due to complex mosaics of local substrate heterogeneity and fire history. However, as this study was based on a taxonomic approach, it could not determine whether the observed floristic differences correspond to deep evolutionary divergences or are the result of more recent ecological processes. While species-based analyses can identify shared and unique species across vegetation types, they do not reveal whether these species represent long-divergent evolutionary lineages or recent colonisation events. By incorporating a phylogenetic framework, our study extends beyond species composition to assess whether vegetation patterns reflect distinct evolutionary histories.
Here, we employ a phylogenetic approach to assess patterns in the evolutionary assembly of the Cerrado Domain flora. Phylogenetic trees provide essential information on the evolutionary relationships among species, and recent developments in phylogenetic techniques, coupled with increased availability of genetic data, allow us to refine biogeographic delineations and evolutionary interpretations (Daru et al. 2016). Despite a high number of endemic species, the Cerrado does not appear to harbour many endemic genera or large amounts of endemic evolutionary history, at least within its woody flora (Segovia et al. 2020; Simon et al. 2009).
The Cerrado flora is relatively young, derived from forest ancestors, primarily from the Atlantic and Amazonian forests (Sarmiento 1983; Prance 1992; Oliveira-Filho and Fontes 2000; Simon et al. 2009). Previous large-scale phylogenetic studies have shown that Cerrado tree assemblages cluster with moist forest assemblages (Segovia et al. 2020), suggesting strong biogeographic connections with tropical forests. Thus, if one analyses patterns in the lineage composition of tree assemblages within the Cerrado Domain, the main evolutionary division may therefore follow a biogeographic split, where sites are affiliated with either the Amazon or Atlantic Domains, driven by historical migration from these forested regions. The study of Segovia et al. (2020) was at an inter-continental scale however, and biogeographic factors may be less relevant in Domain-level analyses. The selective pressures acting on tree species occurring in savanna, for example, fire and high solar radiation, are very different from those acting in forest environments, for example, competition for light, and these divergent selective pressures may be sufficiently strong such that the main evolutionary division within the Cerrado Domain is between savanna and forest assemblages (Hoffmann et al. 2012; Simon and Pennington 2012). Further, if environmental rather than biogeographic factors are the driving force behind variation in the evolutionary lineage composition of tree assemblages (Luize et al. 2024; Rezende et al. 2020a), we may also expect divergence in lineage composition between the tropical dry forest and moist forest assemblages found within the Cerrado Domain.
The evolutionary history of vegetation types will also be reflected in the amount of evolutionary, or phylogenetic, diversity found within them, including overall phylogenetic diversity and unique, or endemic, phylogenetic diversity (Faith 1992). Moist forests, considered older ecosystems and ancestral niches for angiosperms (Crisp et al. 2009), are expected to harbour the highest overall phylogenetic diversity. Dry forests, unlike savannas, appear to contain a high number of unique lineages due to prolonged evolutionary isolation (Segovia et al. 2020; Pennington et al. 2006), suggesting that they may contain a higher proportion of unique phylogenetic diversity. Savannas are thought to be recently colonised (Simon et al. 2009), meaning they have had less time to accumulate unique evolutionary diversity compared to moist and dry forests. Beyond evolutionary history, environmental factors are also key in shaping these evolutionary patterns. Given the relatively homogeneous climate of the Cerrado Domain, we expect edaphic heterogeneity to be a stronger driver of vegetation differentiation than climatic variables (Bueno et al. 2018; Furley 1992; Oliveira-Filho and Ratter 2002; Miranda et al. 2018). Soil characteristics—such as fertility, texture and drainage—strongly influence the distribution of savanna and forest assemblages, with savannas typically occurring in nutrient-poor, well-drained soils, while forests are associated with more fertile and moisture-retentive soils. By integrating a phylogenetic approach, our study assesses the extent to which edaphic factors shape evolutionary structure, providing insights into their role in community assembly and diversification within the Cerrado Domain.
In this context, we aim to test three key predictions to understand the mechanisms underlying the evolutionary differentiation of woody vegetation across the Cerrado Domain: (1) savanna assemblages will form a unique evolutionary group, distinct from forest assemblages, reflecting adaptation to fire and soil conditions, rather than showing a biogeographic division reflecting Amazon versus Atlantic affiliations; (2) moist forests will contain the highest overall and unique phylogenetic diversity, followed by dry forests and savannas; and (3) edaphic variables will be stronger predictors of evolutionary group differentiation than climatic variables. Through this approach, our study contributes to advancing current understanding of the ecological and historical drivers of tropical plant diversification, while offering valuable guidance for conservation strategies that prioritise evolutionary diversity.
2 Material and Methods
2.1 Study Area
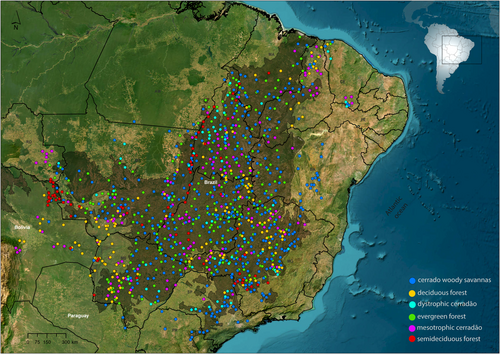
The Cerrado Domain is the second largest phytogeographical domain in South America, surpassed in area only by the Amazon (Ribeiro and Walter 2008). It is spread across the Central Brazilian Highlands, which comprise 1/4 of Brazil's surface, and to smaller areas in northwestern Paraguay and eastern Bolivia (Oliveira-Filho and Ratter 2002; Olson et al. 2001) (Figure 1). The Cerrado Domain extends over 20° of latitude and from altitudes of 100 m in the Pantanal (western floodplains) to 1500 m in the highest tablelands of the Central Brazilian Highlands (Ribeiro and Walter 2008).
2.2 Database
We extracted floristic data from the NeoTropTree database (NTT) (http://www.neotroptree.info/), which consists of checklists for assemblages of woody, freestanding plant species (i.e., lianas excluded), compiled for geo-referenced sites, extending from southern Florida (USA) and Mexico to Patagonia. To ensure consistency, we included only tree species, following NTT's definition of a tree as woody plants capable of growing taller than 3 m without support. NTT holds 7485 sites/checklists; 20,562 woody plant species and 1,206,314 occurrence records (Bueno et al. 2018; Miranda et al. 2018; Pontara et al. 2018; Rezende et al. 2020a, 2020b, 2023). Each assemblage corresponds to a single vegetation type over a 10 km diameter area, and when multiple vegetation types co-occur within the same 10 km diameter area, they are considered as separate assemblages. This means that the species lists for these assemblages only include species recorded within each specific vegetation type, without mixing species from different vegetation types into a single assemblage (see description, history and protocol of NeoTropTree at http://www.neotroptree.info). In addition to the presence and absence data for tree species, each assemblage is characterised by numerous descriptive and environmental data, such as elevation, geo-edaphic and climatic variables (Table S1).
Grass cover (%) was estimated through direct observation of the site on Google Earth images in five 100 × 100 m areas: one at the central coordinates of the NTT site and four at 2.5 km away from it towards the NE, SW, NW and SE (see Neves et al. 2017; Bueno et al. 2018). The data were transformed into ranked mid-class values for each site, which was used as a proxy for fire return interval (i.e., fire frequency; Archibald et al. 2013; Hoffmann et al. 2012; Lehmann et al. 2014). Our dataset consists of 1165 assemblages found in the Cerrado Domain and includes 3072 tree species, 656 angiosperms genera and 121 families. The inclusion of ferns and gymnosperms has a very strong effect on phylogenetic diversity metrics, yet tree ferns and gymnosperms are exceedingly rare over most of the Cerrado, so they were excluded from analyses (sensu Coronado et al. 2015; Kembel and Hubbell 2006). For phylogenetic analysis, we used two complementary approaches to ensure the robustness of our findings: (i) a well-curated genus-level phylogenetic tree, based on the rbcL and matK plastid regions (Neves et al. 2020), and (ii) a species-level phylogeny generated using V.PhyloMaker2 (Jin and Qian 2022).
The genus-level phylogeny encompasses 1100 lowland tropical tree genera from South America, covering 89% of the angiosperm tree genera in our dataset. The 41 genera that were not present in this phylogeny represent only 0.58% of the occurrence records, that is, they are mostly rare genera shared across few sites, meaning their influence on compositional similarity is minimal.
To generate a complete species-level phylogeny, we used V.PhyloMaker2 (Jin and Qian 2022). We opted to use scenario 3, where the tips of a genus or species not included in the mega-tree were bound to the mid-point of the family or genus branch, representing the branch between the family and genus crown and root nodes (see Jin and Qian 2019 for further details). In the main text, we present the results based on the V.PhyloMaker2 phylogeny, as it provides a higher taxonomic resolution, while the results using the genus-level phylogeny are confirmatory and found in the Supporting Information S1.
2.3 Data Analysis
To address our first objective—identifying the main evolutionary groups—we applied the evoPCA approach developed by Pavoine (2016), which allows the placement of assemblages in a multivariate space that reflects both species occurrences and their phylogenetic relationships. Specifically, we conducted a principal component analysis of a Hellinger transformed compositional matrix that not only includes the occurrence of species in assemblages but also the occurrence of phylogenetic nodes. This approach is recommended for having high discriminatory power to detect changes in evolutionary lineage composition over gradients, while also being connected to an ordination that can serve to place lineages and assemblages in the same compositional space. We implemented this approach using the evoPCAHellinger() function in the adiv package (Pavoine 2016).
To determine which ordination axes represented structure that was significantly different from random, we used a parallel test with the fa.parallel() function from the psych package in R. We then quantified the distance between assemblages across the significant axes to perform a K-means clustering which served to determine the main evolutionary groups based on their phylogenetic or lineage composition. For determining the optimal number of groups in the clustering analysis, we used an analysis of gap statistics, average silhouette widths and an elbow plot (see Appendix S1 in Supporting Information S1). The Silhouette width and the gap analyses suggest K = 2 to be the best clustering, while the Elbow analysis suggests K = 3 to be the best clustering. The association between evolutionary clusters and vegetation types was based on the correspondence between the k-means clustering results (applied to the evoPCA scores) and the vegetation type classification, which was already available as part of the original dataset.
2.4 Shared Versus Unique Phylogenetic Diversity (PD)
To address our second objective, we quantified the total and unique phylogenetic diversity (PD) for each evolutionary group identified through clustering analysis, comparing the amount of evolutionary diversity restricted to each group with the diversity shared across groups under both K = 2 and K = 3. PD captures the cumulative evolutionary history present in each group, allowing us to assess how much evolutionary distinctiveness is represented in the savanna group compared to the forest group, highlighting the contribution of each evolutionary group to the overall phylogenetic diversity of the Cerrado.
First, we estimated the association of species with each group by an indicator species analysis following (De Cáceres and Legendre 2009). Specifically, we used the multipatt() function in the R Packages indicspecies (Chave et al. 2014) to allow genera to be associated with more than one group (when K > 2, Table S3). The output of the multipatt () function includes the stat index, which is a function of the specificity (the probability that a surveyed site belongs to the target site group given the fact that the species has been found) and fidelity (the probability of finding the species in sites belonging to the given site group). We constructed pruned phylogenies including those genera with specificity > 0.6 to a group, or combination of groups, to estimate the total PD found in each group or combination of groups. Then, we subtracted these totals from the total PD for the complete, unpruned phylogeny to determine the amount of phylogenetic diversity restricted to each group, or combination of groups, quantifying their unique evolutionary history. Finally, we estimated the PD shared across all groups as that which was not restricted to any particular group or any combination of groups. We fit these different PD totals as areas in a Euler diagram with the euler() function in the ‘eulerr’ package (Bates et al. 2015).
2.5 Identification of Environmental Drivers Using Random Forest Analyses
To address our third objective—identifying the environmental drivers of evolutionary group differentiation—we applied a random forest classification tree approach (Breiman 2001), which evaluates whether the phylogenetic groups can be reliably distinguished using environmental variables. The random forest classification consists of many individual decision trees that operate as an ensemble; it aggregates the votes from different decision trees to decide the final class of each community type. For this, we used elevation and 26 environmental variables available for each site (at 30 arc-second resolution) in the NeoTropTree database (Table 1).
| Variables | Mean decrease Gini coefficient (K = 2) | Mean decrease Gini coefficient (K = 3) |
|---|---|---|
| Grassy cover | 290.44 | 254.27 |
| Ranked soil drainage | 78.87 | 105.14 |
| Soil water storage | 68.54 | 86.54 |
| Ranked total base saturation in soil | 38.15 | 59.46 |
| Ranked sand | 15.79 | 21.69 |
| Elevation | 7.60 | 15.66 |
| Temperature seasonality | 6.33 | 12.41 |
| Temperature annual | 6.45 | 8.44 |
| Minimum temperature | 5.43 | 8.08 |
| Water excess severity | 4.97 | 12.41 |
| Maximum temperature | 5.56 | 8.62 |
| Precipitation annual | 4.51 | 10.63 |
| Hyper seasonality | 4.63 | 10.28 |
| Potential evapotranspiration | 4.44 | 6.20 |
| Precipitation seasonality | 4.00 | 8.08 |
| Water excess duration | 3.07 | 9.53 |
| Precipitation in the wet period | 4.05 | 7.25 |
| Ranked rockiness of soil | 3.88 | 4.47 |
| Isothermality | 3.39 | 6.90 |
| Water deficit duration | 3.07 | 6.65 |
| Temperature annual range | 3.19 | 5.25 |
| Precipitation in the dry period | 3.09 | 6.08 |
| Aridity index | 2.86 | 8.64 |
| Temperature daily range | 2.63 | 4.32 |
| Cloudiness (light interception) | 0.24 | 0.33 |
| Number of days of frost | 0.28 | 0.12 |
In order to evaluate the success rate of the classification tree approach in assigning sites to phylogenetic groups and to determine which phylogenetic groups were incorrectly classified, we generated confusion matrices. We also estimated the importance of each variable for distinguishing vegetation types using Breiman's measure of importance (Breiman 2001). For this, we used the mean decrease in the Gini coefficient when a variable is chosen to split a node. All analyses were conducted in the R 3.2.3 Statistical Environment (R Core Team 2017) using the following packages: ‘picante’ (Kembel et al. 2015), ‘vegan’ (Oksanen et al. 2016), ‘adiv’ (Pavoine 2016), ‘cluster’ (Maechler et al. 2018), ‘factoextra’ (Kassambara and Mundt 2016), ‘ape’ (Paradis et al. 2019), ‘labdsv’ (Roberts 2019) and ‘randomForest’ (Liaw and Wiener 2002).
3 Results
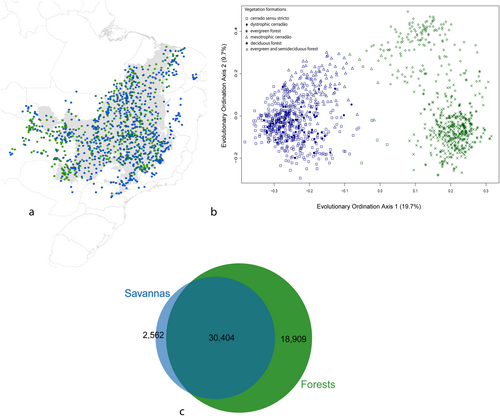
The phylogenetic lineage composition of tree assemblages in the Cerrado Domain contains two distinct evolutionary groups that closely align with vegetation types independently determined by field observations, which are separated by significant turnover in evolutionary lineages. (K = 2, see Figure 2, Figure S4). One of these evolutionary groups corresponds predominantly to the savanna biome and encompasses assemblages in cerrado sensu stricto, mesotrophic cerradão and dystrophic cerradão. The other group largely aligns with forest formations including deciduous, semideciduous and evergreen forests (Figure 2b). These groups are not geographically separated, as evidenced by the spatial interdigitation of these floristic groups across the Cerrado Domain (Figure 2a). Notably, some sites of cerradão were classified within the forest group, reflecting its transitional nature between open savannas and denser forest formations. Our analysis reveals that the majority of evolutionary diversity, as measured by the summed phylogenetic branch length, is found within the forest formations. However, it is important to highlight that there is also unique evolutionary diversity restricted to the savanna biome (see Figure 2c).
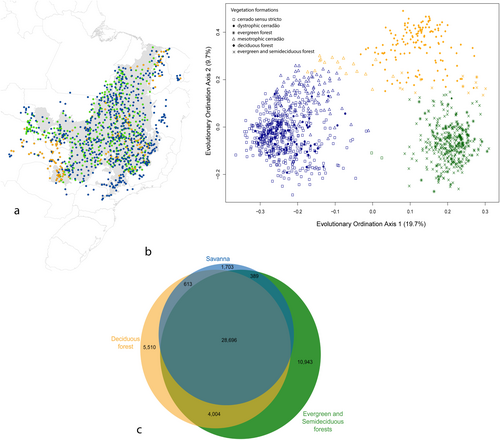
Additional analyses employing the Elbow method suggest support for clusters with K = 3 groups (see Figure 3, Figures S1 and S4). In this scenario, the forest formations were further differentiated into two evolutionary groups: a dry forest group characterised by deciduous forests, and a second group comprising evergreen and semideciduous forests. The third group in k = 3 corresponds to the savanna biome, characterised by more open vegetation and frequent fires, encompassing cerrado sensu stricto and cerradão. Each of these major groups exhibits substantial unique evolutionary diversity (see Figure 3c). To ensure the robustness of our findings, we conducted analyses using a well-curated genus-level phylogeny, which yielded highly similar results to those obtained with the V.PhyloMaker2 phylogeny (see Supporting Information S1). This consistency across presumed variation in the accuracy of the phylogeny and taxonomic resolutions reinforces the robustness of our approach to capture major evolutionary patterns among Cerrado tree assemblages.
Our findings underscore the significance of fire and edaphic factors in distinguishing phylogenetic groups within the Cerrado Domain (see Figures 2 and 3, Table 1). Among the variables considered, the five most influential for classification were associated with fire and edaphic conditions: (1) grassy cover, (2) ranked drainage, (3) soil water storage, (4) ranked TBS (total base saturation) and (5) ranked sand (Table 1). Notably, these variables appear to outweigh climatic factors in their importance for distinguishing evolutionary groups of assemblages within the Cerrado Domain. Of the 1165 assemblages, the evolutionary group of only 15 were misclassified based on environmental variables for K = 2 (1.5% of assemblages), while 40 were misclassified for K = 3 (3.5%).
In the evolutionary composition ordination, the first axis explained 19.7% of the variation and separated assemblages of savanna and forest formations (Figure 2). Axis 1 was related negatively to grassy cover (r2 = 0.81) and positively with ranked soil drainage (r2 = 0.51) and soil water storage (r2 = 0.36, Table S2). Climatic conditions were not as strongly correlated with this axis. In this segregation, the nodes, Inga, Caesalpinioideae and Meliaceae had a positive correlation with Axis 1, which includes many typical forest tree species. On the other hand, Curatella, Davilla, Vochysiaceae and Kielmeyera were associated with the savanna formation and had a negative correlation with Axis 1 (Figure S2). The second axis (explaining 9.7% of variation in lineage composition) separates the savanna formation and evergreen and semideciduous forests from dry forest. This second axis showed a gradient positively linked to soil fertility (ranked total base saturation in soil, r2 = 0.44) and negatively to water availability (r2 = 0.11, Table S2). Negative values for this axis are mainly associated with the genus Inga (Appendix S2).
The evergreen and semideciduous forests group exhibited the highest number of significant indicator lineages (348) and the highest values of the indicator statistic (Table 2, Table S4). The lineage with the highest indicator value (0.80) for the Savanna phylogenetic group was a Dilleniaceae subclade that includes the genera Davilla and Curatella (Table 2). These indicator lineages for the savanna group are scarce to absent in other groups, demonstrating the floristic distinctiveness of savanna formations.
| Phylogenetic group | No. species | No. genera | No. of significant indicator lineages (p < 0.005) | Non-nested li with highest indicator statistic |
|---|---|---|---|---|
| Savanna | 1089 | 350 | 204 | Dilleniaceae; Caesalpinoideae_sub44; Kielmeyera+Caraipa+Marila; Dimorphandra; Connaraceae; Apocynaceae; Malpighiaceae; Annona+Diclinannona; Leptolobium; Caryocaraceae |
| Deciduous forest | 1372 | 439 | 320 | Combretum; Cactaceae; Bauhinia; Handroanthus; Boraginaceae (Cordia+Varronia); Sapindaceae; Brassicales (Capparaceae+Caricaceae); Papilonoideae_39 (Erythrina+Platycyamus+Dahlstedtia+Lonchocarpus+Muellera); Vachelia+Senegalia |
| Evergreen and semideciduous | 2553 | 575 | 897 | Inga+Zygia; Phyllanthceae; Calophyllum; Lauraceae; Meliaceae; Miconia; Psychotria; Peritassa+Cheloclinium; Elaeoluma+Pouteria+Ecclinusa+Micropholis; Piper+Drimys |
4 Discussion
Our results show a clear differentiation in lineage composition between savanna and forest assemblages, pointing to the importance of fire and water availability in driving the evolutionary division in tree assemblages of the Cerrado Domain. Fire is a powerful selective force and, over their evolutionary history, plants have evolved traits that both tolerate and promote fire numerous times and across diverse clades (Archibald et al. 2018; Dantas et al. 2013; Hoffmann et al. 2012). The accumulation of biomass mostly from C4 grasses during the dry season is the main source of fuel for surface fires in modern savannas (Simon and Pennington 2012). This fire regime has shaped the evolution of key traits in savanna trees, such as thick bark, which insulates buds and reduces stem mortality (Hoffmann et al. 2003, 2012). The evolutionary differentiation observed suggests that only certain tree lineages acquired fire-resistant traits. Thus, even though savanna environments in the Cerrado were colonised many times by diverse plant lineages, clearly only a subset of lineages was able to do so.
In contrast, forest tree lineages are associated with traits and vegetation physiognomy that promote shade tolerance, suppress grass growth and reduce fire occurrence, reinforcing biome stability in savanna–forest mosaics (Charles-Dominique et al. 2018). This pattern is consistent with evidence documenting that only angiosperm lineages that evolved fire resistance and/or tolerance traits were able to radiate successfully into the savanna (Archibald et al. 2018). Some examples of these fire-adapted indicator lineages are members of the Dilleniaceae and Connaraceae families (Silva and Batalha 2010), found here associated with the savanna biome. These lineages show a great variety of adaptive mechanisms, such as thick bark to protect their vital tissues from heat or subterranean meristems that protect them from high temperatures and allow resprouting after fires (Gottsberger and Silberbauer-Gottsberger 2006). Functional trait differences have been found between trees of forests and savannas in South America (Hoffmann et al. 2003, 2005, 2012), Australia (Lawes et al. 2013) and Africa (Charles-Dominique et al. 2015), consistently demonstrating the role of fire in shaping savanna assemblages. By refocusing on phylogenetic patterns, our findings emphasise that fire has acted as a key evolutionary filter, selecting for lineages with fire-adapted traits, rather than simply shaping individual plant characteristics.
When we consider the division with three evolutionary groups, the forest evolutionary group was further subdivided into two groups: one associated with deciduous forest and another with evergreen and semideciduous forest (Figure 3b), pointing to an evolutionary divergence between forests adapted to dry versus moist conditions. In the deciduous forest, water stands out as the primary limiting factor, while the semideciduous and evergreen forests thrive in areas where soil water availability is higher. Bueno et al. (2018) observed a similar floristic pattern based only on species composition, but our analysis incorporates phylogenetic relationships, revealing that deciduous forests form an evolutionarily distinct group. This suggests that drought adaptation was acquired in specific evolutionary lineages, rather than being randomly distributed among tree species. Additionally, our findings support the hypothesis proposed by Fine and Lohmann (2018), indicating that some wet-forest taxa might have evolved key traits for survival in dry microhabitats within wet regions before their immigration into dry areas (i.e., a scenario of pre-adaptation). The evolutionary distinctness of the deciduous forests suggests that these adaptive traits emerged in specific evolutionary lineages, reinforcing the role of evolutionary history in shaping the ecological differentiation of forest types in the Cerrado Domain.
The deciduous forests in the Cerrado Domain clearly align with the tropical dry forest biome sensu Dexter et al. (2018). These deciduous forests have no association with streams or lakes, but rather with high soil fertility, for example around calcareous outcrops (Bueno et al. 2018). Tropical dry forests have closed canopies and are generally deciduous in the dry season, and fertility may be the factor promoting tree growth, enabling closed canopies that also suppress grasses and fire (Bueno et al. 2018; Dexter et al. 2018; Pennington et al. 2018; Miranda et al. 2018). Soil fertility may also be the factor that allows for deciduousness, as nutrients for new leaf production can be readily obtained. Furthermore, indicator lineages for dry forest such as Talisia, Melicocus and Dilodendron are characteristic of higher soil fertility. Meanwhile, other indicator lineages, such as fire-intolerant cacti, show the aridity of the tropical dry forest environment and the rarity of regular fire (DRYFLOR et al. 2016).
The evergreen and semideciduous evolutionary group holds the greatest amount of phylogenetic diversity, both overall and unique to them (Figure 3c, Figures S3 and S5). This finding might be explained by the broader representation of clades in the evergreen and semideciduous groups, including basal dicots such as Lauraceae and Hernandiaceae (Rezende et al. 2020a). On the other hand, our findings demonstrate that a significant proportion of angiosperm tree diversity is unique to savanna assemblages. This unique phylogenetic diversity observed in savanna vegetation emphasises the need for targeted conservation measures to preserve these ecosystems and their rich evolutionary heritage. Tree species that can inhabit savanna areas experiencing fire and/or subjected to drainage stress belong to a restricted set of phylogenetic lineages, which gives a unique evolutionary identity to savannas. Furthermore, the savanna biome is home to a rich diversity of non-woody plants, many of which represent unique evolutionary adaptations to the specific environmental conditions of the savanna, contributing to the overall distinctiveness of its biodiversity. Future studies encompassing all plant life forms may show that unique savanna evolutionary diversity rivals that of the dry or moist forest biomes.
It is worth emphasising that the savanna in Cerrado Domain is predominantly associated with poor dystrophic soils, whereas areas with higher soil fertility tend to support tropical dry forest or cerradão (i.e., mesotrophic cerradão on richer soils) (Ribeiro and Walter 2008). This pattern is reflected in our results, which indicate a strong evolutionary differentiation between fire-adapted lineages that dominate savannas and those that characterise forest formations. While dry forests may resemble savannas during the dry season, a key distinction is that savannas experience frequent and natural fires, shaping their floristic and phylogenetic composition over evolutionary timescales. Our findings support this dynamic, showing that cerradão, particularly mesotrophic cerradão, occupies an intermediate evolutionary position, sharing lineages with both savanna and forest assemblages. This reinforces its role as a transitional ecosystem, where shifts in fire regimes and soil fertility can influence species composition and community structure. In areas where fire is suppressed, the accumulation of organic matter through litter deposition and nutrient cycling can gradually increase soil fertility, fostering conditions that favour the establishment of dry forest species (Bueno et al. 2018). These interactions between fire, soil and evolutionary history highlight the complexity of community assembly in the Cerrado Domain and the importance of considering both ecological and phylogenetic perspectives in understanding vegetation dynamics.
Understanding the environmental factors that shape these vegetation types helps us assess their evolutionary history and predict susceptibility to global change. Recent studies indicate that the Brazilian Cerrado is becoming hotter and drier, with increasing drought frequency and declining precipitation and relative humidity (Hofmann et al. 2021). These climatic changes can significantly alter the composition and distribution of vegetation formations, favouring the expansion of savannas at the expense of forests due to the greater drought susceptibility of forest taxa. Additionally, changes in land use and the fragmentation of natural habitats can exacerbate these vulnerabilities. Identifying indicator lineages for each vegetation type and analysing their environmental associations can help develop adaptive conservation strategies to mitigate climate change impacts and enhance ecosystem resilience.
5 Conclusions
Our results demonstrate that savannas constitute a distinct evolutionary group within the Cerrado Domain. Tree species that thrive in fire-prone environments belong to a restricted set of phylogenetic lineages, giving savannas a unique evolutionary identity and revealing a clear evolutionary division between savanna and forest assemblages. Fire has acted as a strong evolutionary filter, selecting for fire-tolerant and fire-dependent lineages, while forest assemblages are more strongly structured by water availability and soil fertility, distinguishing dry forests from evergreen and semideciduous forests. Given the marked evolutionary differentiation of tree assemblages across the Cerrado Domain, it is imperative to recognise and address the specific conservation challenges faced by each evolutionary group of assemblages (savanna, evergreen and semideciduous forest and tropical dry forest). Effective management must account for their differential responses to fire and water availability. In particular, balancing fire regimes is essential –promoting savanna diversity while protecting forests from degradation (Flores et al. 2021).
Author Contributions
V.P., K.G.D. and M.L.B. conceived the ideas and methodological approach and conducted the analyses. V.P. led the writing of the manuscript with contributions from K.G.D., V.L.R. and M.L.B. All authors provided comments on the text and approved the final version of the manuscript.
Acknowledgements
The authors are grateful to FUNDECT (Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul) (71/050.384/2022) for financial support. M.L.B. is supported by a productivity fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (313179/2022-0). No fieldwork permits were required for the activities conducted in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data used in this study are available from: NeoTropTree database (NTT): http://www.neotroptree.info.




