The flickering connectivity system of the north Andean páramos
Abstract
Aim
To quantify the effect of Pleistocene climate fluctuations on habitat connectivity across páramos in the Northern Andes.
Location
Northern Andes.
Methods
The unique páramos habitat underwent dynamic shifts in elevation in response to changing climate conditions during the Pleistocene. The lower boundary of the páramos is defined by the upper forest line, which is known to be highly responsive to temperature. Here, we reconstruct the extent and connectivity of páramos over the last 1 million years (Myr) by reconstructing the upper forest line from the long fossil pollen record of Funza09, Colombia, and applying it to spatial mapping on modern topographies across the Northern Andes for 752 time slices. Data provide an estimate of how often and for how long different elevations were occupied by páramos and estimate their connectivity to provide insights into the role of topography in biogeographical patterns of páramos.
Results
Our findings show that connectivity amongst páramos of the Northern Andes was highly dynamic, both within and across mountain ranges. Connectivity amongst páramos peaked during extreme glacial periods but intermediate cool stadials and mild interstadials dominated the climate system. These variable degrees of connectivity through time result in what we term the ‘flickering connectivity system’. We provide a visualization (video) to showcase this phenomenon. Patterns of connectivity in the Northern Andes contradict patterns observed in other mountain ranges of differing topographies.
Main conclusions
Pleistocene climate change was the driver of significant elevational and spatial shifts in páramos causing dynamic changes in habitat connectivity across and within all mountain ranges. Some generalities emerge, including the fact that connectivity was greatest during the most ephemeral of times. However, the timing, duration and degree of connectivity varied substantially among mountain ranges depending on their topographical configuration. The flickering connectivity system of the páramos uncovers the dynamic settings in which evolutionary radiations shaped the most diverse alpine biome on Earth.
1 INTRODUCTION
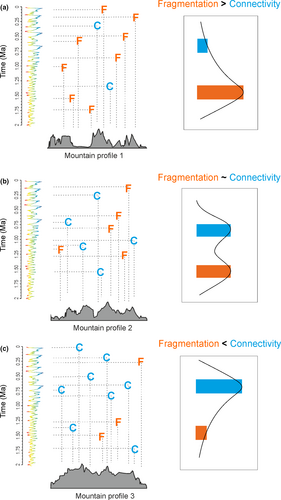
Mountains are regarded as powerhouses of biodiversity in the world (Antonelli et al., 2018; Barthlott, Rafiqpoor, Kier, & Kreft, 2005; Kreft & Jetz, 2007) and harbour numerous examples of very rapid and recent species diversifications (‘radiations’; Hughes & Atchison, 2015). It is thought that a large part of this diversity arose geologically recently, during the Plio-Pleistocene (last 5.3 million years [Myr]), but there is no consensus on the drivers of these radiations. One favoured hypothesis is that the combination of high topographical relief and Plio-Pleistocene climatic oscillations led to rapidly changing distributions of montane species, which generated new lineages (e.g. Graham et al., 2014; Mutke, Jacobs, Meyers, Henning, & Weigend, 2014; Qian & Ricklefs, 2000). However, the relative contributions of isolation (e.g. Schönswetter, Stehlik, Holderegger, & Tribsch, 2005; Wallis, Waters, Upton, & Craw, 2016; Weir, Haddrath, Robertson, Colbourne, & Baker, 2016) versus gene flow and dispersal (e.g. Cadena, Pedraza, & Brumfield, 2016; Knowles & Massatti, 2017; Kolář, Dušková, & Sklenář, 2016; Smith et al., 2014) in driving fast diversification rates (i.e. the ‘species-pump’ effect, Rull, 2005; Rull & Nogué, 2007; Winkworth, Wagstaff, Glenny, & Lockhart, 2005; Ramírez-Barahona & Eguiarte, 2013; Steinbauer et al., 2016; Flantua & Hooghiemstra, 2018) are still debated. It is likely that these radiations have been the results of the interchange between phases of isolation, causing allopatric, in situ speciation, and connectivity, triggering diversification through dispersal and settlement in new areas and hybridization of differentiated taxa from previously isolated populations (Flantua & Hooghiemstra, 2018). The fastest and most spectacular radiations may therefore occur in mountain regions with variable degrees of past connectivity and isolation during climate fluctuations, which, complex in space and time, are inherently related to the mountain topography (Flantua & Hooghiemstra, 2018). It is therefore critical to quantify connectivity of montane habitats using our understanding of topography and past climate fluctuations (Figure 1).
The Northern Andes is an ideal model system to quantify connectivity, due to the large variation in topography and the advanced palaeoecological knowledge on Plio-Pleistocene climate fluctuations derived during the last five decades (Hooghiemstra & Flantua, 2019). The Northern Andes is topographically rich with high elevations, steep ridges and valleys (see illustrations by Von Humboldt during his trips in Latin America, 1773–1858), composed of several mountain ranges, some of which are parallel running from North to South. The area hosts the treeless tundra-like alpine biome, the páramos, regarded as the richest alpine flora in the world in terms of endemism and species richness (Sklenář, Hedberg, & Cleef, 2014) and is known for its bursts of Plio-Pleistocene species diversification amongst plants (Hughes & Atchison, 2015; Madriñán, Cortés, & Richardson, 2013). In terms of quantifying Plio-Pleistocene temperature fluctuations, the palaeoecological history of the páramos has been studied extensively (e.g. Cleef, 1979; Hooghiemstra, 1984; Hooghiemstra & Van der Hammen, 2004; Van der Hammen, 1974; Van der Hammen & Cleef, 1986) because of the unique high elevation fossil pollen records that cover most of the Pleistocene (Bogotá-A, Hooghiemstra, & Berrio, 2016; Bogotá-Angel et al., 2011; Groot, Hooghiemstra, Berrio, & Giraldo, 2013; Groot et al., 2011; Torres, Hooghiemstra, Lourens, & Tzedakis, 2013). Under current conditions, the páramos form isolated archipelagos of ‘alpine (sky) islands’ (McCormack, Huang, & Knowles, 2009; Sklenář et al., 2014) but the rich collection of fossil pollen sequences throughout this region (Flantua et al., 2015) show that the páramos underwent substantial elevational shifts during the Pleistocene, resulting in extensive changes in surface area and connectivity (Flantua et al., 2014; Hooghiemstra & Van der Hammen, 2004; Sklenář et al., 2014; Van der Hammen, 1974). Thus, the topographical diversity and the robust catalogue of palaeoecological reconstructions make the Northern Andes a highly suitable model region to explore patterns of connectivity in mountain biomes in response to Pleistocene climate fluctuations.
In this study, we aim to quantify the biogeographic changes of the páramos in terms of spatial scale and connectivity based on modern topography and pollen-based records of past climate change. Specifically, we developed a novel tool to explore the complex temporal and spatial patterns of páramo connectivity. We constrain our model by using the last 1 Myr of the high-resolution fossil pollen record of Funza09, a composite 586 m deep core taken from the Bogotá basin of Colombia (Torres et al., 2013). Available surface area (Elsen & Tingley, 2015) and connectivity (Bertuzzo et al., 2016; Flantua & Hooghiemstra, 2017; Flantua et al., 2014) is variable along elevational gradients of mountains. We therefore hypothesize that the different mountain ranges that compose the Northern Andes display variable patterns of past páramo connectivity dependent upon their topography (Figure 1). We discuss the implications of our outcomes for evolutionary processes and how defining and quantifying past connectivity in mountain systems is essential to help reveal mechanisms of ecological, biogeographical and evolutionary change. Ultimately, our quantification of páramo connectivity through space and time provides a unique opportunity to disentangle some of the mechanistic drivers (‘modulators’) of radiations in this biome (Bouchenak-Khelladi, Onstein, Xing, Schwery, & Linder, 2015).
2 MATERIAL AND METHODS
2.1 Geographical features
The Northern Andes (ca. 448,000 km2) covers parts of Venezuela, Colombia and Ecuador (Figure 2a), and can be partitioned into six principal mountain ranges or ‘cordilleras’ (Figure 2c), namely the Sierra Nevada de Santa Marta (SNSM), Cordillera de Mérida, Eastern, Central and Western Cordillera and the Ecuadorian Cordilleras. Most of the Northern Andes is considered a highly to extremely high rugged landscape (Figure 2b; See mountain illustrations by Von Humboldt, 1845) where the high peaks and deep inter-Andean valleys cause strong contrasts in climate throughout the region (Flantua et al., 2016). Surface area in mountains does not decrease monotonically with elevation as has been shown previously in southern Colombia by Flantua et al. (2014) and on a global scale by Elsen and Tingley (2015). The Northern Andes shows a decrease of surface area going upslope where there is a slight peak around 900–1,200 m above sea level (a.s.l.) but then continues to decrease up to 6,260 m a.s.l. (Figure 2d), following a typical ‘pyramid shape’. However, the different cordilleras show different patterns of elevational surface area (Figure 2d) where the Eastern Cordillera shows a sharp peak around 2,600 m a.s.l. and the Ecuadorian Cordillera shows high values of surface area at much higher elevations than the other cordilleras (for more details see Table S1.1, Appendix S1 in Supporting Information). The páramos today are spread out over the Northern Andes as a ‘mountain archipelago’ of small and highly fragmented páramo complexes (See Figs S2.1, S2.2 for more details and photos of different páramo complexes) but their full range also cover isolated páramo islands in Costa Rica and northern Peru (Luteyn, 1999). Of all tropical alpine floras, such as in East Africa and New Guinea, the páramos are home to the highest species richness and endemism (Luteyn, 1999; Sklenář et al., 2014), with low between-mountain similarity in species (Sklenář et al., 2014). They also provide numerous ecosystem services on a local and regional scale (Herzog, Martínez, Jørgensen, Tiesse, 2011) and references therein), and especially in terms of hydrological services, they are vital for the provision of fresh water to several large cities in South America, such as Bogotá, Medellín, Quito, Cuenca, Piura and Cajamarca.

2.2 Quantifying temperature and upper forest line based on fossil pollen data
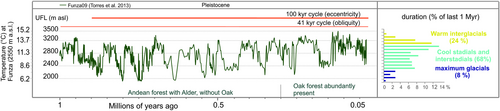
To quantify temperature fluctuations during the Pleistocene (and consequently estimate páramo connectivity), we used fossil pollen data from the Northern Andes. The composite pollen record Funza09 (4.83°N, 75.2°W; 2,550 m a.s.l., Fig. S2.1. Red star) reveals vegetation and climate dynamics over the past 2.25 Myr (Torres et al., 2013). We reconstructed the páramos’ elevational fluctuations, and consequently páramo connectivity, by estimating the upper forest line (UFL; the transition from the upper montane forest to the páramos) from the Funza09 record. Though this record covers the last 2.25 Myr, we only used the last 1 Myr as this interval reflects continuous lake conditions in comparison with variable hydrological conditions between 2.2 and 1 million years ago (Ma) which makes a quantification of changes to the UFL less precise. We follow the methodology described and implemented by Hooghiemstra (1984), Groot et al. (2011) and Hooghiemstra et al. (2012) to derive the Andean UFL and palaeotemperature curve (for detailed methodology on the UFL reconstruction see Appendix S3).
2.3 Calculations of connectivity per páramo ‘island’
To calculate the degree of connectivity between páramos, we used a graph-based habitat availability index called probability of connectivity (PC) metric. This metric takes into account the area of the páramo ‘island’ itself and the distances to other islands where a user-defined distance threshold defines the ‘reachability’ of other islands (Saura, Estreguil, Mouton, & Rodríguez-Freire, 2011; Saura & Pascual-Hortal, 2007), even if they are not physically connected (i.e. ‘structural connectivity’, Tischendorf & Fahrig, 2000). The metric assigns a value to each páramo island representing its contribution in maintaining the overall connectivity of the páramo biome (Saura & Pascual-Hortal, 2007; Saura et al., 2011). The total PC is built up in three ‘fractions’, namely the ‘intrapatch’, the ‘flux’ and the ‘connector’ fractions (Saura & Rubio, 2010). The first fraction focuses on the available surface area and habitat quality (if applicable) within the individual island. The second fraction assesses how well the individual island is connected to other islands given additional importance to the other islands’ attributes (surface and quality) and its strategic position to other páramo islands. The third fraction quantifies the contribution of the island to maintain connectivity between the rest of the islands, in other words its role as an intermediate stepping stone between non-adjacent islands. Additionally, we calculated the equivalent connected area (ECA), which is derived directly from the PC, as a measure of the overall connectivity of a region (Saura et al., 2011). Here, Conefor Sensinode (V2.2; Saura & Pascual-Hortal, 2007; Saura & Torné, 2009) and ESRI ArcGIS 10.3 (ESRI, 2014) were used to calculate the straight-line distances between islands, the PC and ECA. We calculated connectivity for the entire Northern Andes and for each mountain range separately.
2.4 Calculations of corridors between páramo islands
We identified corridors between páramo islands within and between cordilleras under different climatic conditions. We used the Gnarly Landscape Utilities (V0.1.3; McRae, Shirk, & Platt, 2013) with ESRI ArcGIS 10.3 to create a raster grid of ‘landscape resistance’ based on ruggedness (Figure 2b) and habitat suitability. We assumed an increased landscape resistance with increased ruggedness, assigning values between 0 (no resistance) to 100 (maximum resistance) using an equal interval classification. For the habitat suitability map, we started by assigning a ‘perfectly suitable’ score of 100 to each páramo island, while outside the island the score of 0 reflects maximum unsuitability. To soften this boundary, an exponential decay function was then used by increasing resistance in five elevational steps of 100 m where we assigned a suitability score of 40 to the boundary of the páramo. As a result of the decay function the highest suitability of páramo – its core area – was restrained 200 m above the UFL and 200 m below the snowline.
We used Linkage mapper to calculate the least-cost pathways, or corridors, based on the produced raster grid of landscape resistance (McRae & Kavanagh, 2011). These corridors are expressed as ‘conductance maps’ that represent gradients of cumulative corridors. Where the densities of corridors is highest, it is assumed that there is a high probability of dispersal and migration possible between islands (McRae, Dickson, Keitt, & Shah, 2008). The full landscape of the Northern Andes is considered an area where corridors could exist, with exception of the region between SNSM and the Sierra de Perijá (Fig. S2.1).
We resampled the 30 m Digital Elevation Model (DEM, Figure 2) to a 1 km resolution to reduce computing time for each Linkage mapper down to on average 2 hr. We allowed Linkage mapper to create corridors through (instead of only between) core areas to represent the full arsenal of connectivity through the landscape. Only corridors between páramo islands larger than 1 km2 were considered at any given moment in time. From the final output maps, only values lower than 200k conductance (default threshold) are selected to highlight the strongest corridors. The outputs were weighted according to the percentage of time they occurred during the last 1 Myr.
3 RESULTS
3.1 A million years of temperature fluctuations
Temperatures at Funza (2,550 m a.s.l.) are estimated to have fluctuated between ca. 15 and 6°C causing an estimated maximum 1,600 m elevational shift of the UFL between ca. 3,500 and ca. 1,900 m a.s.l. (Figure 3). The Pleistocene glacial-interglacial dynamics were not replicated cycles of temperature change showing repeated patterns of high and lows, but display a high temporal variability between each glacial-interglacial cycle. Conditions similar to the current warm, interglacial conditions occurred several times during the last 1 Myr and accounted for around a quarter of the time. Extreme cool glacial conditions, ~6–8°C cooler than today, were relatively rare, occurring less than 10 percent of the time. On the whole, intermediate cool stadials and mild interstadials dominated the last 1 Myr, occurring over two-thirds of the time.
3.2 Calculations of páramo connectivity
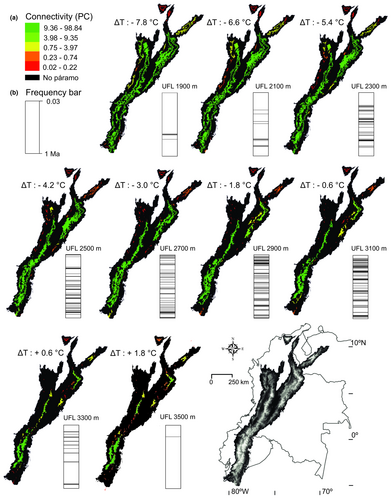
Our estimations on the spatial and elevational extent of ancient páramos and their connectedness at different times in the past reveals that páramos underwent frequent spatial alterations between fragmented and connected spatial configurations, but the exact patterns were highly dependent on mountain chain topography (Figure 4a,b. See Appendices S4 and S5). The páramos in the Ecuadorian Cordillera generally maintained a high degree of connectivity over the last 1 Myr, rarely enduring severe fragmentation. Fragmentation did however occur when the snowline plunged significantly during colder and wetter glacial periods, causing a break up of páramo areas on lateral flanks of the mountains. Likewise, the level of connectivity between páramos on the Central Cordillera fragmented substantially through a descending snowline, breaking the upper elevation limit of páramo connectivity. In contrast, the Eastern Cordillera shifted substantially between periods of connectivity and fragmentation, always, however, maintaining two large páramo islands surrounded by smaller ‘satellite islands’. Páramos in the Cordillera de Mérida seem to have been restricted during interglacials to one core area only, while during colder periods a relatively high fragmentation is observed possibly due to glaciers pushing páramos to lateral distributions. Here, connectivity increased mainly towards the southwest and during colder periods (UFL ≤ 2,300 m a.s.l.). The páramos of the SNSM and the Western Cordillera endured the highest degree of rates of change in fragmentation of all ranges. In the latter, páramo habitats are estimated to have often completely disappeared. In contrast, páramos of the Central Cordillera maintained a long latitudinal distribution, forming a chain of isolated populations in small patches that on the whole remained connected. Even in very cold conditions, no continuous connectivity of core areas seems to have been possible between the Eastern Cordillera and Cordillera de Mérida, or the region of Sierra de Perijá. Towards the south of the Eastern Cordillera a low-elevation barrier was possibly crossed at 1,900 m a.s.l. forming a brief bridge suitable for páramo habitat into the Macizo Colombiano of the Central Cordillera.
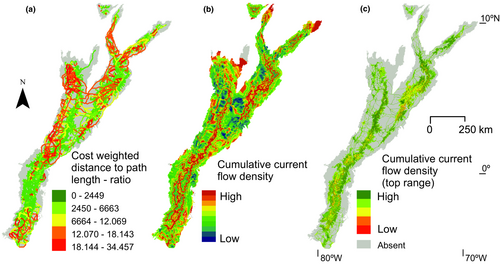
The reconstruction of putative corridors shows a complex spatial pattern through the mountainous landscapes of the Northern Andes (Figure 5a,b). The long ridge of the Central Cordillera forms the starting point of numerous corridors to the páramos in the Western Cordillera. The Eastern Cordillera shows a complex internal pattern of corridors, where there are neither strong corridors towards Sierra de Perijá in the North, nor towards the Cordillera de Mérida, while a high concentration of corridors is found between the large páramos complexes in the Eastern Cordillera (Páramos of Boyacá and Cundinamarca, Fig. S2.1). In the Ecuadorian Cordillera a more lateral pattern of high/low potential corridors is observed following the intra-Andean valleys and peaks within this mountain range. Corridors to the southernmost páramos of Ecuador as also the northernmost páramos of the Western Cordillera are weak and occurred infrequent during the last million years, shown by the thin lines.
3.3 Flickering connectivity systems
Páramo connectivity through time shows a highly variable pattern (Figure 6a) introduced by Flantua and Hooghiemstra (2018) as a flickering connectivity system (see visualization video in Appendix S6). We find support for the hypothesis that this system with fluctuating, highly variable connectivity in spatial and temporal dimension is unique for each mountain range of the Northern Andes (Figure 1). For example, changes in connectivity within the Ecuadorian Cordillera are substantial but the system ‘flickers’ around a high average when compared to other mountain ranges. The flickering connectivity systems within the Eastern and Central Cordillera are surprisingly similar, though the peaks of connectivity during glacial periods and the dips of connectivity during interglacials are more extreme in the former (Figure 6a). The Western Cordillera is a larger mountain range than the Cordillera of Mérida and the SNSM (Table S1.1), and its variation of connectivity has been correspondingly larger (Figure 6b) but with the lowest occurrence of connectivity compared to the other mountain ranges (Figure 6a). Considering only the frequency in the distribution of data (Figure 6b), the Ecuadorian Cordillera and the SNSM stand out for their relatively small within-mountain range variation in connectivity, compared to the Eastern and Central Cordillera (similar patterns) and the Western Cordillera.
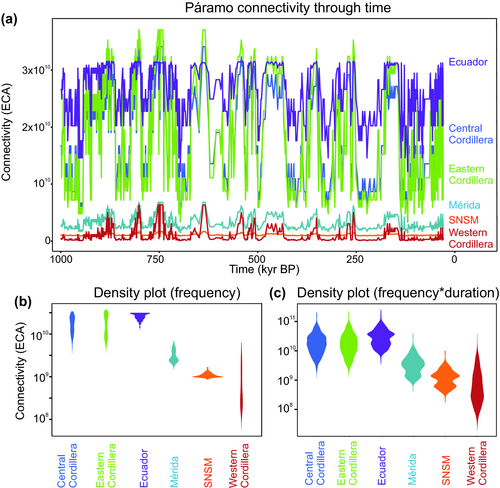
When frequencies of connectivity are weighted by the amount of time that connectivity lasted two main patterns emerge (Figure 6c). The first is shared by the Western, Central and Eastern Cordilleras, which all display an elongated pattern where the highest values are around a centroid, resembling a ‘humming top’ or, as Elsen and Tingley (2015) recognized in mountain hypsographies, a ‘diamond’ shape. Ecuadorian Cordilleras, Cordillera de Mérida and SNSM instead reveal a different pattern with a narrower centroid that widens towards the upper and lower section, resembling an ‘hourglass’ shape. Here, the Ecuadorian Cordillera and SNSM show a surprising similarity though at different connectivity ranges. The Central and Eastern Cordilleras are strikingly similar overall.
4 DISCUSSION
4.1 Variable degrees of past connectivity of different mountain ranges
Although currently isolated, evolutionary radiations and the assembly of the páramo ecosystem formed during times when the páramos were flickering in and out of different degrees of connectivity (Figure 6). The concept of ‘mountain fingerprints’ (Flantua & Hooghiemstra, 2018) proposes that the region's complex topography would have meant that páramos in different mountain regions would have fragmented and connected at different periods of time, at different elevations, and with different rates and frequencies (as summarized in Figure 1). This means that in some mountain ranges the páramos are a mix of somewhat even occurrence of connectivity and fragmentation events through time (Figure 1b, exemplified by the Eastern Cordillera), or could have been dominantly fragmented (Figure 1a, e.g. Western Cordillera), or more connected (Figure 1c, e.g. Ecuadorian Cordilleras). These regional differences in the temporal and spatial variation in past páramo connectivity (Figures 4-6) are likely to have resulted not only in regional differences in biogeographical patterns through time, but also varying ecological and evolutionary processes. We therefore propose that our data and models can be used to test hypotheses of the drivers of species richness, endemism and degrees of Pleistocene diversification in the Northern Andes, and the approach applicable to other mountain regions around the world.
4.2 Evolutionary implications of the flickering connectivity system
Several insightful schematic representations of Pleistocene diversification models in the Neotropics have been developed in recent years (Flantua & Hooghiemstra, 2018; Hazzi, Moreno, Ortiz-Movliav, & Palacio, 2018; Ramírez-Barahona & Eguiarte, 2013; Rull, 2005). Phylogeographical and phylogenetic synthesis work within and among páramo taxa is currently still largely lacking (see for instance Yu et al., 2019 for the Qinghai-Tibet Plateau), inhibiting the direct testing of these models. However, here we highlight several recent studies that are considered valuable in the light of the flickering connectivity system reconstruction (see Table 1), emphasizing the expectation that the rapidly growing body of phylogeographical/phylogenetic literature in the region will support future comparative analyses.
| Level of analysis/Mountain range | Group | Taxon | Family | Dataset/markers | Approach | Result/finding | Reference |
|---|---|---|---|---|---|---|---|
| Ecuadorian Cordillera | Birds | Spinus | Fringillidae | cyt b, ND2, ND3, MUSK, MYO2 | Phylogenetic | Recent radiation | Beckman and Witt (2015) |
| Andes | Plants | Valeriana | Valerianaceae | psbA-trnH intron, trnK-matK intron, trnL-F, ITS | Phylogenetic | Recent radiation | Bell and Donoghue (2005) |
| Eastern and Central Cordillera | Birds | Buarremon | Emberizidae | ND2, cyt b, ATPase 6, ATPase 8, ACO1, MUSK | Phylogeographic | High genetic divergence | Cadena, Klicka, and Ricklefs (2007) |
| Western and Central Cordillera | Birds | Buarremon | Emberizidae | ND2, cyt b, ATPase 6, ATPase 8, ACO1, MUSK | Phylogeographic | High genetic resemblance and likely high migration | Cadena et al. (2007) |
| Andes | Plants | Oreobolus | Cyperacea | ITS, trnL inron and trnL-F intergenic spacer | Phylogenetic | Recent radiation; South to north migration | Chacón, Madriñán, Chase, and Bruhl (2006) |
| Andes | Birds | Muscisaxicola | Tyrannidae | COII and ND3 | Phylogenetic | Recent radiation | Chesser (2000) |
| Central and Eastern Cordillera, Ecuadorial Cordillera | Plants | Lupinus | Leguminosae | Genome-scale nextRADseq | Phylogeographic/Phylogenomic | High genetic divergence | Contreras-Ortiz, Atchison, Hughes, and Madriňán (2018) |
| Central and Eastern Cordillera | Plants | Espeletia | Asteraceae | Genotyping by sequencing | Phylogeographic | Rapid morphological radiations | Cortés, Garzón, Valencia, and Madriñán (2018) |
| Northern Andes | Plants | Calceolaria | Calceolariaceae | ITS, matK and morphology | Phylogenetic | Recent radiation | Cosacov et al. (2009) |
| Northern Andes | Plants | Espeletiinae | Asteraceae | n.a. | Biogeographic | Step-wise but irregular migration of species | Cuatrecasas (1979, 2013) |
| Eastern Cordillera | Plants | Espeletiinae | Asteraceae | ITS, ETS, rpl16, AFLP data | Phylogenetic | High genetic diversity in the larger páramo complexes with multiple distinct clades somewhat related to each other | Diazgranados and Barber (2017) |
| Eastern Cordillera | Plants | Espeletiinae | Asteraceae | ITS, ETS, rpl16, AFLP data | Phylogenetic | Hybridization | Diazgranados and Barber (2017) |
| Ecuadorian Cordillera | Plants | Senecio | Asteraceae | ITS, AFLP data | Phylogenetic/Phylogeographic | Genetic differences between northern and southern populations within Ecuador | Dušková et al. (2017) |
| Northern Andes | Plants | Oxalis | Oxaliaceae | ITS and ncpGS | Phylogenetic | Recent radiation | Emshwiller (2002) |
| Northern Andes | Plants | Oreobolus | Cyperacea | ITS, trnL-F, trnH-psbA and rpl32-trnL | Phylogeographic | Incomplete lineage sorting, cryptic speciation, genetic divergence, suggests evidence of repeated vicariance and secondary contact | Gómez-Gutiérrez et al. (2017) |
| Andes | Birds | Many | n.a. | n.a. | Phylogenetic | Bioregion formations correlates with Andean uplift and mountain dispersal facilitated by temperature oscillations of the Pleistocene | Hazzi et al. (2018) |
| Central and Eastern Cordillera | Plants | Lupinus | Leguminosae | ITS/LEGCYCIA genes | Phylogenetic | Recent radiation, higher diversification at higher elevations | Hughes and Eastwood (2006); Drummond, Eastwood, Miotto, and Hughes (2012); Hughes and Atchison (2015) |
| Eastern and Central Cordillera | Plants | Puya | Bromeliaceae | AFLP data | Phylogenetic | Genetic divergence, suggest multiple migration events from the Eastern Cordillera to the Western Cordillera | Jabaily and Sytsma (2013) |
| Northern Andes | Plants | Puya | Bromeliaceae | AFLP data | Phylogenetic | Step-wise but irregular migration of páramo plant species, recent rapid radation | Jabaily and Sytsma (2013) |
| Western and Central Cordillera | Plants | Puya | Bromeliaceae | AFLP data | Phylogenetic | Frequent gene flow events | Jabaily and Sytsma (2013) |
| Ecuadorian Cordillera | Plants | Loricaria | Asteraceae | AFLP and plastid DNA | Phylogeographic | Step-wise but irregular migration of páramo plant species | Kolář et al. (2016) |
| Ecuadorian Cordillera | Plants | Loricaria | Asteraceae | AFLP and plastid DNA | Phylogeographic | Lack of genetic divergence. Suggests extensive gene flow. | Kolář et al. (2016) |
| Andes | Plants | Many | n.a. | n.a. | Phylogenetic | Environmental change, adaptation and biotic interactions as drivers of Andean radiations | Luebert and Weigend (2014) |
| Andes | Plants | Polystichum | Dryopteridaceae | Cytosolic phosphoglucose isomerase gene | Phylogenetic | Gene evolution during radiation | Lyons, McHenry, and Barrington (2017) |
| Northern Andes | Plants | Many | Asteraceae | n.a. | Phylogenetic | Recent radiation | Madriñán et al. (2013) |
| Andes | Plants | Polystichum | Dryopteridaceae | trnS-rps4, rbcL | Phylogenetic | Recent radiation, multiple secondary dispersal events from central Andes to Northern Andes | McHenry and Barrington (2014) |
| Northern Andes | Plants | Lachemilla | Rosaceae | ITS, trnL-F | Phylogenetic | Recent radiation | Morales-Briones, Romoleroux, Kolář, and Tank (2018) |
| Central and Eastern Cordillera | Plants | Lupinus | Leguminosae | Genomic and transcriptomic data | Phylogenomic/Phylogeographic | High genetic divergence but events of gene flow detected | Nevado et al. (2018) |
| Eastern Cordillera | Plants | Lupinus | Leguminosae | Genomic and transcriptomic data | Phylogenomic/Phylogeographic | High genetic diversity in the larger páramo complexes with multiple distinct clades somewhat related to each other | Nevado et al. (2018) |
| Eastern Cordillera | Plants | Lupinus | Leguminosae | Genomic and transcriptomic data | Phylogenomic/Phylogeographic | Hybridization | Nevado et al. (2018) |
| Andes | Plants | Hypericum | Hypericaceae | n.a. | n.a. | Suggests niche expansion/evolution and shifts in temperature optima that may have facilitated páramo radiations | Nürk, Michling, and Linder (2017) |
| Andes | Plants | Hypericum | Hypericaceae | ITS | Phylogenetic | Recent radiation | Nürk, Scheriau, and Madriñán (2013) |
| Eastern Cordillera and Merida Cordillera | Plants | Espeletia | Asteraceae | Metabolomics | n.a. | Metabolic fingerprints linked to high genetic divergence but with events of gene flow | Padilla-González, Diazgranados, and Da Costa (2017) |
| Western and Central Cordillera | Plants | Espeletia | Asteraceae | Metabolomics | n.a. | Apparent clustering | Padilla-González et al. (2017) |
| Merida Cordillera, northern tip of Eastern Cordillera | Plants | Espeletiinae | Asteraceae | Whole plastomes, de novo assembly | Phylogenomic | Hybridization, suggests two independent centers of radiations and no dispersal between cordilleras. Increase of diversification during last 1 Myr | Pouchon et al. (2018) |
| Andes | Birds | Many | n.a. | n.a. | Phylogenetic | Higher diversification rates at higher elevations | Quintero and Jetz (2018) |
| Northern Andes | Plants | Espeletia complex | Asteraceae | ITS | Phylogenetic | Recent radiation | Rauscher (2002) |
| Andes | Birds | Pionus | Psittacidae | cyt a and ND2 | Phylogenetic | Recent radiation | Ribas, Moyle, Miyaki, and Cracraft (2007) |
| Northern Andes | Plants | Jamesonia-Eriosorus Complex | Pteridaceae | ETS, 18S–26S rDNA, rps4, intergenic spacer rps4-trnS | Phylogenetic | Fast speciation of páramo species, possibly linked to morphological adaptation | Sánchez-Baracaldo and Thomas (2014) |
| Northern Andes | Plants | Jamesonia, Eriosorus | Pteridaceae | ETS, 18S–26S rDNA, rps4, intergenic spacer rps4-trnS | Phylogenetic | Recent radiation | Sánchez-Baracaldo (2004) |
| Andes | Plants | Astragalus | Leguminosae | ITS and chloroplast spacers trnD-trnT and trnfM-trnS1 | Phylogenetic | Recent radiation | Scherson, Vidal, and Sanderson (2008) |
| Andes | Plants | Bartsia | Orobanchaceae | trnT–trnF region and the rps16 intron | Phylogenetic | Recent radiation, suggests to be related to dispersification | Uribe-Convers and Tank (2015) |
| Andes | Plants | Diplostephium | Asteraceae | ITS, rpoB, rpoC1, and psbA-trnH | Phylogenetic | Radiation originated in páramo, with diversification slowdowns associated with colonization of Andean forests. Phylogeny shows large uncertainty. | Vargas and Madriñán (2012) |
| Northern Andes | Plants | Diplostephium | Asteraceae | Complete nuclear ribosomal cistron, the complete chloroplast genome, a partial mitochondrial genome and nuclear-ddRAD | Phylogenomic | Hybridization, recent radiation | Vargas, Ortiz, and Simpson (2017) |
| Eastern Cordillera, Cordilleras of Colombia | Plants | Lupinus alopecuroides | Leguminosae | 11 microsatellite markers | Phylogeographic | High genetic diversity in the larger páramo complexes with multiple distinct clades somewhat related to each other | Vásquez, Balslev, Hansen, Sklenář, and Romoleroux (2016) |
| Andes | Plants | Gentianella, Halenia | Gentianaceae | ITS, matK, rpl16 intron | Phylogenetic | Recent radiation | von Hagen and Kadereit (2001, 2003) |
| Andes | Birds | Many | n.a. | n.a. | Phylogenetic | Recent radiation, increase in diversification in last 1 Myr | Weir (2006) |
| Northern Andes | Plants | Escallonia | Escalloniaceae | trnH-psbA, MYC, NIA | Phylogenetic | Recent radiation | Zapata (2013) |
The dynamic history of the páramos elucidated by the flickering connectivity system can provide three important insights in terms of evolutionary processes. First of all, the regional differences in past páramo connectivity – the mountain fingerprint – support temporally and spatially discordant phylogeographical patterns (Massatti & Knowles, 2014; Papadopoulou & Knowles, 2015, 2016; Pennington et al., 2010). This means that the timing of diversification in the different mountain regions would not be expected to have occurred synchronously, even if all phylogenetic studies on páramo species could overcome current issues in model inference, taxonomy and distribution, spatial resolution and time-calibration points (Rull, 2011). Secondly, diversification rates might differ along the elevational gradient and this might be the rule rather than the exception. Elevational differences in surface availability and connectivity (Bertuzzo et al., 2016; Flantua & Hooghiemstra, 2017; Flantua et al., 2014) are likely to influence at what elevation the strongest geographical processes will occur, and these processes are thus expected to differ between mountain systems resulting in elevational differences of diversification (see e.g. Hughes & Eastwood, 2006; Kropf, Kadereit, & Comes, 2003; Lagomarsino, Condamine, Antonelli, Mulch, & Davis, 2016; Quintero & Jetz, 2018). Furthermore, the climate fluctuations of the Pleistocene caused connectivity to occur at different moments through time (Figure 1), a process facilitating the step-wise but irregular migration of páramo plant species throughout the landscape, such as Puya, Loricaria and Espeletiinae (Table 1). Thirdly, the flickering connectivity system, which is expected to cause phases of increased isolation followed by increased connectivity of populations, is expected to result in pulses of diversification (Knowles, 2000), possibly resulting in series of sub-radiations in the páramos. Where isolation resulted in allopatric, in situ speciation, connectivity triggered diversification through dispersal and settlement in new areas (‘dispersification’, Moore & Donoghue, 2007), and hybridization of previously isolated populations (Grant, 2014; Petit et al., 2003). Much evidence suggests that hybridization is not the processes of species becoming ‘reabsorbed’ into their parental forms but contributes by bringing evolutionary novelty and gene flow operating at different introgression rates (Dušková et al., 2017; Nevado, Contreras-Ortiz, Hughes, & Filatov, 2018; Pouchon et al., 2018), and thus a likely trigger of speciation and morphological diversity. Interestingly, population-level processes such as gene flow, dispersification and hybridization, alongside periods of isolation, have been increasingly recognized to play out at the phylogenetic scale, leading to (rapid) lineage diversification, for example in mountains (e.g. Hazzi et al., 2018; Knowles & Massatti, 2017), tropical rain forests (e.g. Onstein et al., 2017) and islands (e.g. Ali & Aitchison, 2014). Interestingly, the Funza09 pollen record shows a clear increase in the amplitude of climate change around the mid-Pleistocene transition (ca. 0.9 Ma) coinciding with accelerated diversification of high elevation birds (Weir, 2006) and the Espeletiinae in the Cordillera de Mérida (Pouchon et al., 2018; Table 1). Indeed, these studies signal a potential link between the intensity of the flickering connectivity system and biological radiations (Flantua & Hooghiemstra, 2018). Thus, the flickering connectivity system is expected to have left an imprint on geographical patterning of genetic divergence (between populations) and within-populations genetic diversity with obvious inter-cordillera differences. Furthermore, extinction events may further complicate the observed patterns of divergence between cordilleras.
4.3 Future research
Our spatio-temporal estimates of past connectivity lay a foundation for further research on elucidating the causal mechanisms of mountain diversifications (see also Appendix S7). Models of past connectivity (Figures 4-6), when combined with phylogeographic data, could help reveal the role of interspecific gene flow and allopatric speciation in driving radiations in the high Andes and contribute to a better understanding of the relative importance of geography versus adaptive radiation that underpin Andean diversifications. In such a complex system it may also be useful to pay attention to commonalities. For example, when considering both frequency and duration, our data show that two connectivity patterns emerge (i.e. hourglass versus non-hourglass; Figure 6c). Research could explore if cordilleras with shared connectivity patterns also share phylogenetic histories and contemporary (endemic) species’ biogeographies to test for universal mechanisms that have shaped present day alpine biomes. This would be especially useful if used in conjunction with information on the reproductive life histories, growth and dispersal capacities of specific taxa.
Finally, past patterns of connectivity are critical to interpret biogeographical patterns of currently isolated or fragmented systems in a wide variety of terrestrial ecosystems including mountains (Flantua & Hooghiemstra, 2018), islands (e.g. Simpson, 1974; Weigelt, Steinbauer, Cabral, & Kreft, 2016; Norder et al., 2018), fresh water systems (e.g. Dias et al., 2014), rain forests (e.g. Graham, Moritz, & Williams, 2006), grasslands (e.g. Lindborg & Eriksson, 2004; Münzbergová et al., 2013) and marine coastal ecosystems (Hoeksema, 2007) that similarly experienced major spatial changes during rapid sea-level fluctuations over the Pleistocene. The approach developed here, to quantify historical connectivity, can therefore provide a platform for interpreting contemporary biogeographies and past drivers of diversification in a wide array of both marine and terrestrial ecosystems where available space has been altered by climatic fluctuations. We postulate that quantifying flickering connectivity systems will facilitate a much more detailed and much needed quantitative basis to compare phylogeographic/phylogenetic patterns, e.g. the Tibeto-Himalayan region (Muellner-Riehl, 2019), and species (endemic) richness (e.g., Sklenář et al., 2014), from different mountain regions of the world.
5 CONCLUSIONS
We present a pollen record-based biogeographical model for the páramo biome spanning the northern Andes (Venezuela, Colombia and Ecuador) over the last 1 Myr. Our models suggest substantial temperature oscillations where extreme temperature lows were ca. 8°C cooler than today, causing a total amplitude of the UFL of up to 1,600 vertical meters. These extreme cold events were, however, rare (See frequency bars in Figure 4) and during glacial periods most of the time cool stadial and interstadial climate conditions prevailed (Figure 3). Our analysis shows that páramos on all mountain ranges underwent frequent alterations between fragmented and connected configurations (Figures 4 and 5), but the estimated degrees and amount of connectivity varied among mountain ranges (Figure 6). Most páramos expanded during glacial periods even though extensive glaciers were present. To a large extent the current páramo distribution (located near their highest Pleistocene elevational position) was replaced by the lowermost ice extensions during the cool stadials, and during the coldest events replaced by the thick ice masses of mountain glaciers, implicating a substantial range size change of populations and a highly dynamic system during Pleistocene times. Depending on the location of initial dispersal – originating from ancestral areas – species would have experienced the flickering connectivity system differently and thus a mosaic of contrasting patterns of genetic divergence and diversity is expected among cordilleras mirroring the mountain fingerprint signatures.
There the different climates are ranged the one above the other, stage by stage, like the vegetable zones, whose succession they limit; and there the observer may readily trace the laws that regulate the diminution of heat, as they stand indelibly inscribed on the rocky walls and abrupt declivities of the Cordilleras.
(Von Humboldt, 1877 (1845), I, p 46)
ACKNOWLEDGEMENTS
This work was part of SGAF's doctoral thesis funded by Netherlands Organization for Scientific Research (NWO, grant 2012/13248/ALW to HH.). The Hugo de Vries foundation (Amsterdam) is acknowledged for financially supporting multiple grant proposals during the project including the development of the visualization accompanying this paper. The Sistema Nacional de Investigadores (SNI) de SENACYT supported AO. REO acknowledges the support of the German Centre for Integrative Biodiversity Research (iDiv) Halle- Jena-Leipzig funded by the Deutsche Forschungsgemeinschaft (DFG)—FZT 118. CG thanks NUFFIC (the Hague) for financial support to obtain her master degree and Piet Zwart Institute (Rotterdam) advisors for designing and technical support. UvA-IBED colleagues Carina Hoorn and Daniel Kissling are thanked for the educational environment shaped by the parallel paper on mountain diversity (Antonelli et al., 2018). Colin Hughes is thanked for comments on previous versions of this paper. We thank Mauricio Bermúdez for help with the geological delimitation of the Northern Andes and Francisco Cuesta for facilitating the map by Josse et al. (2009).
REFERENCES
BIOSKETCHES
Suzette Flantua has a background in palaeoecology, biogeography, landscape ecology and spatial analyses, and enjoys integrating them all. She is interested in a wide range of topics from the Miocene to the present, from islands to mountains, to understand contemporary patterns of species richness and endemism.
Henry Hooghiemstra is a terrestrial and marine tropical palynologist with interest in climate change, evolution of ecosystems, and how civilizations coped with environmental change. He is working on time-scales from the full Quaternary to the Anthropocene. His research focuses on a wide variety of biomes in Latin America, Saharan and East Africa, and in Mauritius.
Aaron O'Dea is a marine palaeobiologist who uses the marine fossil record of Tropical America to explore drivers of macroevolution in the seas, and takes cores on coral reefs from French Polynesia to the Dominican Republic to reconstruct how reefs changed over millennia with the aim of improving their future resilience.
Renske E. Onstein is an evolutionary ecologist who enjoys collecting (and eating) tropical megafaunal fruits, e.g. on Borneo and Madagascar, while studying how fruit functional traits interact with frugivores to affect diversification dynamics. She is generally interested in the broad-scale distribution and diversification of functional and taxonomic diversity of flowering plants.
Catalina Giraldo is an environmental artist (www.catalhinagiraldo.com/) who combines her scientific background with visual media to raise awareness of environmental issues. She co-founded Fundación Biodiversa Colombia (www.fundacionbiodiversa.org), a biodiversity foundation that carries out research and educational projects in Colombia to protect the environment and help local communities develop a sustainable way of living.
Author contributions: S.G.A.F. and H.H. conceived the ideas. H.H. provided the AP% of the Funza09 dataset. S.G.A.F. performed the spatial analyses. S.G.A.F and H.H. led the writing and figure design with critical contributions by all authors. C.G. developed the visualization with S.G.A.F. and H.H.




