On the origin of endemic species in the Red Sea
Abstract
Aim
The geological and palaeo-climatic forces that produced the unique biodiversity in the Red Sea are a subject of vigorous debate. Here, we review evidence for and against the hypotheses that: (1) Red Sea fauna was extirpated during glacial cycles of the Pleistocene and (2) coral reef fauna found refuge within or just outside the Red Sea during low sea level stands when conditions were inhospitable.
Location
Red Sea and Western Indian Ocean.
Methods
We review the literature on palaeontological, geological, biological and genetic evidence that allow us to explore competing hypotheses on the origins and maintenance of shallow-water reef fauna in the Red Sea.
Results
Palaeontological (microfossil) evidence indicates that some areas of the central Red Sea were devoid of most plankton during low sea level stands due to hypersaline conditions caused by almost complete isolation from the Indian Ocean. However, two areas may have retained conditions adequate for survival: the Gulf of Aqaba and the southern Red Sea. In addition to isolation within the Red Sea, which separated the northern and southern faunas, a strong barrier may also operate in the region: the cold, nutrient-rich water upwelling at the boundary of the Gulf of Aden and the Arabian Sea. Biological data are either inconclusive or support these putative barriers and refugia, but no data set, that we know of rejects them. Genetic evidence suggests that many endemic lineages diverged from their Indian Ocean counterparts long before the most recent glaciations and/or are restricted to narrow areas, especially in the northern Red Sea.
Main conclusions
High endemism observed in the Red Sea and Gulf of Aden appears to have multiple origins. A cold, nutrient-rich water barrier separates the Gulf of Aden from the rest of the Arabian Sea, whereas a narrow strait separates the Red Sea from the Gulf of Aden, each providing potential isolating barriers. Additional barriers may arise from environmental gradients, circulation patterns and the constriction at the mouth of the Gulf of Aqaba. Endemics that evolved within the Red Sea basin had to survive glacial cycles in relatively low salinity refugia. It therefore appears that the unique conditions in the Red Sea, in addition to those characteristics of the Arabian Peninsula region as a whole, drive the divergence of populations via a combination of isolation and selection.
Introduction
The Red Sea is a semi-enclosed basin at the north-western corner of the Indian Ocean that harbours one of the highest levels of endemism for marine organisms. Although continually being revised (e.g. due to cryptic species; Tornabene et al., 2014), a recent review reports that 12.9% of fishes, 12.6% of polychaetes, 8.1% of echinoderms, 16.5% of ascidians and 5.8% of scleractinian corals in the Red Sea are endemic (see DiBattista et al., in press). In the Indo-West Pacific, this level of endemism is exceeded only in the Hawaiian Archipelago (25.0%; Randall, 2007) and Easter Island (21.7%; Randall & Cea, 2011), with the Marquesas Islands close behind (11.6%; Randall & Earle, 2000), for the well-characterised shore fish fauna. The level of endemism among shore fish in the Red Sea exceeds those of all other localised hotspots identified in the Indian Ocean, including the Mascarene Islands (3.4%; Fricke, 1999; Eschmeyer et al., 2010), the Arabian Gulf (4.0%; DiBattista et al., in press) and southern Oman (2.8%; DiBattista et al., in press).
Recent research has demonstrated the importance of peripheral regions, such as the Red Sea, the Hawaiian Archipelago and the Marquesas Islands as “evolutionary incubators” that contribute unique genetic lineages to other regions of the Indo-West Pacific (Gaither et al., 2010, 2011; Malay & Paulay, 2010; DiBattista et al., 2011, 2013; Eble et al., 2011; Skillings et al., 2011; Hodge et al., 2012; Bowen et al., 2013). Peripheral endemism can be driven by isolation or selection, and both are of potential importance in the Red Sea. Indeed, the Red Sea is isolated by a narrow, shallow sill in the south, whereas broad areas of upwelling create a habitat barrier for reef-associated taxa in the Arabian Sea. Large spatial gradients and temporal fluctuations in physical conditions make this one of the most variable regions in the tropical marine environment, with a high potential for ecological speciation. Even greater environmental variation is evident through glacio-eustatic cycles, with the Red Sea basin becoming isolated and hypersaline at glacial maxima. The geological and palaeo-climatic forces that gave rise to shallow-water reef fauna in the Red Sea are therefore topics of biogeographical importance and the origins of the endemics are still the subject of much debate (see Rasul & Stewart, 2015). After describing the regional setting, we outline key components of this debate below.
Geological history of the Red Sea
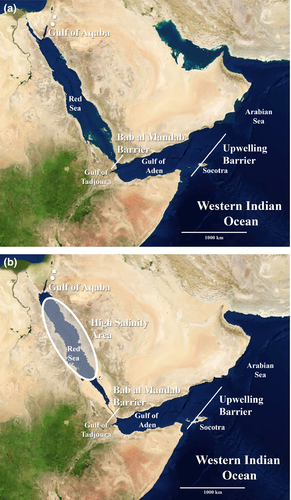
The dimensions and configuration of the Red Sea reflect the influence of a combination of geological and climatic processes, which include rifting (spreading) between the African and Arabian plates, associated volcanism in the mid-Tertiary and eustatic sea level fluctuations, which intensified in the Pliocene but more so since the mid-Pleistocene (Rihm & Henke, 1998). The present-day result is a narrow ocean basin with a north–south orientation, modest surface area (438,000 km2) and limited continuity with the greater Indo-West Pacific as it is connected only at the Strait of Bab al Mandab (Sheppard et al., 1992). Many of the present-day Red Sea reefs have a characteristic structure of shallow flat tops, steeply sloping sides and an elongated north–south axis as a result of the geologic history in this region (Dullo & Montaggioni, 1998).
Major events that led to the present-day configuration of the Red Sea were initiated by Oligocene episodes of sea floor spreading 41 to 34 Ma (Girdler & Styles, 1974). The rifting processes were associated with episodes of volcanism at the Afar Traps near present-day Ethiopia, with major activity around 31 Ma near the Afar Plume (Bosworth et al., 2005). During this time, saltwater replenished the Red Sea initially from the north but subsequently from the south via episodic connections to the Mediterranean Sea and the Gulf of Aden, respectively. The stratigraphy of this period is characterised by thick evaporate deposits interleaved with marine deposits of a northern origin indicating periods of intense evaporation interspersed with marine incursions from the Mediterranean. Uplift of the Suez area (i.e. Sinai Peninsula), driven by the collision of Eurasia with Arabia 14 Ma, shifted the location of the main oceanic connection to the south via the Gulf of Aden (Girdler & Styles, 1974; Hubert-Ferrari et al., 2003). During this period, the Red Sea environment was highly saline and unstable, subject to episodes of high temperature and evaporation and therefore unlikely to have supported a diverse tropical reef fauna.
Marine conditions re-established in the Red Sea during a second major phase of seafloor spreading 5 to 4 Ma. This produced the deep axial trough that characterises the basin today and established a more permanent connection with the Gulf of Aden through the Strait of Bab al Mandab (Bailey et al., 2007). The variable pattern of rifting and localised uplift since the Pliocene is reflected in the latitudinal variation of reef formation. The Red Sea is characterised by a vertical offshore profile and limited reefs in the northern Gulf of Aqaba, but extensive shallow areas in the southern Farasan Islands and Dahlak Archipelago. The different reef structures within the Red Sea are also reflected in the distinctive northern versus southern distribution of the reef fauna (Winterbottom, 1985; Spalding et al., 2001).
The Red Sea reef fauna, having been established during the Pliocene and Pleistocene (4 to 3 Ma), is relatively recent and has been subjected to environmental variation (e.g. temperature and salinity) of a magnitude not experienced by reefs in the Indian and Pacific Oceans (Taviani, 1998). The biological impacts of this dynamic environment have been exacerbated by the distinctive reef environments of the eastern Gulf of Aden and southern Oman, which act as filters for potential colonists due to major fluctuations in temperature and primary productivity as a result of episodic monsoonal-driven upwelling (Currie et al., 1973; Smeed, 1997; Kemp, 2000; Jung et al., 2001).
Isolation and barriers to dispersal
The Red Sea reef biota is isolated from potential propagules by its narrow entrance and by the environmental conditions of bordering waters. The Strait of Bab al Mandab is a narrow (29 km) and shallow (137 m) channel that constitutes the only connection between the Red Sea and Indian Ocean (Eshel et al., 1994; Bailey, 2009). There is a seasonal variation in the ebb and flow of Red Sea waters influenced by the Indian Ocean monsoon system. The water exchange between the Red Sea and Gulf of Aden changes from a two-layer surface flow in the winter to a three-layer flow in the summer (i.e. intrusion of Gulf of Aden Intermediate Water), with surface and deeper layers often taking on very different properties (Murray & Johns, 1997; Siddall et al., 2002; Sofianos et al., 2002; Smeed, 2004; Yao et al., 2014). Circulation models investigating the variability of the monsoonal activity from the early Holocene (Biton et al., 2010) suggest that the two-layer exchange pattern remained constant throughout glaciation periods.
While the southern Red Sea and western Gulf of Aden are relatively similar, the eastern Gulf of Aden, including northeast Africa (south of Ras Hafun) and southern Oman have markedly different contemporary environments with limited reef development that pose an environmental barrier for potential colonists. Currently, this area experiences major fluctuations in temperature and primary productivity driven by episodic monsoonal-driven upwelling (and temperatures as low as 13 °C; Currie et al., 1973; Smeed, 1997; Kemp, 2000; Jung et al., 2001). Because many Red Sea endemics also occur in the Gulf of Aden (Türkay, 1996; Janssen & Taviani, 2015; DiBattista et al., in press), some believe that the adjacent regions of cold-water upwelling off Somalia and Oman, as well as seasonal current patterns, are of greater importance as isolating mechanisms than the physical isolation at Bab al Mandab (Kemp, 1998, 2000; Zajonz et al., 2000).
Pleistocene conditions
During the last (and presumably previous) glacial maxima (20 to 15 ka), the Red Sea was physically isolated by the shoaling of Bab al Mandab, which was further exacerbated by changing winds and marine currents associated with the Indian Ocean monsoon system (Fig. 1; Siddall et al., 2003; Ludt & Rocha, 2015). The result was substantially increased salinity and residence times of the water masses within the Red Sea (Biton et al., 2008), which led some authors to suggest a scenario where the Red Sea resembled a hypersaline lake (Klausewitz, 1989). Such a shift in oceanographic conditions within the Red Sea is likely to have recurred repeatedly during Pleistocene glaciations, and with greatest frequency and amplitude during the last 430 kyr (Rohling et al., 2009).
Although the physical isolation of the Red Sea through the Strait of Bab al Mandab increased greatly during low sea level stands, glacial impacts on Arabian Sea environments are less clear. Studies on sediment cores in the Arabian Sea have shown that the upwelling was increased during glacial periods (Singh et al., 2011), but this point is contentious. Ivanova (2009) reported the opposite effect, an intensification of upwelling during interglacial stages, based on the palaeo-record of foraminifera.
Competing hypotheses
The primary unanswered question concerning Red Sea biogeography has been to what extent the marine biota was able to maintain a continuous presence in the Red Sea through the major environmental fluctuations of the Pleistocene. Loss of the planktonic fauna in most of the Red Sea suggests extirpation of many species, but survival of some plankton and occurrence of relatively old endemics with restricted ranges within the basin (e.g. Türkay, 1996; Grill & Zuschin, 2001; Choat et al., 2012; Janssen & Taviani, 2015; DiBattista et al., in press) suggest otherwise. There are thus two distinct, but not mutually exclusive hypotheses: marine organisms survived glacial conditions (1) within the Red Sea or (2) just outside of the Red Sea (i.e. Gulf of Aden). In the following section, we review palaeontological, biological and genetic evidence for and against these hypotheses. This evidence is in turn related to hypotheses of endemism – whether it is conditions in the Red Sea basin per se or the Arabian Peninsula region as a whole that drive the divergence of populations, and how isolation and selection contribute to the divergence of endemics.
Palaeontological Evidence
Core data and microfossils
Deep-sea cores taken along the length of the Red Sea, including the Gulf of Aqaba, provide a microfossil, isotopic, geochemical and petrographic record of environmental and biotic changes over the glacial cycles of the mid to late Pleistocene (Reiss et al., 1980; Almogi-Labin, 1982; Hoffmann et al., 1998; Fenton et al., 2000; Badawi et al., 2005). The most striking aspect of these data is that glacial maxima are associated with an ‘aplanktonic’ period, when most, if not all, planktonic foraminiferans, as well as many coccolithophorids and pteropods, disappeared, indicating unsuitable environmental conditions (Fenton et al., 2000). Although plankton diversity decreased sharply during glacial maxima, some taxa increased in abundance, especially benthic, miliolid forams, the euryhaline pteropod Creseis acicula and, locally, siliceous diatoms and some sponges (Reiss et al., 1980; Almogi-Labin et al., 2008). Increases in δ18O, development of sapropels and carbonate crusts, together with considerations of salinity tolerance for extirpated and persisting microfossils, indicates that salinity levels reached and potentially exceeded 50‰ (Reiss et al., 1980; Hoffmann et al., 1998; Taviani, 1998). Temperatures also fell between 3 and 5 °C, but the decrease in the diversity and abundance of planktonic microfossils is primarily attributed to hypersaline conditions versus lowered temperatures (Reiss et al., 1980).
Variation in the occurrence and diversity of microfossils in cores during glacial maxima along the length of the Red Sea and Gulf of Aqaba suggests that conditions varied significantly within the basin. Siliceous diatoms and sponge spicules are common during glacial maxima in the northern Red Sea, suggesting upwelling, but not in cores from the Gulf of Aqaba (Fenton et al., 2000). Planktonic foraminiferans persisted through the Last Glacial Maximum in cores from the southern Red Sea and the northern Gulf of Aqaba, which has been interpreted to indicate that salinity remained below 45‰ there (Fenton et al., 2000). In contrast, salinities were estimated to have reached c. 55‰ in most of the Red Sea basin (Fenton et al., 2000). The combined evidence here suggests that Red Sea fauna may have survived salinity crises of the Pleistocene, particularly through refugia in the Gulf of Aqaba and southern Red Sea.
The drastic changes in the planktonic foraminifera communities and productivity of the Red Sea (including the Gulf of Aqaba) during Pleistocene glacial periods were much greater than that in the Gulf of Aden (Deuser et al., 1976). The salinities in the Gulf Aden during glacial periods were similar to present-day salinities (36‰; Duplessy, 1982; Locke, 1986; Thunell et al., 1988) and, with the exception of a few species, the foraminifera and pteropod assemblages in this region were similar between glacial and interglacial periods, suggesting no large changes in productivity in the surface waters over the last 50 kyr (Ivanova, 1985; Locke & Thunell, 1988). This cumulative evidence supports the idea that the Gulf of Aden may have served as a refuge for Red Sea fauna during these times of harsh environmental conditions.
Physical closure of the Red Sea at the Strait of Bab al Mandab
A question of primary interest in the recent evolutionary history of the Red Sea fauna is the possibility of complete closure of the Red Sea during periods of low sea level associated with Pleistocene glacial cycles. Given that sea levels fell 115 m below present levels during five periods over the last 430 kyr (Rohling et al., 2009), the complete emergence of the Hanish Sill (137 m depth) at Bab al Mandab is a definite possibility. That said, the current consensus is that although Bab al Mandab was reduced to a narrow channel no less than 260 km in length, with a minimum depth of 15 m and an approximate width of 4 km at its narrowest point, complete closure probably did not occur (Bailey et al., 2007; Fig. 1). Even without complete closure, the restriction of water flow to the Gulf of Aden is so effective (Lambeck et al., 2011) that any residual flow through the narrower channel would have been insufficient to prevent major increases in salinity and temperature in the Red Sea during glacial maxima.
Climatic reconstruction of rainfall regimes
Support for tolerable marine conditions in the northern and southern Red Sea comes from climatic reconstructions. The Red Sea appears to be influenced by two rainfall regimes: (1) a Mediterranean system in the northern Red Sea (deMenocal & Rind, 1996) and (2) a monsoonal system in the southern Red Sea (Murray & Johns, 1997; Siddall et al., 2002; Sofianos et al., 2002; Smeed, 2004). Evidence of palaeorivers and palaeolakes, such as the vast Mudawwara depression (2000 km2) on the border of Saudi Arabia and Jordan, can be seen across the Arabian Peninsula (e.g. Parton et al., 2010). Studies using oxygen isotope records have also reported the occurrence of five periods of increased wetness in the Arabian Peninsula (10.5 to 6 ka, 82 to 78 ka, 135 to 120 ka, 200 to 180 ka and 325 to 300 ka; Fleitmann et al., 2003). Additional evidence for increased precipitation in the region comes from anthropological research. Studies show that during wet periods, lakes and rivers formed, resulting in increased vegetation during the Pleistocene (McLaren et al., 2009; Rosenberg et al., 2011; Groucutt & Petraglia, 2012). These wetter periods appear to have enhanced the capacity of humans to migrate out of Africa and into Arabia, and could also have had a substantial effect on the nearshore reefs of the eastern Red Sea if a physical connection with the Indian Ocean persisted. That said, peaks in precipitation rarely coincide with glacial periods; they favour interglacial periods instead.
Survival within the Red Sea may have been possible in the Gulf of Aqaba, owing to this area being wetter (and less saline) during glacial maxima. In this scenario, southern Jordan functioned as a fresh water reservoir lowering salinity in the Gulf of Aqaba to a tolerable level, as suggested by the existence of palaeolakes Hasa, Jafr, Jurf ed Darawish and those in the Mudawwara and Umari depressions (Rech, 2013; also see Fig. 1). There is no indication, however, that the Nile ever flowed into and impacted the northern Red Sea region (i.e. Gulf of Suez; Stanley & Warne, 1993; Krom et al., 2002). Regardless of rainfall level, the existence of Pleistocene reefs at 98 m depth (Hoffmann et al., 1998) provides evidence of coral survival during advanced glacial conditions in the Gulf of Aqaba (also see Fricke, 1996). Evidence from foraminifera also indicates that conditions in the Gulf of Aqaba may have provided a refuge for shallow-water organisms during glacial maxima (Locke & Thunell, 1988). This scenario is supported by an endemic fish fauna that is restricted to the Gulf of Aqaba (4.1%, DiBattista et al., in press; but also see Winterbottom, 1985; Fricke et al., 2014).
Evidence suggests that milder environmental conditions (i.e. temperature and salinity) may also have prevailed in the southern Red Sea due in part to the remaining connection with the Gulf of Aden. Rivers, such as the Hawash River, may have drained along the coast of Eritrea and into the series of lakes feeding the Gulf of Tadjoura in Djibouti, but also further north into the southern Red Sea (De Lattin, 1967). Extensive wadi systems (desert valleys or dry riverbeds), most frequent in the southern Red Sea, provide ‘geological evidence’ of increased precipitation and ancient river courses (Gabriel, 1978) that may have contributed to decreased salinity in the region. The exact timing of this increase in precipitation remains unclear.
Counter evidence to the idea of a wetter Arabian Peninsula is provided by Rohling et al. (2013), who report no significant rainfall associated with the Indian Ocean monsoon but do note that the regions affected by summer rainfall may have shifted to the south-east margin of the Arabian Peninsula (i.e. Yemen and Oman; Conroy & Overpeck, 2011). Parton et al. (2015) argues that increased monsoonal activity occurred during Marine Isotope Stage (MIS) six (c. 160 to 150 ka), MIS five (c. 130 to 75 ka) and early MIS three (55 ka), but these were not linked to particular aspects of the glacial cycle. Vast areas of the Arabian Peninsula have not been explored in terms of Quaternary environmental change (Fleitmann et al., 2004) or geoarchaeology (Rose, 2004). Based on this equivocal evidence, we argue that although wet periods are probably not directly linked to glacial cycles and the Arabian landscape remains incompletely surveyed, it is clear that the Red Sea nearshore environment was subject to high variance in temperature, salinity and perhaps nearshore turbidity during the late Pleistocene.
Biological Evidence
Salinity tolerances for marine organisms
During glacial maxima, salinity in the Red Sea was more than 10‰ higher than current levels (which are 37–41‰), and much greater in the central and northern regions (reaching up to 55–57‰) than in the southern region (which remained below 45‰) (Thunell et al., 1988; Geiselhart, 1998). These episodes of elevated salinity correspond with the virtual disappearance of planktonic foraminifera in parts of the Red Sea (Locke & Thunell, 1988); however, other marine species may have persisted (e.g. some fish can tolerate salinities up to 60‰; Bayly, 1972). The Red Sea biota thrives today at salinities near 42‰ in the Gulf of Aqaba, but species rapidly drop out between 45‰ to 50‰ in marginal lagoons (Kinsman, 1964; Por, 1972, 2008). For corals, the key habitat-forming organism that currently supports much of the Red Sea biodiversity, the upper threshold to salinity tolerance appears to be 50‰, with few corals surviving salinities in excess of 45‰ (Coles, 2003). Some shallow-water macroinvertebrates can tolerate high salinities (> 50‰; Por, 1972) and may have persisted in parts of the Red Sea during interglacial periods. There is also evidence that endemic deep water bivalves persisted and evolved during glacial periods (Türkay, 1996), whereas shallow-water species contracted to refugia outside of the Red Sea (Grill & Zuschin, 2001). Thus, evidence suggests that tolerance to elevated salinities allowed some species to survive through glacial cycles within the Red Sea and evolve into endemics.
Species distributions and distribution of sister taxa
The most compelling evidence for survival of endemics within the Red Sea comes from the large number of species that appear to be restricted to the basin (DiBattista et al., in press). Whereas some of these species may have been overlooked outside the basin and others could have become recently restricted, the sheer diversity of species suggests that some have persisted in the Red Sea through glacial periods.
Sister taxon relationships provide further evidence for the origin of Red Sea endemics. The Red Sea reef fish fauna are relatively well-characterised (Golani & Bogorodsky, 2010), and consist primarily of species of Indo-West Pacific origin in addition to a small number of species (along with some Arabian Peninsula endemics) that lack clearly identifiable close relatives. The latter groups are likely relicts of palaeo-Mediterranean Tethyan ancestry that persisted in the north-western Indian Ocean long after the mass extinction of their Mediterranean relatives in the late Miocene (i.e. Messinian salinity crisis, c. 6 Ma; Krijgsman et al., 1999). An updated list of Red Sea endemics with presumed sister species of reef fish are provided in Table 1. We excluded species with no known close relatives given that these may be Tethyan relicts. We focused on fish because this is the group with the most resolved taxonomy among the reef fauna. The final list was constructed by examining the list of endemic species and choosing those pairs that we had some knowledge of, or could systematically check within the literature for, information on presumed relationships. In some, but not all cases, the information is backed by molecular evidence, and the naming convention follows Eschmeyer (2014) unless otherwise noted.
| Species | Sister species | Distribution of sister species |
|---|---|---|
| Red Sea endemic | ||
| Narkidae (sleeper rays) | ||
| Heteronarce bentuviai (Baranes & Randall, 1989) | Heteronarce garmani Regan, 1921 | Gulf of Aden to South Africa |
| Torpedinidae (torpedos) | ||
| Torpedo alexandrinsis Mazhar, 1987 | Torpedo adenensis Carvalho, Stehmen & Manilo, 2002 | Gulf of Aden |
| Muraenidae (moray eels) | ||
| Gymnothorax corallinus (Klunzinger, 1871) | Gymnothorax buroensis (Bleeker, 1857) | Indo-Pacific |
| Uropterygius golanii McCosker & Smith, 1997 | Uropterygius xenodontus McCosker & Smith, 1997 | W Pacific |
| Congridae (conger and garden eels) | ||
| Gorgasia sillneri Klausewitz, 1962 | Gorgasia naeocepaeus (Böhlke, 1951) | W Papua and Philippines |
| Clupeidae (herrings, sprats, & sardines) | ||
| Etrumeus golanii DiBattista, Randall & Bowen,2012 | Etrumeus wongratanai DiBattista, Randall & Bowen, 2012 | Gulf of Aden to South Africa |
| Herklotsichthys punctatus (Rüppell, 1837) | Herklotsichthys lossei Wongratana, 1983 | Arabian Gulf |
| Synodontidae (lizardfishes) | ||
| Synodus randalli Cressey, 1981 | Synodus fasciapelvicus Randall, 2009 | Indonesia and Philippines |
| Batrachoididae (toadfishes) | ||
| Barchatus cirrhosa (Klunzinger, 1871) | Barchatus indicus Greenfield, 2014 | Gulf of Aden (N Somalia) |
| Atherinidae (silversides) | ||
| Atherinomorus forskalii (Forster, 1801) | Atherinomorus lacunosus (Schneider, 1801) | Indo-Pacific (sympatric in C Red Sea) |
| Hypoatherina golanii Sasaki & Kimura, 2012 | Hypoatherina klunzingeri Smith, 1965 | W Indian Ocean (E Somalia) to South Africa |
| Holocentridae (soldierfishes & squirrelfishes) | ||
| Sargocentron marisrubri Randal, Guezé & Diamant, 1989 | Sargocentron melanospilos Bleeker, 1858 | Indo-West Pacific |
| Syngnathidae (pipefishes & seahorses) | ||
| Corythoichthys cf nigripectusa | Corythoichthys nigripectus Herald, 1953 | W Pacific |
| Corythoichthys cf schultzib | Corythoichthys schultzi Herald, 1953 | W Pacific |
| Micrognathus brevirostris (Rüppell, 1838) | Micrognathus pygmaeus Fritzsche, 1981 | Indo-West Pacific |
| Scorpaenidae (scorpionfishes) | ||
| Scorpaenodes steinitzi Klausewitz & Fröiland, 1970 | Scorpaenodes parvipinnis (Garrett, 1864) | Indo-Pacific |
| Aploactinidae (velvetfishes) | ||
| Ptarmus gallus Kossman & Rauber, 1877 | Ptarmus jubatus (Smith, 1935) | E Africa to Natal, South Africa |
| Serranoidea (seabasses) | ||
| Plectropomus marisrubri Randall & Hoese, 1986 | Plectropomus pessuliferus (Fowler, 1904) | Indian Ocean and Fiji |
| Pseudanthias taeniatus (Klunzinger, 1855) | Pseudanthias townsendi (Boulenger, 1897) | Gulf of Aden to Gulf of Oman |
| Pseudogramma megamycterum Randall & Baldwin, 1997 | Pseudogramma astigmum Randall & Baldwin, 1997 | Indo-Pacific |
| Pseudochromidae (dottybacks) | ||
| Pseudochromis fridmani Klausewitz, 1968 | Pseudochromis sankeyi Lubbock, 1975 | S Red Sea and Gulf of Aden |
| Pseudochromis pesi Lubbock, 1975 | Pseudochromis melas Lubbock, 1977 | E Africa |
| Plesiopidae (prettyfins/longfins) | ||
| Acanthoplesiops cappuccino Gill, Bogorodsky & Mal, 2013 | Acanthoplesiops indicus (Day, 1888) | Indian Ocean |
| Opisthognathidae (jawfishes) | ||
| Stalix davidsheni Klausewitz, 1985 | Stalix histrio Jordan & Snyder, 1902 | W Pacific |
| Apogonidae (cardinalfishes) | ||
| Cheilodipterus pygmaios Gon, 1993 | Cheilodipterus quinquelineatus (Cuvier, 1828) | Indo-Pacific (sympatric in Red Sea) |
| Taeniamia lineolata (Cuvier, 1828) | Taeniamia flavofasciata (Gon & Randall, 2003) | E Africa to Madagascar |
| Malacanthidae (sand tilefishes) | ||
| Hoplolatilus oreni Clark & Ben-Tuvia, 1973 | Hoplolatilus fourmanoiri Smith, 1964 | Indonesia, Brunei, and Solomon Islands |
| Caesionidae (fusiliers) | ||
| Caesio suevica Klunzinger, 1884 | Caesio xanthonota Bleeker, 1853 | Indian Ocean (sympatric S of Gulf of Aqaba) |
| Sparidae (sea breams) | ||
| Argyrops megalommatus (Klunzinger, 1870) | Argyrops filamentosus (Valenciennes, 1830) | W Indian Ocean, including Gulf of Aden |
| Diplodus noct (Valenciennes, 1830) | Diplodus capensis (Smith, 1884) | W Indian Ocean, including Gulf of Aden |
| Pempheridae (sweepers) | ||
| Parapriacanthus guentheri (Klunzinger, 1871) | Parapriacanthus ransonneti Steindachner, 1870 | Indo-West Pacific, including Gulf of Aden |
| Pomacentridae (damselfishes) | ||
| Chromis dimidiata (Klunzinger, 1871) | Chromis fieldi Randall & DiBattista, 2013 | Indian Ocean, including Gulf of Aden |
| Chromis pelloura Randall & Allen, 1982 | Chromis axillaris (Bennett, 1831) | Somalia to S Mozambique and Mauritius |
| Pomacentrus albicaudatus Baschieri-Salvadori, 1955 | Pomacentrus adelus Allen, 1991 | Andaman Sea to W Pacific |
| Labridae (wrasses) | ||
| Chlorurus gibbus (Rüppell, 1829) | Chlorurus strongylocephalus (Bleeker, 1864) | Indian Ocean, including Gulf of Aden |
| Cirrhilabrus blatteus Springer & Randall, 1974 | Cirrhilabrus lanceolatus Randall & Masuda, 1991 | W Pacific |
| Iniistius n sp cf balwinib | Iniistius baldwini (Jordan & Evermann, 1903) | Indo-West Pacific |
| Macropharyngodon marisrubri Randall, 1978 | Macropharyngodon bipartitus Smtih 1957 | W Indian Ocean, including Gulf of Aden |
| Thalassoma rueppellii (Klunzinger, 1828) | Thalassoma quinquevitattum (Lay & Bennett, 1839) | Indo-Pacific, including Gulf of Aden |
| Trichonotidae (sand-divers) | ||
| Limnichthys marisrubri Fricke & Golani, 2012 | Limnichthys nitidus (Smith, 1958) | Indian Ocean |
| Tripterygiidae (triplefins) | ||
| Enneapterygius altipinnis Clark, 1980 | Enneapterygius tutuilae Jordan & Seale, 1906 | Indo-West Pacific |
| Blenniidae (blennies) | ||
| Alticus magnusi (Klausewitz, 1964) | Alticus kirki (Günther, 1868) | WC Indian Ocean, including Gulf of Aden |
| Entomacrodus solus Williams & Bogorodsky, 2010 | Entomacrodus epalzeocheilos (Bleeker, 1859) | Indo-Pacific |
| Istiblennius rivulatus (Rüppell 1830) | Istiblennius dussumieri (Valenciennes, 1836) | Indo-Pacific |
| Gobiidae (gobies) | ||
| Oxyurichthys petersi (KLunzinger, 1871) | Oxyurichthys papuensis (Valenciennes, 1837) | Indo-West Pacific |
| Tomiyamichthys dorsostigma Bogorodsky, Kovacic & Randall, 2011 | Tomiyamichthys smithi (Chen & Fang, 2003) | W Pacific |
| Trichiuridae (hairtails) | ||
| Evoxymetopon moricheni Fricke, Golani & Appelbaum-Golani 2014 | Evoxymetopon taeniatus Gill, 1863 | W Pacific and W Atlantic |
| Monacanthidae (filefishes/leatherjackets) | ||
| Oxymonacanthus halli Marshall, 1952 | Oxymonacanthus longirostris (Bloch & Schneider, 1801) | Indo-Pacific, excluding Arabian Peninsula |
| Paraluteres arqat Clark & Gohar, 1953 | Paraluteres n spc | Andaman Sea |
| Thamnoconus erythraensis Bauchot & Mauge, 1978 | Thamnoconus modestoides (Barnard, 1927) | Indo-West Pacific |
| Tetraodontidae (pufferfish) | ||
| Arothron diadematus (Rüppell, 1829) | Arothron nigropunctatus (Bloch & Schneider, 1801) | Indo-Pacific, including Gulf of Aden |
| Red Sea to Gulf of Aden endemic | ||
| Holocentridae (soldierfishes & squirrelfishes) | ||
| Myripristis xanthacra Randall & Gueze, 1981 | Myripristis hexagona (Lacepède, 1802) | Indo-West Pacific |
| Platycephalidae (flatheads) | ||
| Thysanophrys springeri Knapp, 2013 | Thysanophrys chiltonae (Schultz, 1966) | Indo-West Pacific |
| Serranoidea (seabasses) | ||
| Diploprion drachi Esteve, 1955 | Diploprion bifasciatum Cuvier, 1828 | Indo-West Pacific to Maldives |
| Epinephelus geoffroyi (Klunzinger, 1870)d | Epinephelus chlorostigma (Valenciennes, 1828) | Indo-Pacific, including Gulf of Aden |
| Epinephelus summana (Forsskål, 1775) | Epinephelus caeruleopunctatus (Bloch, 1790) | Indo-West Pacific |
| Pseudochromidae (dottybacks) | ||
| Chlidichthys auratus Lubbock, 1975 | Chlidichthys johnvoelckeri Smith, 1953 | East Africa |
| Pseudochromis sankeyi Lubbock, 1975 | Pseudochromis fridmani Klausewitz, 1968 | N to C Red Sea |
| Apogonidae (cardinalfishes) | ||
| Cheilodipterus lachneri Klausewitz, 1959 | Cheilodipterus arabicus (Gmelin [ex Forsskål], 1789) | Red Sea and W Indian Ocean |
| Nectamia zebrinus (Fraser, Randall & Lachner, 1999) | Nectamia luxuria Fraser, 2008 | Indo-West Pacific to Maldives |
| Mullidae (goatfishes) | ||
| Parupeneus forsskali (Fourmanior & Gueze, 1976) | Parupeneus barberinus (Lacepède, 1801) | Indo-Pacific |
| Pempheridae (sweepers) | ||
| Pempheris flavicycla marisrubri (Randall, Bogorodsky & Alpermann, 2013) | Pempheris flavicycla flavicycla (Randall, Bogorodsky & Alpermann, 2013) | W Indian Ocean |
| Chaetodontidae (butterflyfishes) | ||
| Chaetodon fasciatus Forsskål, 1775 | Chaetodon lunula (Lacepède, 1802) | Indo-Pacific |
| Chaetodon paucifasciatus Ahl, 1923 | Chaetodon madagascariensis Cuvier, 1831 | W Indian Ocean |
| Pomacentridae (damselfishes) | ||
| Amblyglyphidodon flavilatus Allen & Randall, 1981 | Amblyglyphidodon indicus Allen & Randall, 2002 | Indian Ocean |
| Neopomacentrus xanthurus Allen & Randall, 1981 | Neopomacentrus nemurus (Bleeker, 1857) | W Pacific |
| Pristotis cyanostigma Rüppell, 1835 | Pristotis obtusirostris (Günther, 1862) | Indo-Pacific |
| Labridae (wrasses) | ||
| Cetoscarus bicolor (Rüppell, 1829)e | Cetoscarus ocellatus (Valenciennes, 1840) | Indo-West Pacific |
| Cheilinus abudjubbe Rüppell, 1835 | Cheilinus chlororurus (Bloch, 1791) | Indo-Pacific |
| Cheilinus quinquecinctus Rüppell, 1835 | Cheilinus fasciatus (Bloch, 1791) | Indo-Pacific |
| Coris variegata (Rüppell, 1835) | Coris batuensis (Bleeker, 1856) | Indian Ocean |
| Gomphosus caeruleus klunzingeri Klausewitz, 1962 | Gomphosus caeruleus caeruleus Lacepede, 1801 | Indian Ocean |
| Hemigymnus sexfasciatus Rüppell, 1835 | Hemigymnus fasciatus (Bloch, 1792) | Indo-West Pacific |
| Trichonotidae (sand-divers) | ||
| Trichonotus nikii Clark & Schmidt, 1966 | Trichonotus marleyi (Smith, 1936) | W Indian Ocean |
| Blenniidae (blennies) | ||
| Antennablennius n spf | Antennablennius variopunctatus (Jatzow & Lenz, 1898) | W Indian Ocean, including Gulf of Aden |
| Siganidae (rabbitfishes/spinefoots) | ||
| Siganus rivulatus (Forsskål, 1775) | Siganus sutor (Valenciennes, 1835) | Indian Ocean, including Gulf of Aden |
| Siganus stellatus (Forsskål, 1775) | Siganus laqueus von Bonde, 1934 | Indian Ocean |
| Acanthuridae (surgeonfishes & unicornfishes) | ||
| Acanthurus gahhm Forsskål, 1775 | Acanthurus nigricauda Duncker & Mohr, 1929 | Indo-Pacific |
| Balistidae (triggerfishes) | ||
| Sufflamen albicaudatum (Rüppell, 1829) | Sufflamen chrysopterum (Bloch & Schneider, 1801) | Indo-Pacific |
| Tetraodontidae (pufferfish) | ||
| Canthigaster margaritata (Rüppell, 1826) | Canthigaster petersii (Bianconi, 1854)c | Indian Ocean, including Gulf of Aden |
Klausewitz (1989) provided a framework for classifying the historical origins of Red Sea fish based on the relative age of differentiation: (1) postglacial fish that invaded the basin during the past 10 kyr, (2) interglacial fish that invaded during earlier interglacial periods and (3) fish with a high degree of differentiation that invaded much earlier and persisted in the basin. Although Klausewitz (1989) considered the question of the viability of the Red Sea as a habitat for tropical reef fish during glacial maxima to be unresolved, he also suggested that the southern part of the Red Sea could have served as a refuge. Whereas current ecological conditions in the southern Red Sea may not be ideal for many species, it is clear that a number of them effectively disperse through the Bab al Mandab barrier in both directions. It therefore seems more likely that speciation of true Red Sea endemics (those not found in the Gulf of Aden), with clearly identifiable Indo-West Pacific sister species, occurred when isolation of the basin was greater if not complete. Furthermore, we now know that the relative ages of differentiation of many endemics in the Red Sea precede the end of the Last Interglacial, and in rare cases the entire Pleistocene [examples from Table 1: Chlorurus gibbus and C. strongylocephalus (0.5 Ma), Choat et al., 2012; Chromis dimidiata and C. fieldi (0.95 Ma), Randall & DiBattista, 2013; Etrumeus golanii and E. wongratanai (1.65 Ma), DiBattista et al., 2012; Pomacentrus albicaudatus and P. adelus (~3.5 Ma), Litsios et al., 2012; Thalassoma rueppellii and T. quinquevitattum (~12.5 Ma), Hodge et al., 2014].
The presence of refugia outside of the Red Sea is supported by the distribution of reef fish and other marine organisms in the region. While 138 of the 189 (73%) Red Sea endemics are only known from the Red Sea, 45 (24%) occur in the Red Sea and Gulf of Aden, which indicates that the Gulf of Aden represents a biogeographical extension of the Red Sea (Kemp, 1998; DiBattista et al., in press). Moreover, 13.5% of Red Sea to Gulf of Aden endemics (e.g. Myripristis xanthacra, Neopomacentrus xanthurus, Pristotis cyanostigma) are restricted to the southern portion of the Red Sea (DiBattista et al., in press), presumably due to a greater similarity between environments. The Gulf of Aden may therefore have served as a refuge for local endemics during periods of lowered sea level and hypersalinity within the Red Sea.
Genetic Evidence
Phylogenetic dispersion and taxonomic sampling
Time-calibrated phylogenetic trees for coral reef fish allows an examination of the evolutionary history of the Red Sea fauna. Phylogenetic hypotheses for angelfish (Gaither et al., 2014), butterflyfish (Fessler & Westneat, 2007; Bellwood et al., 2010), damselfish (Cooper et al., 2009; Frédérich et al., 2013), parrotfish (Choat et al., 2012) and wrasses (Westneat & Alfaro, 2005), as well as integrated higher-level phylogenies among reef fish (Hodge et al., 2014), provide templates for exploring the historical patterns of biogeography in the Red Sea in two ways. First, calibrated phylogenetic time trees provide estimates of the timing of the origin of species groups, and can further bracket the minimum and maximum timing of splits between species pairs. Second, phylogenetic trees combined with regional species composition data enable exploration of the evolutionary history of community composition.
Phylogenetic analyses show that Red Sea reef fish, including endemic species, have repeatedly evolved within major reef fish groups over the past 25 Myr, have primarily evolved from Indian Ocean relatives and represent highly ‘over-dispersed’ communities sampled from the phylogenies of reef fish families. Using a multi-family fish phylogeny, Hodge et al. (2014) showed that Red Sea endemic reef fish species have originated frequently and steadily over the past 16 Myr, with most endemics originating within the past 5 Myr. Using a time-calibrated phylogeny of the butterflyfish, Fessler & Westneat (2007) showed that four of the six Red Sea endemics split from their sister species 4 to 1 Ma, and perhaps even more recently. Similarly, endemic Red Sea parrotfish have originated within the past 4 Myr, and several species within the past 500 kyr (Choat et al., 2012). Pomacentridae (see Fig. 2) show a more complex pattern of deeper origins for Red Sea species, as well as more recent speciation in the region, with time calibrations of some Red Sea components dating back as much as 25 Myr across the Miocene/Oligocene boundary.
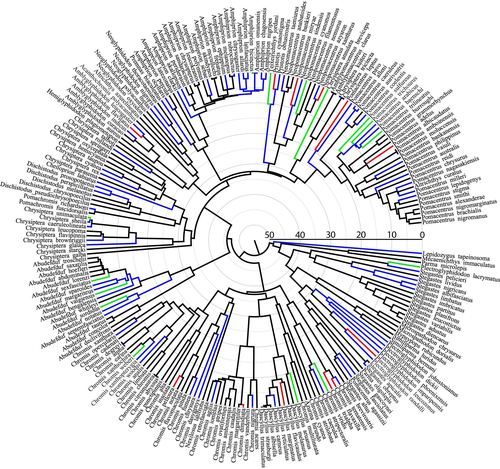
Red Sea coral reef fish communities can be viewed as having been ‘sampled’ from the phylogenies of major family groups (Webb et al., 2002; Emerson & Gillespie, 2008; Rabosky et al., 2011). The assembly of Red Sea reef fish in several families (Chaetodontidae, Labridae and Pomacentridae) is a non-random sample from the phylogenetic history of these groups (Fig. 2; M.W. Westneat, unpublished data). For the damselfish, the Red Sea community is composed of members of almost all major clades, and endemics have arisen from various parts of the phylogeny (Fig. 2). This pattern is considered phylogenetically over-dispersed, with a significantly negative net relatedness index (−2.2). Such patterns are characteristic of systems with high competition for resources or species interactions based on key ecological traits (Emerson & Gillespie, 2008). These patterns may also be explained by an alternate hypothesis where endemics are related to widespread species rather than to each other as a result of little adaptive radiation in the Red Sea.
Phylogenetic patterns suggest that periods of inhospitable conditions in the Red Sea, from the Miocene through the Pleistocene, were survived by many lineages. Current phylogenetic time tree resolution among species does not usually have the accuracy to resolve species origins on the order of thousands of years, but some of the youngest speciation events in the Red Sea occurred within the past 50 kyr. Most of the Red Sea reef fish community, including many endemics, however, originated much earlier. Future work should broaden our sample of time-calibrated phylogenies for fishes, explore trait evolution that may have driven the patterns of over dispersion that are emerging, test for adaptive radiation and attempt to integrate species-level phylogenetic patterns with finer-scale phylogeographical studies to increase resolution on the timing of recent splits in this dynamic region.
Phylogeography and population genetics
Phylogeographical analyses are powerful tools for detecting population level divergences, identifying cryptic lineages and providing insight into historical processes that may not be apparent from contemporary species distributions or higher-level phylogenies (Palumbi, 1997; Avise, 2000). Most phylogeographical studies of broadly distributed species indicate some level of genetic distinction of Red Sea populations (Table 2), and a few studies have resulted in the description of new species endemic to the region (e.g. Terranova et al., 2007; DiBattista et al., 2012). There are only a handful of cases where Red Sea populations demonstrate little to no genetic distinction from populations in the Indian Ocean, including the widespread checkerboard wrasse (Halichoeres hortulanus; DiBattista et al., 2013), the bluestripe snapper (Lutjanus kasmira; DiBattista et al., 2013), the African coris wrasse (Coris cuvieri; P. Ahti, pers. comm.) and several species of elasmobranchs (Spaet et al., 2015) (Table 2). Red Sea populations of lionfish (Pterois miles and Dendrochirus brachypterus) also show no distinction, however, limited sample sizes preclude a final conclusion (Kochzius et al., 2003; Kochzius & Blohm, 2005).
| Species | DNA fragment | ΦST, FST or % divergence | Reciprocally monophyletic | Source of tissue samples | Reference |
|---|---|---|---|---|---|
| Lutjanus kasmira (bluestripe snapper) | COI & Cyt b | FST = 0.05 (1 of 6) | No |
RS: Thuwal and Al Lith (KSA) IO: Diego Garcia, Seychelles, Sodwana Bay (South Africa) |
DiBattista et al., 2013 |
| Halichoeres hortulanus (checkerboard wrasse) | COI & Cyt b | FST = 0.07–0.12 (2 of 4) | No |
RS: Thuwal and Al Lith (KSA) IO: Diego Garcia, Seychelles |
DiBattista et al., 2013 |
| Cephalopholis argus (peacock hind) | COI & Cyt b | FST = 0.20–0.45 (5 of 6) | No |
RS: Thuwal and Al Lith (KSA) IO: Al Hallaniyats (Oman), Diego Garcia, Seychelles |
DiBattista et al., 2013 |
| Acanthurus nigrofuscus (brown surgeonfish) | COI & Cyt b | FST = 0.18–0.28 (4 of 4) | No |
RS: Thuwal and Al Lith (KSA) IO: Diego Garcia, Seychelles |
DiBattista et al., 2013 |
| Chaetodon auriga (threadfin butterflyfish) | COI & Cyt b | FST = 0.17–0.23 (4 of 4) | No |
RS: Thuwal and Al Lith (KSA) IO: Diego Garcia, Seychelles |
DiBattista et al., 2013, 2015 |
| Neoniphon sammara (Sammara squirrelfish) | COI & Cyt b | FST = 0.12–0.16 (4 of 4) | No |
RS: Thuwal and Al Lith (KSA) IO: Diego Garcia, Seychelles |
DiBattista et al., 2013 |
| Scylla serrata (mud crab) | COI | FST = 0.42–1.0 (6 of 6) | Yes |
RS: Jeddah (KSA) IO: Kenya, Zanzibar, Madagascar, Mauritius, South Africa |
Frantini et al., 2002; Gopurenko et al., 1999 |
| Pygoplites diacanthus (regal angelfish) | COI & Cyt b | FST = 0.65–0.67 (2 of 2), < 1.0%a | Yes |
RS: Thuwal and Al Lith (KSA) IO: Diego Garcia |
DiBattista et al., 2013 |
| Leucetta chagosensis (lemon sponge) | ITS/18S & ATPSb | 0.7%b | Yes |
RS: Sinai Peninsula (Egypt) IO: Maldives |
Wörheide et al., 2008 |
| Tridacna maxima (giant clam) | COI | 2.5%c | Yes |
RS: not specified IO: Indonesia |
Nuryanto & Kochzius, 2009 |
| Acanthaster planci (crown-of-thorns starfish) | COI | 8.80% | Yes |
RS: Al Wajh (KSA), Sinai Peninsula (Egypt) IO: Indonesia, Thailand, Christmas and Cocos-Keeling Islands, Maldives, UAE, Oman, Kenya, South Africa, Reunion, Mauritius |
Vogler et al., 2008 |
| Mulloidichthys flavolineatus (yellowstripe goatfish) | Cyt b | ΦST = 0–0.86 (3 of 6) | No |
RS: Eilat (Israel), Magna and Jeddah (KSA), Sudan IO: Djibouti, Madagascar |
Fernandez-Silva et al., in press |
| Coris cuvieri (African coris) | COI | ΦST = 0.04-0.11 (1 of 3) | No |
RS: N to C Red Sea (KSA) IO: Djibouti, Diego Garcia, Seychelles |
P. Ahti, pers. comm. |
| Carcharhinus limbatus (blacktip shark) | Control region | FST = 0.003 (P = 0.24) | No |
RS: Red Sea (KSA) IO: Oman, UAE, Bahrain |
Spaet et al., 2015 |
| Carcharhinus sorrah (spot-tail shark) | Control region | FST = 0.006 (P = 0.10) | No |
RS: Red Sea (KSA) IO: Oman, UAE, Bahrain |
Spaet et al., 2015 |
| Rhizoprionodon acutus (milk shark) | Control region | FST = 0.06 (P = 0.58) | No |
RS: Red Sea (KSA) IO: Oman, UAE, Bahrain |
Spaet et al., 2015 |
| Sphyrna lewini (scalloped hammerhead) | Control region | FST = 0.01 (P = 0.58) | No |
RS: Red Sea (KSA) IO: Oman, UAE |
Spaet et al., 2015 |
| Cephalopholis hemistiktos (yellowfin hind) | COI & S7 |
ΦST = 0–0.85 FST = 0–0.41 (10 of 15 at both) |
Yes for COI no for S7 |
RS: Magna, Thuwal, Al Lith, and Farasan Islands (KSA), Sudan IO: Djibouti, Oman, Jubail (KSA – Arabian Gulf) |
M. Priest, pers. comm. |
| Panulirus penicillatus (pronghorn spiny lobster) | COI | ΦST = 0.74 (1 of 1) | Yes |
RS: Jeddah (KSA) IO: Zanzibar, Seychelles, India |
M. Iacchei, pers. comm. |
- KSA, Kingdom of Saudi Arabia; UAE, United Arab Emirates.
- a Calculated as 1 fixed difference in 634 bp.
- b Calculated as 7 fixed differences in 1049 bp.
- c Calculated as 12 fixed differences in 484 bp.
Levels of genetic divergence detected in these studies vary by an order of magnitude across species (Table 2), indicating that colonisation of the Red Sea did not coincide with a specific geologic event but perhaps multiple stochastic events. Deep phylogenetic partitions have been detected in the Red Sea populations of the mud crab (Scylla serrata; Gopurenko et al., 1999; Fratini & Vannini, 2002), regal angelfish (Pygoplites diacanthus; DiBattista et al., 2013), lemon sponge (Leucetta chagosensis; Wörheide et al., 2008), yellowfin goatfish (Mulloidichthys flavolineatus; Fernandez-Silva et al., in press), yellowfin hind (Cephalopholis hemistiktos; M. Priest, pers. comm.), giant clam (Tridacna maxima; Nuryanto & Kochzius, 2009), pronghorn spiny lobster (Panulirus penicillatus; M. Iacchei, pers. comm.) and crown-of-thorns starfish (Acanthaster planci; Vogler et al., 2008). The oldest of these lineages may represent cryptic species complexes (e.g. A. planci and P. diacanthus; Table 2). Red Sea and Indian Ocean lineages in these examples are reciprocally monophyletic with no evidence of gene flow between regions, and levels of divergence that represent hundreds of thousands (e.g. P. diacanthus) to millions of years (e.g. A. planci). In the Sammara squirrelfish, Neoniphon sammara, shallower divergences have been recorded that date to about 125 kyr of isolation, with near monophyly (DiBattista et al., 2013). Other species demonstrate significant population level structure between the Red Sea and Indian Ocean, but with shared haplotypes among regions (brown surgeonfish, Acanthurus nigrofuscus; peacock hind, Cephalopholis argus; threadfin butterflyfish, Chaetodon auriga) and perhaps represent more recent colonisation of the Red Sea (DiBattista et al., 2013; also see DiBattista et al., in press).
Only four phylogeographical studies conducted thus far in reef fish include samples from the Gulf of Aden, and these show a variable pattern that parallels what we know from species distributions. The African coris wrasse (C. cuvieri, a Red Sea to Indian Ocean species, P. Ahti, pers. comm.) and threadfin butterflyfish (C. auriga, an Indo-West Pacific species, DiBattista et al., 2015) demonstrate modest differentiation among Red Sea, Gulf of Aden and Indian Ocean populations, whereas the other two species, the yellowfin goatfish (M. flavolineatus; Fernandez-Silva et al., in press) and the yellowfin hind (C. hemistiktos; M. Priest, pers. comm.), have an endemic genetic lineage that extends between the Red Sea and the Gulf of Aden. We see similar patterns at the species level for some invertebrates (echinoderms: G. Paulay, unpublished data; coral gall crabs: S. van der Meij, pers. comm.), which are characterised by high levels of endemism, with many endemics making it into the Gulf of Aden or also into the Arabian Sea. This pattern suggests that many of the lineages thought to be unique to the Red Sea may be found outside the basin with increased sampling. This pattern, however, does not allow us to conclude whether lineages originate from glacial refugia within or just outside the Red Sea owing to the potential for bidirectional post-glacial expansion.
Conclusion and Future Directions
Limited water exchange between the Red Sea and Indian Ocean at the Strait of Bab al Mandab led to drastic changes in environmental conditions within the Red Sea during glacial maxima. Coupled with regional climate shifts, the Red Sea (particularly the central region) experienced fluctuations in salinity and temperature that may have presented exceptional physiological challenges to resident marine life. However, apart from sediment cores from a few locations indicating a loss of most planktonic organisms, there is little direct evidence supporting the complete loss of species within the entire Red Sea. The spatial variability of environmental conditions in the Red Sea during glacial maxima therefore requires further study, especially in groups with adequate fossil records, to conclusively resolve whether one or more refugia existed and how effective these were. The shallow-water molluscan fauna would be a suitable target given the excellent fossil record that can be interpreted in a biogeographical context (Paulay, 1996; Grill & Zuschin, 2001).
Perhaps the most compelling evidence for the persistence of some Red Sea taxa during glaciation events is the genetic evidence that many endemic taxa (or lineages) diverged from their Indian Ocean counterparts long before the most recent glaciations, and the restriction of some endemics to narrow areas, especially in the northern Red Sea. The range of ages of Red Sea endemics suggests that peripatric speciation has been an ongoing process in this region, a pattern shown at other hotspots of endemism in the Indo-West Pacific (e.g. Hawaiian Archipelago; Craig et al., 2010) that may be the rule rather than the exception.
The evolutionary history of the Red Sea, Gulf of Aden and Arabian Sea is much more complex than previously believed. The abrupt transition among distinctive habitats and Pleistocene fluctuations in temperature, salinity and productivity has all contributed to an evolutionary dynamic theatre. Future genetic work, particularly studies using advanced genomic approaches (e.g. RADs, UCEs or whole genome sequencing) in this under-studied region (Berumen et al., 2013) could provide greater resolution to particular taxa of interest. We additionally suggest that the endemism of the Red Sea may not be solely driven by isolation related to the narrow strait of Bab al Mandab, but linked to other barriers in the Arabian Sea punctuated with pulses of ecological selection.
Acknowledgements
The “Red Sea and Western Indian Ocean Biogeography Workshop” was funded by the KAUST Office of Competitive Research Funds (OCRF) under Award no. 59130357. This synthesis was further supported by the KAUST OCRF under Award No. CRG-1-2012-BER-002 and baseline research funds to M.L.B. We thank Richard Ladle, Martin Zuschin and two anonymous referees for their constructive comments on earlier versions of the manuscript. We acknowledge logistic support from L. Chen, C. Nelson and A. Macaulay.
References
Biosketch
This paper arose from a workshop on ‘Red Sea and Western Indian Ocean Biogeography’ in the Division of Biological and Environmental Science and Engineering at King Abdullah University of Science and Technology (KAUST), Saudi Arabia. The authors' interests are based on elucidating the evolutionary processes that generate and maintain marine biodiversity in the tropical Indo-Pacific, with a particular focus on characterising endemism in marginal habitat like the Red Sea and Western Indian Ocean.
Author contributions: J.D.D. led the writing. All other authors listed here performed literature reviews and contributed to writing. R.F.M. produced a table of Red Sea endemic reef fish species and their presumed sister species. M.W.W. produced a time-calibrated phylogeny for the damselfish.




